Abstract
Background
Sedation to preterm neonates receiving less invasive surfactant administration (LISA) for respiratory distress syndrome is controversial.
Methods
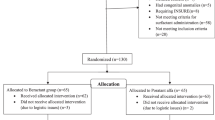
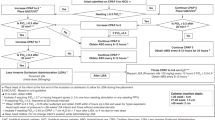
Systematic review and meta-analysis of randomized controlled trials (RCTs) and observational studies (OS) to evaluate the effect of sedative drugs for LISA on respiratory outcomes and adverse effects.
Results
One RCT (78 neonates) and two OS (519 neonates) were analyzed in pairwise meta-analysis and 30 studies (2164 neonates) in proportion-based meta-analysis. Sedative drugs might not affect the duration of the procedure [RCT: mean difference (MD) (95% CI); −11 (−90; 67) s; OS: MD 95% CI: −60 (−178; 58) s; low certainty of evidence (CoE)]. Evidence for success at the first attempt and rescue intubation was uncertain (very low CoE). The risk of nasal intermittent positive pressure ventilation [RCT: 1.97 (1.38–2.81); OS: RR, 95% CI: 2.96 (1.46; 6.00), low CoE], desaturation [RCT: RR, 95% CI: 1.30 (1.03; 1.65), low CoE], and apnea [OS: RR, 95% CI: 3.13 (1.35; 7.24), very low CoE] might be increased with sedation. Bradycardia, hypotension, and mechanical ventilation were comparable between groups (low CoE).
Conclusions
Use of sedative drugs for LISA temporarily affects the newborn’s breathing. Further trials are warranted to explore the use of sedation for LISA.
Impact
-
The effect of sedative drugs (analgesics, sedatives, anesthetics) compared to the effect of no-sedation for LISA in preterm infants with RDS is underexplored.
-
This systematic review and meta-analysis assesses the impact of sedative drugs compared to no-sedation for LISA on short-term pulmonary outcomes and potential adverse events.
-
Sedative drugs for LISA temporarily affect the newborn’s breathing (desaturation, apnea) and increase the need for nasal intermittent positive pressure ventilation. For most outcomes, certainty of evidence is low/very low.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary information files.
References
Hentschel, R., Bohlin, K., van Kaam, A., Fuchs, H. & Danhaive, O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr. Res. 88, 176–183 (2020).
Verder, H. et al. Surfaktantbehandling af nyfødte med respiratorisk distress-syndrom primaert behandlet med nasalt kontinuerligt positivt luftvejstryk. En pilotundersøgelse [Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study]. Ugeskr. Laege. 154, 2136–2139 (1992).
More, K., Sakhuja, P. & Shah, P. S. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr. 168, 901–908 (2014).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 115, 432–450 (2019).
Göpel, W. et al. German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kribs, A. et al. NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
Abdel-Latif, M. E., Davis, P. G., Wheeler, K. I., De Paoli, A. G. & Dargaville, P. A. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst. Rev. 5, CD011672 (2021).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 102, F17–F23 (2017).
Rigo, V., Lefebvre, C. & Broux, I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur. J. Pediatr. 175, 1933–1942 (2016).
Lau, C. S. M., Chamberlain, R. S. & Sun, S. Less invasive surfactant administration reduces the need for mechanical ventilation in preterm infants: a meta-analysis. Glob. Pediatr. Health 4, 2333794X17696683 (2017).
Cao, Z. L. et al. Less invasive surfactant administration in preterm infants with respiratory distress syndrome-an updated meta-analysis. J. Chin. Med Assoc. 83, 170–179 (2020).
Bellos, I., Fitrou, G., Panza, R. & Pandita, A. Comparative efficacy of methods for surfactant administration: a network meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 106, 474–487 (2021).
Dargaville, P. A. et al. OPTIMIST-A Trial Investigators. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the OPTIMIST-A randomized clinical trial. JAMA 326, 2478–2487 (2021).
Klotz, D., Porcaro, U., Fleck, T. & Fuchs, H. European perspective on less invasive surfactant administration-a survey. Eur. J. Pediatr. 176, 147–154 (2017).
Herting, E., Härtel, C. & Göpel, W. Less invasive surfactant administration: best practices and unanswered questions. Curr. Opin. Pediatr. 32, 228–234 (2020).
Kribs, A. et al. Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klin. Padiatr. 222, 13–17 (2010).
Kribs, A., Pillekamp, F., Hünseler, C., Vierzig, A. & Roth, B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks). Paediatr. Anaesth. 17, 364–369 (2007).
Dargaville, P. A., Aiyappan, A., Cornelius, A., Williams, C. & De Paoli, A. G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child Fetal Neonatal Ed. 96, F243–F248 (2011).
Dargaville, P. A., Ali, S. K., Jackson, H. D., Williams, C. & De Paoli, A. G. Impact of minimally invasive surfactant therapy in preterm infants at 29–32 weeks gestation. Neonatology 113, 7–14 (2018).
Dargaville, P. A. et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch. Dis. Child Fetal Neonatal Ed. 98, F122–F126 (2013).
Vento, M., Bohlin, K., Herting, E., Roehr, C. C. & Dargaville, P. A. Surfactant administration via thin catheter: a practical guide. Neonatology 116, 211–226 (2019).
Balakrishnan, A., Sanghera, R. S. & Boyle, E. M. New techniques, new challenges—the dilemma of pain management for less invasive surfactant administration? Paediatr. Neonatal Pain 3, 2–8 (2021).
Peterson, J., den Boer, M.C., & Roehr, C.C. To sedate or not to sedate for less invasive surfactant administration: an ethical approach. Neonatology. 118, 639–646 (2021).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189 (2021).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012 (2000).
Cochrane Handbook for Systematic Reviews of Interventions Chapter 8. https://methods.cochrane.org/risk-bias-2. Accessed 2021.
Cochrane Handbook for Systematic Reviews of Interventions Chapter 25. https://methods.cochrane.org/methods-cochrane/robins-i-tool. Accessed 2021.
Santesso, N. et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 119, 126–135 (2020).
Dekker, J. et al. Sedation during minimal invasive surfactant therapy: a randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 104, F378–F383 (2019).
Dekker, J. et al. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 109, 308–313 (2016).
Krajewski, P., Szpecht, D. & Hożejowski, R. Premedication practices for less invasive surfactant administration—results from a nationwide cohort study. J. Matern Fetal Neonatal Med. 25, 1–5 (2020).
Akcay, N. et al. Comparison of LISA vs INSURE technique using nasal intermittent positive pressure ventilation (NIPPV) support In preterm infants: a randomized controlled trial. Med J. Bakirkoy. 17, 1–6 (2021).
Bao, Y., Zhang, G., Wu, M., Ma, L. & Zhu, J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr. 15, 21 (2015).
Boskabadi, H., Maamouri, G., Gharaei Jomeh, R. & Zakerihamidi, M. Comparative study of the effect of the administration of surfactant through a thin endotracheal catheter into trachea during spontaneous breathing with intubation (intubationsurfactant-extubation method). J. Clin. Neonatol. 8, 227–231 (2019).
Choupani, R., Mashayekhy, G., Hmidi, M., Kheiri, S. & Khalili Dehkordi, M. A comparative study of the eJicacy of surfactant administration through a thin intratracheal catheter and its administration via an endotracheal tube in neonatal respiratory distress syndrome. Iran. J. Neonatol. 9, 33–40 (2018).
Gupta, B. K., Saha, A. K., Mukherjee, S. & Saha, B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur. J. Pediatr. 179, 1287–1293 (2020).
Halim, A., Shirazi, H., Riaz, S., Gul, S. S. & Ali, W. Less invasive surfactant administration in preterm infants with respiratory distress syndrome. J. Coll. Physicians Surg. Pak. 29, 226–330 (2019).
Han, T. et al. Minimally invasive surfactant administration for the treatment of neonatal respiratory distress syndrome: a multicenter randomized study in China. Front Pediatr. 8, 182 (2020).
Jena, S. R. et al. Surfactant therapy in premature babies: SurE or InSurE. Pediatr. Pulmonol. 54, 1747–1752 (2019).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131, e502–e509 (2013).
Mirnia, K. et al. Comparison outcome of surfactant administration via tracheal catheterization during spontaneous breathing with INSURE. Med J. Islamic World Acad. Sci. 21, 143–148 (2013).
Mohammadizadeh, M., Ardestani, A. G. & Sadeghnia, A. R. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. J. Res Pharm. Pract. 4, 31–36 (2015).
Mosayebi, Z. et al. A randomized trial comparing surfactant administration using InSurE technique and the minimally invasive surfactant therapy in preterm infants (28 to 34 weeks of gestation) with respiratory distress syndrome. J. J. Compr. Pediatr. 8, e60724 (2017).
Okur, N. et al. Neonatal pain and heart rate variability in preterm infants treated with surfactant: a pilot study. Arch. Argent. Pediatr. 117, 397 (2019).
Olivier, F. et al. Efficacy of minimally invasive surfactant therapy in moderate and late preterm infants: a multicentre randomized control trial. Paediatr. Child Health 22, 120–124 (2017).
Pareek, P. et al. Less invasive surfactant administration (LISA) vs. intubation surfactant extubation (InSurE) in preterm infants with respiratory distress syndrome: a pilot randomized controlled trial. J. Trop. Pediatr. 67, fmab086 (2021).
Sabzehei, M.K., Basiri, B., Shokouhi, M., Ghremani, S., & Moradi, A. Comparison of minimally invasive surfactant therapy with intubation surfactant administration and extubation for treating preterm infants with respiratory distress syndrome: a randomized clinical trial. Clin. Exp. Pediatr. 65, 188–193 (2022).
Yang, G. et al. Effects of less invasive surfactant administration (LISA) via a gastric tube on the treatment of respiratory distress syndrome in premature infants aged 32 to 36 weeks. Medicine (Baltimore) 99, e19216 (2020).
Bourgoin, L. et al. Administering atropine and ketamine before less invasive surfactant administration resulted in low pain scores in a prospective study of premature neonates. Acta Paediatr. Int J. Paediat. 107, 1184–1190 (2018).
Brotelande, C. et al. Premedication with ketamine or propofol for less invasive surfactant administration (LISA): observational study in the delivery room. Eur. J. Pediatr. 180, 3053–3058 (2021).
de Kort, E. et al. Quality assessment and response to less invasive surfactant administration (LISA) without sedation. Pediatr. Res. 87, 125–30 (2020).
Descamps, C. S. et al. Propofol for sedation during less invasive surfactant administration in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 102, F455 (2017).
Höck, M., Posod, A., Waltner-Romen, M., Kiechl-Kohlendorfer, U. & Griesmaier, E. Less invasive surfactant administration is associated with a higher need for nonpharmacological pain-relieving interventions compared to the intubation-surfactant extubation technique in preterm infants. Paediatr. Neonatal Pain. 3, 29–35 (2021).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 103, 252–8 (2013).
Shetty, S. et al. Less invasive surfactant administration in very prematurely born infants. AJP Rep. 11, e119–e122 (2021).
Tinoco Mendoza, G., Allgood, C. & Tan, A. Minimally invasive surfactant therapy for moderate to late premature neonates with respiratory distress syndrome born in a non-tertiary unit. J. Paediatr. Child Health 57, 1658–1661 (2021).
Kumar, P., Denson, S. E. & Mancuso, T. J. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics 125, 608–615 (2010).
Ancora, G. et al. Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatr. 108, 208–217 (2019).
Wycoff, C. C. Endotracheal intubation: effects on blood pressure and pulse rate. Anesthesiology 21, 153–158 (1960).
Marshall, T. A., Deeder, R., Pai, S., Berkowitz, P. & Austin, T. L. Physiologic changes associated with endotracheal intubation in preterm infants. Crit. Care Med. 12, 501–503 (1984).
Friesen, R. H., Honda, A. T. & Thieme, R. E. Changes in anterior fontanel pressure in preterm neonates during tracheal intubation. Anesth. Analg. 66, 874–878 (1987).
Oei, J., Hari, R., Butha, T. & Lui, K. Facilitation of neonatal nasotracheal intubation with premedication: a randomized controlled trial. J. Paediatr. Child Health 38, 146–150 (2002).
De Luca, D. et al. Less invasive surfactant administration: a word of caution. Lancet Child Adolesc. Health 4, 331–340 (2020).
Mehler, K. et al. German Neonatal Network (GNN). Use of analgesic and sedative drugs in VLBW infants in German NICUs from 2003 to 2010. Eur. J. Pediatr. 172, 1633–1639 (2013).
Jeffreys, E., Hunt, K., Dassios, T. & Greenough, A. UK survey of less invasive surfactant administration. Arch. Dis. Child Fetal Neonatal Ed. 104, F567 (2019).
Heiring, C., Jonsson, B., Andersson, S. & Björklund, L. J. Survey shows large differences between the Nordic countries in the use of less invasive surfactant administration. Acta Paediatr. 106, 382–386 (2017).
Fernandez, C., Boix, H., Camba, F., Comuñas, J. J. & Castillo, F. Less invasive surfactant administration in Spain: a survey regarding its practice, the target population, and premedication use. Am. J. Perinatol. 37, 277–280 (2020).
Szczapa, T., Hożejowski, R. & Krajewski, P. Implementation of less invasive surfactant administration in clinical practice—experience of a mid-sized country. PLoS One 15, e0235363 (2020).
Öncel, M. Y. & Erdeve, Ö. A national survey on use of less invasive surfactant administration in Turkey. Turk. J. Pediatr. 62, 787–794 (2020).
Bhayat, S., Kaur, A., Premadeva, I., Reynolds, P. & Gowda, H. Survey of less Invasive Surfactant Administration in England, slow adoption and variable practice. Acta Paediatr. 109, 505–510 (2020).
Kurepa, D., Perveen, S., Lipener, Y. & Kakkilaya, V. The use of less invasive surfactant administration (LISA) in the United States with review of the literature. J. Perinatol. 39, 426–432 (2019).
Reynolds, P. et al. Less-invasive surfactant administration for neonatal respiratory distress syndrome: a consensus guideline. Neonatology 118, 586–592 (2021).
Tribolet, S., Hennuy, N., Snyers, D., Lefèbvre, C., & Rigo, V. Analgosedation before less-invasive surfactant administration: a systematic review. Neonatology. 119, 137–150 (2022).
Chevallier, M., Durrmeyer, X., Ego, A. & Debillon, T. PROLISA Study Group Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double-blind, placebo controlled trial (PROLISA). BMC Pediatrics. 20, 1–9 (2020). NCT04016246.
EUCTR2018-002876-41-FR. Assesment of Propofol Sedation during Intra Tracheal Surfactant Administration by the LISA Method (Less Invasive Surfactant Administration). http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-002876-41-FR (2018).
NCT. Premedication for Less Invasive Surfactant Administration. https://clinicaltrials.gov/show/NCT03735563 (2018).
NCT. The Use of Sedation Drugs in the Procedure of Administering Surfactant Without Intubation (LISA/MIST) (LISA KGS). https://clinicaltrials.gov/ct2/show/NCT04409665 (2020).
NCT. Premedication for Less Invasive Surfactant Administration Study (PRELISA). NCT05065424 https://clinicaltrials.gov/show/NCT05065424 (2021). Added to CENTRAL:2021 | 2021 Issue 10.
NCT. Stress Assessment With and Without Analgesia During Surfactant Therapy in Preterm Infants. https://clinicaltrials.gov/show/NCT04073173 (2019).
NCT. Study on the Effects of Different Premedication for LISA on Stress and Cerebral Tissue Oxygenation in Preterm Infants. https://clinicaltrials.gov/show/nct03718507 (2018).
Acknowledgements
This paper was written as part of the European Society for Pediatric Research (ESPR) Young Investigators Mentoring Program 2019. No financial assistance was received in support of this manuscript, although the ESPR financially supported L.M. and S.H.P.S. for the YIMP Meeting at the Joint of European Neonatal Societies (JENS) 2019. The authors would like to thank Sven Wellman and Heike Rabe for the initiative of this program and the ESPR for their support. The authors wish to thank Wichor Bramer from the Erasmus MC Medical Library for developing and updating the search strategies.
Author information
Authors and Affiliations
Contributions
L.M., V.V.R., C.C.R., and S.H.P.S. gave substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data of this manuscript. L.M., V.V.R., I.K.M.R., E.B., C.C.R., and S.H.P.S. drafted the article and revised it critically for important intellectual content. All the authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Moschino, L., Ramaswamy, V.V., Reiss, I.K.M. et al. Sedation for less invasive surfactant administration in preterm infants: a systematic review and meta-analysis. Pediatr Res 93, 471–491 (2023). https://doi.org/10.1038/s41390-022-02121-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02121-9
This article is cited by
-
Low invasive surfactant administration by videolaringoscopy: a feasibility study
Italian Journal of Pediatrics (2025)
-
Outcomes, safety and health economics of introduction of video laryngoscopy-assisted less invasive surfactant administration
Journal of Perinatology (2025)
-
Can non-pharmacological comfort care replace fentanyl in LISA? The NONA-LISA feasibility study
Pediatric Research (2025)
-
Dexmedetomidine for Less Invasive Surfactant Administration: A Pilot Study
Pediatric Drugs (2025)
-
NON-pharmacological Approach Less Invasive Surfactant Administration (NONA-LISA) trial: protocol for a randomised controlled trial
Pediatric Research (2024)