Abstract
Background
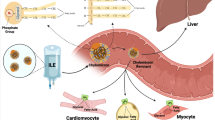
Long-term parenteral nutrition in children often results in intestinal failure-associated liver disease (IFALD). Phytosterols are plant steroids in vegetable oil-based intravenous lipid emulsions (ILEs) that are associated with IFALD. We investigated whether a phytosterol-depleted soybean oil ILE, compared to standard soybean oil ILE, prevented hepatotoxicity in a murine IFALD model.
Methods
Eight-week-old male C57BL/6 J mice were provided a fat-free high carbohydrate liquid diet for 19 days. Mice were intravenously administered ILEs as the sole fat source: Intralipid® (commercially available soybean oil ILE), Omegaven® (commercially available fish oil ILE), a low phytosterol soybean oil ILE (L-SOLE) or a high phytosterol soybean oil ILE (H-SOLE) with matched alpha tocopherol content. On days 6, 12, and 18 mice were administered escalating intraperitoneal doses of lipopolysaccharide.
Results
Compared to chow controls, mice that received Intralipid® demonstrated elevated plasma biomarkers of liver injury and histologic liver disease (hepatosteatosis, histologic inflammation, F4/80 staining). L-SOLE prevented both biochemical and histologic liver injury. Administration of H-SOLE also prevented biochemical liver injury, but not steatosis.
Conclusion
The combination of phytosterol removal and alpha tocopherol supplementation may reduce the toxicity associated with parenteral use of soybean oil-based ILE. Low phytosterol soybean oil may be a valuable component in safer next generation ILEs.
Impact
-
Half of children receiving long-term parenteral nutrition develop intestinal failure-associated liver disease (IFALD).
-
Standard intravenous lipid emulsions (ILEs) in parenteral nutrition are vegetable oil based and high in phytosterols (plant steroids); no low phytosterol vegetable oil-based ILE is available.
-
Phytosterols in ILEs are associated with IFALD.
-
In this study, a new phytosterol-depleted soybean oil was utilized in a laboratory-generated ILE. Use of the phytosterol-depleted soybean oil ILE prevented liver injury in a murine model of IFALD.
-
Phytosterol-depleted soybean oil may be utilized as a component of less toxic next-generation ILEs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article as a supplementary file.
References
Lauriti, G. et al. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J. Parenter. Enter. Nutr. 38, 70–85 (2014).
Secor, J. D. et al. Current strategies for managing intestinal failure-associated liver disease. Expert Opin. Drug Saf. 20, 307–320 (2021).
El Kasmi, K. C. et al. Macrophage-derived IL-1β/NF-κB signaling mediates parenteral nutrition-associated cholestasis. Nat. Commun. 9, 1393 (2018).
El Kasmi, K. C. et al. Phytosterols Promote Liver Injury and Kupffer Cell Activation in Parenteral Nutrition–Associated Liver Disease. Sci. Transl. Med 5, 206ra137 (2013).
Clayton, P. T. et al. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology 105, 1806–1813 (1993).
Mutanen, A. et al. Serum plant sterols, cholestanol, and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 100, 1085–1094 (2014).
Fell, G. L. et al. Alpha-tocopherol in intravenous lipid emulsions imparts hepatic protection in a murine model of hepatosteatosis induced by the enteral administration of a parenteral nutrition solution. PLoS One 14, e0217155 (2019).
Guthrie, G. et al. Depletion and enrichment of phytosterols in soybean oil lipid emulsions directly associate with serum markers of cholestasis in preterm parenteral nutrition-fed pigs. JPEN J. Parenter. Enter. Nutr. 46, 160–171 (2022).
Guide for the Care and Use of Laboratory Animals: Eighth Edition. 12910 (National Academies Press, Washington, D.C., 2011). https://doi.org/10.17226/12910.
Chapter <729>. Globule Size Distribution in Lipid Injectable Emulsions. in USP37 - NF32 2014: U.S. Pharmacopeia National Formulary 360–363 (United States Pharmacopeia, Rockville, MD, 2014).
Baker, M. A. et al. Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury. Am. J. Clin. Nutr. 109, 1038–1050 (2019).
Meisel, J. A. et al. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J. Pediatr. Surg. 46, 666–673 (2011).
Hamesch, K., Borkham-Kamphorst, E., Strnad, P. & Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 49, 37–46 (2015).
Xu, Z. et al. Steroidal compounds in commercial parenteral lipid emulsions. Nutrients 4, 904–921 (2012).
Calkins, K. L. & Robinson, D. T. Intravenous Lipid Emulsions in the NICU. Neoreviews 21, e109–e119 (2020).
Liang, W. et al. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS One 9, e115922 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Shu, J., Qiu, G. & Mohammad, I. A Semi-automatic Image Analysis Tool for Biomarker Detection in Immunohistochemistry Analysis. In 2013 Seventh International Conference on Image and Graphics 937–942 https://doi.org/10.1109/ICIG.2013.197 (2013).
Dao, D. T. et al. Vascular endothelial growth factor accelerates compensatory lung growth by increasing the alveolar units. Pediatr. Res 83, 1182–1189 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Beurel, E. & Jope, R. S. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J. Neuroinflammation 6, 1–11 (2009).
Nandivada, P. et al. Treatment of Parenteral Nutrition-Associated Liver Disease: The Role of Lipid Emulsions123. Adv. Nutr. 4, 711–717 (2013).
Mutanen, A., Lohi, J., Merras-Salmio, L., Koivusalo, A. & Pakarinen, M. P. Prediction, identification and progression of histopathological liver disease activity in children with intestinal failure. J. Hepatol. 74, 593–602 (2021).
Keefe, G. et al. Long-term assessment of bilirubin and transaminase trends in pediatric intestinal failure patients during the era of hepatoprotective parenteral nutrition. J. Pediatr. Surg. 57, 122–126 (2022).
Carter, B. A. et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 62, 301–306 (2007).
Fligor, S. C. et al. Inflammation drives pathogenesis of early intestinal failure-associated liver disease. Sci. Rep. 14, 4240 (2024).
Gura, K. M., Premkumar, M. H., Calkins, K. L. & Puder, M. Fish Oil Emulsion Reduces Liver Injury and Liver Transplantation in Children with Intestinal Failure-Associated Liver Disease: A Multicenter Integrated Study. J. Pediatr. 230, 46–54.e2 (2021).
Acknowledgements
This study received funding from Lipoid GmbH under a sponsored research agreement, National Institutes of Health grants 5T32HL007734 (SCF, TIH) and 2T32DK007754-22 (STT), the Beth Israel Deaconess Medical Center Richard and Sandra Cummings Research Fellowship (SCF), the Boston Children’s Hospital Vascular Biology Program, the Boston Children’s Hospital Surgical Foundation, the Hannah Lillie Fund, The Maisie Ellis & Friends Fund, and the Luke Raymond Celaya Research Fund. GmbH did not design the study, participate in the collection, analysis, or interpretation of data, and did not contribute to the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SF, KG, and MP designed the study. SF, TH, ST, AP, and MQ acquired the data. SF analyzed the data. SF, TH, ST, and MP interpreted the data. The original draft was written by SF. All authors revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
A patent application has been filed by SF, KG, and MP entitled “Methods and Compositions Relation to Treatment and Prevention of Fatty Acid Deficiencies.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fligor, S.C., Hirsch, T.I., Tsikis, S.T. et al. Intestinal failure-associated liver disease model: a reduced phytosterol intravenous lipid emulsion prevents liver injury. Pediatr Res 97, 2454–2461 (2025). https://doi.org/10.1038/s41390-024-03753-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03753-9
This article is cited by
-
Depletion of phytosterols from intravenous lipid emulsions: to be or not to be
Pediatric Research (2025)