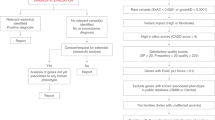
Abstract
Neurodevelopmental disorders (NDDs) include a broad spectrum of phenotypes spanning from intellectual disability (ID) to developmental delay (DD) and autism spectrum disorder (ASD). As neurodevelopmental phenotypes are a common presenting feature of an underlying genetic condition, professional medical organizations recommend genetic testing for all individuals with a NDD. When testing is pursued, identified genetic differences can lead to personalized clinical management with early diagnosis supporting the development of surveillance and intervention for co-occurring adverse health outcomes. Despite this, barriers to testing have prevented individuals from receiving a genetics referral and testing. Current therapeutic modalities including small molecule drugs, gene therapies, and antisense oligonucleotide therapies have emerged and shown promise in preclinical trials with therapeutic drugs gaining FDA approval. However, translational challenges are extensive, especially for identifying biomarkers of drug effects in the CNS. In this review, we discuss diagnostic approaches and clinical utility of genetic testing for rare genetic neurodevelopmental disorders, emerging development of individualized therapies, and progress for current therapeutics in addition to challenges with clinical translation and delivery. We will highlight opportunities for early diagnosis and treatment that are steadily gaining ground in favor of optimizing long-term health outcomes and improving quality of life for neurodiverse individuals.
Impact
-
The path from genomics to therapeutics for neurodevelopmental disorders continues to present multiple opportunities and challenges.
-
While emerging genome-wide sequencing and gene editing technologies deliver increased diagnostic yields and alternatives to life-long small molecule therapies, clinical translation has been challenging due to inherent cost and genetic heterogeneity.
-
Limited access to genetic testing despite practice guidelines remains a barrier towards precision therapeutics for rare neurodevelopmental disorders, while pre-clinical investigations face obstacles when translating to human subjects.
-
This review will summarize the impact of existing successes in diagnosis and therapeutics for neurodevelopmental disorders while highlighting ongoing challenges and areas of future opportunities.
Similar content being viewed by others
Introduction
Neurodevelopmental disorders (NDDs) represent a diverse spectrum of phenotypes ranging from intellectual disability (ID) to behavioral differences. Defined as delays in expected timelines for developmental milestones, developmental delay (DD) presents early in childhood, and when persistent and coupled with cognitive impairment by the age of 5 yo, often precedes an ID diagnosis. In a subset of individuals with DD, behavioral differences that affect social interactions establish a diagnosis of autism spectrum disorder (ASD). Ranging in presentation and severity with onset as early as the first year of life, core autism characteristics include enduring challenges in social and communication development alongside restrictive, repetitive behaviors.1 Common comorbidities between ASD and behavioral and psychiatric disorders such as anxiety and attention deficit hyperactivity disorder (ADHD) have been well-evidenced in the growing literature.2 For subsets of autistic individuals more significantly affected, additional symptoms may extend beyond behavioral differences to include cognitive and motor deficits, DD, as well as medical comorbidities.
With rising prevalence in the past two decades, ASD approximately affects 1 in 36 children in the United States according to the Centers for Disease Control and Prevention’s Autism and Developmental Disabilities Monitoring (ADDM) Network.3 Heritability estimates support ASD as the most heritable NDD,4 based on large differences in concordance rates between monozygotic and dizygotic twins, with monozygotic twins having rates approximately three times higher than those of dizygotic twins.1 These population-based twin studies have consistently observed a strong genetic correlation between autism traits and other neurodevelopmental diagnoses.5 Increased prevalence of clinically significant autism characteristics have similarly been shown in individuals with genetic syndromes associated with ID compared to individuals in the general population.6
Intellectual disability is characterized by early-onset below-average intellectual functioning in addition to noticeable limitations to adaptive functioning.7 ID typically presents alongside global DD, defined as a delay in two or more developmental domains such as motor, speech, and cognition; many children with ID are initially diagnosed with global DD.8 Individuals with ID have a higher prevalence of physical conditions such as neurological disorders, congenital malformations, and sensory impairments when compared to the general population.9
NDDs manifest with vast phenotypic and genetic heterogeneity that extends across presentations and genetic syndromes. An emerging distinction in their phenotypic presentation is the likelihood of an underlying genetic diagnosis depending on the presence of distinct signs or phenotype combinations that help to identify underlying genetic conditions. The likelihood of an associated genetic diagnosis increases with additional co-occurring medical diagnoses, in contrast to cases with isolated neurodevelopmental phenotypes.10 It is estimated that up to 40% of autistic individuals with complex phenotypic presentations may carry a diagnosis of a genetic syndrome or chromosomal abnormality.11 Similarly, a genetic diagnosis is more likely in cases of ID that present associated with congenital malformations or comorbidities, or additional clinical features such as epilepsy and metabolic manifestations, compared to those that manifest ID or global DD as the only presenting feature.8
One of the major findings and subsequent challenges in identifying risk genes for NDDs is that most of the risk, at the population level, derives from common genetic variation.12 Genetic changes that are causally linked to disease and are thus rare in the population remain missing in larger-scale genomics data. However, rare genetic changes have recently emerged repeatedly across patients through informed investigations of shared similar symptom profiles. As ASD and ID are often a presenting feature of a genetic condition, professional medical organizations such as The American College of Medical Genetics and Genomics (ACMG) initially recommend that all individuals with an ASD diagnosis be offered genetic testing, with chromosomal microarray (CMA) testing suggested as first-tier testing for all patients and Fragile X testing suggested for males.11 In cases where genetic testing is pursued, an identified genetic diagnosis can lead to clinical utility, with early diagnosis supporting the development of personalized surveillance and intervention plans. Development of a personalized treatment plan based on precise genetic findings can likewise aid clinicians in anticipating the development of associated co-occurring conditions.13,14,15,16,17,18,19,20 However, for many genetic disorders that fall beyond the classification of rare to ultra-rare, limitations remain on informed clinical management because of their scarcity in the population which creates a paucity of natural history data.
In this review, we will discuss the diagnostic approaches and clinical utility of genetic testing in early diagnosis of rare genetic NDDs and emerging development of individualized precision therapies. This review will integrate existing successes in diagnosis and therapeutics for rare genetic NDDs while highlighting ongoing challenges and areas of future opportunities (Fig. 1). We emphasize the application of these recommendations in identifying and treating the medical comorbidities that disproportionately affect individuals with NDDs in favor of optimizing long-term health outcomes and improving quality of life.
Gene discoveries and translational advances define rare neurodevelopmental disorders
Over the past decade a flurry of discoveries has expanded the number of novel single gene genetic conditions associated with neurodevelopmental phenotypes.13 Advances in genome sequencing technologies, coupled with improvements in reference genomes and databases of natural population variants, have led to the identification of thousands of rare and ultra-rare genetic disorders.21,22 Emergence of genome-wide sequencing tools and their improvements over time has iteratively increased the spectrum of genomic alterations associated with human phenotypes.23
Early on, genetic testing was restricted to evaluations of chromosome number and structures (ploidy) using karyotypes, coupled with fluorescence in situ hybridization for detection of deletions and duplications of chromosomal segments.24 This led to the recognition of fragile sites on the X chromosome and the identification of the FMR1 gene associated with Fragile X syndrome, one of the most common NDDs in males.25 Chromosomal microarray allowed for the detection of smaller losses and gains of chromosomal material (copy number variations, CNVs) and revealed many common genetic etiologies now commonly associated with ASD and ID, such as deletions or duplications in 1q21.1, 7q11.23, 15q11.2, 16p11.2 and 22q11.2.26,27,28 Characterizing single gene contributions to NDDs, however, proved more challenging, as it often required large families (pedigrees) of affected individuals to link a specific gene to the observed NDD (linkage analysis). The advent of next generation sequencing (NGS), the ability to massively sequence all regions of the genome in parallel, allowed for the generation of complete genomes and thus identification of all protein-coding genes.29 This disruptive technology allowed for expansion of the known repertoire of single nucleotide gene variants (single nucleotide variations) known in the population.30,31 NGS can be used to sequence a whole human genome (genome sequencing, GS), or by capturing only the gene sequences that code for proteins (exome), generate whole exome sequences (exome sequencing, ES) to identify variants that are distinct from reference genomes.32 As more genomes were sequenced in unaffected individuals, collection of the data in centralized databases like the Genome Aggregation Database (gnomAD33) allowed for population frequency estimates for each single nucleotide variant (SNV), establishing prevalence in the general population. These efforts have facilitated identifying SNVs rarely observed in the population, which statistically represent gene variants under negative selection (not passed down from generation to generation).22 It is these rare variants that, in combination with the shared phenotypes observed across individuals that carry them, have established new diseases and genetic syndromes associated with NDDs.34 Analysis of family trios, in which ES for both unaffected biological parents is included in the variant analysis, has allowed identification of de novo variants only present in the affected proband as one of the most common diagnostic etiology.35 All of the above-mentioned genomic sequencing technologies have successfully transitioned as diagnostic tests with demonstrated impact on identifying genetic associations for greater than 40% of syndromic NDDs.15
Genetic heterogeneity
Genome-wide diagnostic tests implemented in the clinical care of individuals with NDDs include chromosomal microarray, ES, and GS. Genome-wide chromosomal microarray screens the entire genome for CNVs and has been recommended as a first-tier test among patients with NDDs.13 CMA technologies can detect CNVs, but face limitations in the size of the deletion or duplication that can be detected. In the last decade, technologies that have enabled sequencing large amounts of DNA have emerged, allowing for testing beyond single or several genes to test panels that include extensive gene lists. In ES, exons of the genome are sequenced facilitating the detection of sequence level, single gene variants, while GS allows for comprehensive assessment of both CNVs and single gene variants across the genome.
The phenotypic heterogeneity of NDDs extends to their genetic architecture as recent analyses utilizing modern genome scanning have identified hundreds of genes worthy of further investigation. ES studies have revealed an estimated 400–1000 genes to be involved in ASD susceptibility,36 with studies of large genotyped cohorts having recognized several pathogenic chromosomal copy number variants (CNVs) as major contributors of risk.37 Prevalence of pathogenic CNVs are estimated to be 10 to 15% in individuals with ASD.38 Although rare versus common genetic variants continue to be studied in their contributions towards autism risk, common variation contributes significantly at the population level.39
Phenotypic heterogeneity
Approximately 80% of ASD cases are non-syndromic with no clear connection to a single genetic condition.40 However, growing research studies on the genetic architecture of ASD have supported that different individuals with the same or similar clinical presentations may have varying and distinct underlying genetic etiologies.41 Thus, co-occurring symptoms in combination with an ASD or DD diagnosis suggest that a genetic condition should be considered within these populations.
An ASD or NDD diagnosis accompanied by additional medical comorbidities is highly suggestive of underlying genetic conditions. Studies have measured the incidence of cancer in ASD to be globally equal to that of the general population,42 highlighting cancer as an additional phenotype not directly associated with autism and its related mechanisms. However, considered rare in combination, an ASD diagnosis with such medical findings should prompt a patient’s referral to a geneticist. Lysosomal storage disorders (LSDs) are metabolic disorders characterized by reduced or absent levels of lysosomal enzymes, leading to accumulation of macromolecules and resulting in organomegaly. In individuals with an ASD diagnosis, medical concerns aligning with distinct presentations seen in patients with LSD should, likewise, lead to a genetic evaluation. The presence of neurodegenerative symptoms, characterized by developmental regression or progressive loss of milestones, alongside ASD are further examples of potential co-occurring diagnostic concerns. In the setting of individuals with diagnosed DD or ASD, presenting with symptoms of regression unique to known genetic syndromes should prompt physicians to seek genetic evaluation and testing.
Early-onset, concomitant neurodevelopmental and medical diagnoses can serve as key markers for identifying an associated genetic condition and, in some instances, are amenable to screening and intervention. Significant motor differences can be exhibited in infants with elevated likelihoods of autism between 6 and 36 months of age, including delays in reaching motor milestones as well as fine and gross motor problems.43 In a retrospective review of 316 patient charts in a neurodevelopmental clinic, hypotonia and motor delays emerged as being strongly associated with a genetic diagnosis, with an approximately 5 to 11% increase in likelihood for an associated pathogenic variant with each 1 month delay in walking onset.44 Motor phenotypes have thus emerged as highly predictive of an associated genetic condition, highlighting cases where genetics diagnostics may be pursued even when a corresponding neurodevelopmental diagnosis is missing. Similarly, a common association has been recognized in children with epileptic encephalopathies (such as infantile spasms) and ASD for tuberous sclerosis complex (TSC),45 a genetic disorder characterized by the development of benign tumors across several organs.46 Congenital anomalies and cardiomyopathies are among additional co-occurring phenotypes that, in a patient diagnosed with a NDD, should trigger a referral for a genetic evaluation as emerging therapeutic opportunities depending on genetic diagnosis, particularly genes associated with the RAS signaling pathway (rasopathies), are having an impact on health outcomes.47,48,49
Broad heterogeneity in phenotypic presentation across genes creates additional complexities when considering syndrome specificity and developing informed interventions. Individuals with a diagnosed genetic condition may not present with the same symptoms despite having identical genetic etiologies. This can be due to differences in penetrance, the likelihood of observing a specific phenotype associated with a genetic condition, or variable expressivity, the variety of symptoms observed in individuals affected by a specific genetic condition. An example is TSC, which presents both genetic heterogeneity and variable expressivity. Tuberous sclerosis complex can be caused by either the TSC1 or TSC2 genes, and individuals carrying the same disease-causing gene variant within or between families might present with a variable spectrum of phenotypic findings.50 Considering this diversity and reduced penetrance within groups of dominant genetic diseases, recommendations for genetic testing may require to be bolder and more expansive, encompassing all individuals with a known NDD as this diagnosis, even in isolation, may represent a milder and earliest manifestation of an otherwise more complex genetic syndrome.
Diagnostic outcomes and gene networks
To date, there are more than 3000 genes with unique associations to neurodevelopmental phenotypes as part of a genetic syndrome. As more gene variants are discovered associated within each of these genes, current practice guidelines direct their classification into three major categories: pathogenic/likely pathogenic, benign, and variants of uncertain significance.51 A positive diagnostic test result is usually associated with a pathogenic or likely pathogenic variant in a well-established gene associated with neurodevelopmental symptoms. A negative result does not report any variants for the patient; however, this does not rule out the possibility of a genetic association. Typically, additional research needs to take place for the number of genes associated with NDDs to expand. Therefore, patients are usually recommended to follow up over the years as more genes are identified and more comprehensive tests are developed. Finally, variants of uncertain significance present a unique challenge to patients as they typically are neither associated with pathogenic or benign status. This also requires follow-up over time and additional research to take place to eventually resolve the variant uncertainty. Furthermore, current gene variant reference databases lack comprehensive diversity, which increases the likelihood of reporting variants of uncertain significance in instances where those variants are prevalent in specific ancestries that are not represented in the databases.52
For those with a pathogenic variant, the genes associated with NDDs have emerged as important in several developmental processes during brain formation as well as development and function of other organs. Large ASD genetic screening studies have identified broad categories of gene pathways that have been identified recurrently across NDDs. Amongst these, chromatin modifying proteins and signaling pathways have emerged as the most common and most likely targetable genetic associations. Dysfunction in the ERK/MAPK signaling pathway has been implicated in numerous NDDs including ASD, ADHD, and Fragile X syndrome, with mutations in the signaling machinery correlating to both upregulation and downregulation of proteins that make up the ERK/MAPK cascade.53 Additionally, the PI3K/mTOR pathway has emerged with a central role in numerous developmental processes associated with autism as mutations of several genes involved in components of the pathway such as TSC1 and PTEN are required for crucial roles in protein synthesis and mRNA translation, all common cellular phenotypes that when altered are associated with ASD.54
As a result of many of these gene families and their co-occurring conditions emerging as frequent associations with NDD, many patient-focused organizations as well as academic institutions have developed clinics that provide care to patients with these rare conditions. Examples include Centers for Excellence for Angelman syndrome and Tuberous Sclerosis supported by private foundations like the Tuberous Sclerosis Alliance. Similar clinics that focus on chromatinopathies, metabolic disorders, or are genetic syndrome-specific have emerged across medical centers to provide precision care unique to these conditions. Providing access to highly trained specialists for specific conditions and a genetics-first approach to care, the UCLA Care and Research in Neurogenetics (CARING) Clinic serves as one such model for delivering neuropsychiatric care for patients.55 Among 316 patients over a 5-year period, approximately 78% received a genetic testing before, during, or after CARING clinic and, of the 152 patients with an identified pathogenic or likely pathogenic variant, 36 patients received their genetic diagnosis from the CARING Clinic. The clinical management of all 36 patients with pathogenic or likely pathogenic variants was also affected in at least one domain. Clinics that utilize a genomics-informed approach to care agnostic to a specific diagnosis provide significant clinical benefits to patients with NDDs, directly affecting management across multiple domains. As the number of genetic syndromes associated with NDDs expands over time, emphasizing care around core principles of genomic medicine over individual syndromes will likely become the norm.
Barriers to access to genetic testing
Although genome-wide technologies have translated into the clinic as genetic diagnostic tools, there remain multilevel barriers to access that prevent affected individuals from receiving genetic testing. An empirical study examining parental experiences and challenges with pathways to ASD genetic testing highlighted a broad lack of insurance coverage as discouraging many providers from ordering testing, simultaneously raising concerns about the utility of such testing.56 Of the remaining 20% of patients who did not receive genetic testing, these individuals were similarly reported to have been denied by insurance for not meeting specific insurance policy requirements for testing, or to have been lost to follow-up.55 Insurance denial rates can be as high as 18% in many instances.57 Additionally, many patients and their families may never receive access to a specialist who can recognize certain medical concerns as requiring additional testing, either because of their specific policy coverage or through geographic barriers preventing access to large academic and clinic centers. These geographic barriers include lack of transportation, concentration of specialists in urban centers, lower health literacy, digital divide, and lack of primary care provider knowledge.58 Lack of knowledge amongst providers on what tests to order and when, as well as how to interpret genetic test results, likewise remains a significant barrier to patients gaining a genetic testing referral.59 Perhaps most challenging are perceptions that genetic testing is unlikely to affect clinical management. However, evidence-based guidelines and position statements have demonstrated the clinical utility of a referral to a genetics provider and genetic testing.60
Clinical utility of genetic testing
Identification of an individual’s underlying genetic diagnosis can provide both personal and clinical utility to patients and their families.61 The utility that arises from genetic testing, however, varies in many ways from other diagnostic techniques. As patients undergo medical testing and receive a diagnosis, the subsequent expectation is to receive an intervention for their condition. The complexities of a genetic diagnosis are exemplified here, in that there may not be an identified, direct action to intervene. Thus, the question of clinical utility is better answered when considering the potential benefits patients experience from receiving an early diagnosis of a genetic condition. Beyond providing a diagnostic answer (which many patients and their families find valuable in and of itself as the end of a diagnostic journey), a genetic diagnosis can lead to positive changes in surveillance, medical management, and access to clinical trials/research for individuals with rare genetic conditions.38 For example, a genetic diagnosis of GLUT1 deficiency can lead to the implementation of a therapeutic intervention like the ketogenic diet. In a systematic evidence-based review of literature describing the clinical outcomes resulting from ES and GS in patients with congenital anomalies, DD, or ID, 95% of the 167 included studies reported a change in the clinical management of patients.61 Genetic counseling as part of the diagnosis process can also have an impact on patient decision-making and family planning.62,63 Additionally, access to support and advocacy groups as well as the potential for reduced healthcare costs highlight some of the personal impacts patients and their families may experience. As research continues to be generated regarding the best practices and guidelines for the medical management of rare genetic NDDs, in addition to the development of precision therapies, rates of changes in clinical management can be expected to increase as a result of patients receiving an early genetic diagnosis.
Therapeutic approaches to rare genetic neurodevelopmental disorders
Advances in therapeutic approaches for rare genetic NDDs have seen varying levels of success in distinct patient populations. Small molecule drugs have been a primary focus for developing therapeutics due to their ease of delivery and demonstrated high efficacy in specific conditions.64 Through oral delivery in the form of a pill, such small molecules can penetrate cell membranes and modulate the function of specific enzymes and receptors, allowing for potentially therapeutic effects by directly or indirectly targeting the altered protein or signaling pathway. However, most current treatments target symptoms rather than underlying pathophysiology. Alternatively, gene therapies have emerged as a novel technique with the potential to reverse genetic abnormalities underscoring disease pathogenesis.
Natural history studies have emerged as an essential component in the development and approval of therapeutic interventions for rare diseases. These studies collect longitudinal phenotypic data to identify specific clinical endpoints amenable to inclusion in clinical trials to facilitate drug development and approval.65 For gene therapy to be a viable treatment option, the condition must have a well-established natural history with the potential for correction through the administration of functional genes copies or reversion of sequence variants; thus, NDDs generally associated with highly penetrant single gene variants, such as Angelman syndrome, are the ideal candidates for gene therapeutics.66
Delivery methods and administration routes are a few of the current barriers when considering the therapeutic approach most amenable for treatment in rare genetic conditions. The use of small molecule drugs is characterized by short-term effects, requiring continuous oral intake of the drug across an individual’s lifetime. In contrast, gene therapies provide long-term effects through a single dose but require delivery via an injection into the body. Despite systemic injection being a suitable and attractive option for delivery in cases where the genetic condition is characterized by genetic abnormalities outside of the brain, low blood-brain barrier (BBB) permeability creates additional obstacles with injection delivery for central nervous system (CNS) disorders.67 For such cases, an intrathecal injection would be administered to bypass the BBB, allowing the therapy to reach the CNS directly. The complexities involved in the delivery of therapeutics to the brain similarly impacts the efficacy of small molecule drugs, as success rates of CNS drug development remains at 8% in comparison to rates for cardiovascular drugs at approximately 20%.68 Ensuring the ability of a drug to cross the BBB is one of the major barriers limiting the use and efficacy of these medications. A number of emerging approaches are currently under development to overcome this barrier, including nanoparticle carriers, receptor-mediated transcytosis, focused ultrasound, cell-penetrating peptides, and intranasal delivery.69,70,71
Outcome measures and biomarkers guiding intervention
The development and implementation of therapeutic interventions relies largely on the identification of outcomes that can be measured accurately and reproducibly amongst individual patients, providing quantitative and objective metrics of relevant clinical processes.72 While treatments are continually developed for many genetic NDDs, relevant clinical outcome assessments and biomarkers have been limited. In more recent years, electroencephalography (EEG) has been used as a potential diagnostic or translatable biomarker in clinical translational studies of genetic NDDs as well as idiopathic ASD, with qualitative analyses of EEGs in patients with Angelman syndrome revealing rhythmic delta activity as a reliable EEG biomarker.73 Additionally, protein and peptide-based biomarkers in ASD have been an emerging focus with studies highlighting the analysis of both protein expression levels and posttranslational modifications as having potential to increase the specificity and accuracy of markers.74 Similarly, MRI-based assessments are emerging as a reliable biomarker for clinical trial response.72
Behavioral assessments have similarly been used as primary methods for assessing therapeutic trial efficacy endpoints when administered pre and post intervention. The Autism Diagnostic Observation Schedule (ADOS) is a formal assessment with a standardized, semi-structured approach to evaluate social and communication skills, play, and creativity for individuals suspected of having ASD.75 When evaluating the efficacy of targeted behavioral interventions for individuals with an ASD diagnosis, re-administering the ADOS may serve as an evaluation of behavioral changes that are clinically significant. Alternatives to the ADOS include the Vineland Adaptive Behavior Scale and the Repetitive Behavior Scale-Revised and Social Responsiveness Scale.76 However, the vast majority of outcome measures lack reliability, validity, and sensitivity to treatment, as evidenced by multiple failures in Fragile X syndrome therapeutic trials.77 As such, the identification and use of objective outcome measures and biomarkers is of paramount importance when developing therapeutic interventions to establish mechanisms of evaluating change.
Existing and emerging therapeutic interventions for genetic neurodevelopmental disorders
As previously discussed, the extensive phenotypic heterogeneity in genetic NDDs presents challenges for identifying reliable endpoints for efficacy in the development of therapeutic interventions. However, advances in small molecule drugs and gene therapies offer promising approaches to treating co-occurring conditions in these populations. While not disease modifying, small molecule therapies have shown great potential in alleviating specific symptom domains, such as seizures, and having a large impact on quality of life.78 In the past decade, a few small molecules and gene therapies have been developed and FDA-approved for the treatment of genetic disorders (Table 1). Despite only a few, several examples of these successes have been recently reported for specific genetic syndromes associated with NDDs.
Rett syndrome (RTT) is a severe, progressive rare genetic NDD that predominantly affects females, and is one of the leading genetic causes of ID in females.79 RTT presents with a distinct natural history characterized by a developmental regression period after seemingly typical early development, followed by the onset of autistic-like features such as intense hand stereotypes, impairment in communication skills, and loss of fine motor skills.80 Diagnosis of Rett syndrome is primarily clinical with additional features ranging from epilepsy to growth restrictions, and treatments have long been limited to symptom management. Most individuals with RTT carry loss of function mutations in the X-linked gene methyl-CpG binding protein 2 (MECP2), found to be the primary cause of RTT.81 In March 2023, the United States Food and Drug Administration (USFDA) authorized Trofinetide--an oral, small molecule, synthetic analog of glycine-proline-glutamate (GPE) able to penetrate the blood-brain barrier--as the first treatment of Rett syndrome for adults and pediatric patients 2 years of age or older.82 For patients below a certain age threshold, Trofinetide may reduce the progression of RTT; for those above the age threshold, this treatment may allow neuronal networks to improve in functioning.80
Glucose transporter type 1 (GLUT1) deficiency results in deficient glucose transport over the blood-brain barrier due to mutations in the SLC2A1 gene, leading to reduced glucose availability in the brain and thus causing movement disorders, cognitive impairment, DD, and early onset epilepsy.83 In the absence of glucose in the brain, ketones serve as the only alternative energy source. As such, a ketogenic diet (high-fat, low-carbohydrates) is the treatment of choice for GLUT1 deficiency as a mechanism of providing ketones to the brain and restoring brain energy metabolism.84 Early initiation of a ketogenic diet would allow for increased cerebral metabolism of the developing brain11 and reduction of neuroinflammation in patients, contributing to improved quality of life and decreased risk of comorbidities.85 Of note, the long term use of the ketogenic diet can be associated with cardiovascular risks and metabolic disturbances, including changes in BMI, lipid profiles, and metabolic acidosis.86
Timothy syndrome (TS) is an extremely rare genetic syndrome primarily characterized by prolonged QT intervals and hand/foot syndactyly alongside multiple system malfunctions, affecting individuals early in life and resulting in severe cardiac arrhythmias.87 Caused by heterozygous, gain of function variants in the CACNA1C gene, treatments include avoiding QT prolonging medications as well as introducing medications that help maintain normal QT intervals such as beta blockers.13 Early diagnosis is of paramount importance in this high-risk patient population.
Smith-Magenis syndrome (SMS) is characterized by distinct facial features that evolve with age, DD and ID, and a typical behavioral phenotype such as maladaptive and self-injurious behaviors alongside significant sleep disturbances.88 SMS results from haploinsufficiency of the retinoic acid induced 1 (RAI1) gene which maps on the short arm of chromosome 17. Chromosome 17p11.2 microdeletions have been identified as causative in 90% of patients diagnosed with SMS, with the remaining 10% evidenced to have heterozygous pathogenic variants of RAI1.89 Inversions of typical melatonin secretion have been well-evidenced in patients with SMS in addition to disrupted nocturnal sleep, lending to the considered mechanisms for sleep disturbances in this population. Typically, management of these disturbances requires joint behavioral and medication-based intervention. Tasimelteon, a selective melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) agonist, was approved by the FDA in 2014 for treatment of individuals with non-24 h sleep/wake disorders and was shown in a double-blind randomized two-period crossover trial of 26 SMS patients to improve total sleep time and quality of sleep.90
In these select genetic NDDs, advancements in understanding disease pathogenesis have led to the development of considerable therapeutics to treat co-occurring medical concerns. Furthermore, this insight can inform symptom management across different genetic syndromes where individuals may exhibit similar co-occurring symptoms despite varying genetic diagnoses and etiologies.
Alongside small molecule drugs, advancements in gene therapies have seen an exponential rise over the last decade, encompassing techniques with the potential to alter the expression of genes and therapeutically restore homeostasis.91 Angelman syndrome (AS) is a rare genetic NDD characterized by impaired communication skills, ID, and a high prevalence of seizures. AS is caused by insufficient expression of the maternal copy UBE3A gene, which is primarily expressed in the brain while the paternal copy is silenced. Several molecular mechanisms can cause AS, such as deletion of the maternal 15q11.2-13.1 region and mutations of the maternal UBE3A gene.92 Because Angelman syndrome is genetically defined by the loss of functional UBE3A, gene therapies that introduce a functional copy of the gene are highly promising. For example, activation of the normally silenced paternal copy of the gene could rescue the absence of functional UBE3A in the brain. This approach has been explored through adeno-associated virus (AAV) mediated activation of the paternal UBE3A gene in mouse models, resulting in localized reinstatement of Ube3a to typical levels.78 Pharmaceutical companies are currently in preclinical phases exploring therapeutics using such viral vector gene replacement mechanisms.92
DNA- or RNA-based targeted therapies such as antisense oligonucleotides (ASOs) have also gained more attention for therapeutic approaches due to their high target sequence recognition specificity.93 ASOs work by targeting RNA or DNA to modulate translation, alter splicing, or impact mRNA degradation. For the treatment of epilepsy phenotypes in Angelman syndrome, targeting of the UBE3A NAT mRNA antisense transcript that normally silences paternal allele expression for degradation at the transcriptional level is possible, and studies have shown evidence of decreased UBE3A NAT expression and rescue of sense UBE3A expression in mouse models, in addition to improved cognitive and behavioral phenotypes.94 Currently, multiple experimental drugs utilizing NAT-targeting ASOs are in phase 1 or phase 2 clinical trials in adult and pediatric AS patients.91
Challenges to translational approaches for rare neurodevelopmental genetic disorders
Rare genetic disorders, defined as less than 1 in 200,000 in the United States, present unique and extensive challenges for the development of both drug and biologic-based therapeutic interventions. Designing and implementing standard randomized controlled trials are often impossible due to small and typically geographically dispersed patient populations, creating issues in the recruitment of a sufficient number of subjects.95 As such, only an estimated 5% of 10,000 recognized rare disease have an approved pharmacotherapy despite millions of affected individuals worlwide.96 Clinical practice guidelines for rare disorders have similarly met their own obstacles as a result of limited resources, a lack of published evidence, and small or virtually absent expert groups.97 Implementation of the Orphan Drug Act of 198398 facilitated development of therapeutics for rare diseases by incentivizing pharmaceutical companies to pursue therapies for these conditions.99,100 Academia partnerships with the pharmaceutical industry have been instrumental in facilitating development of most of these orphan drugs.101 Although the current model of drug development for rare diseases has led to the approval of many drugs through various therapeutic modalities, this model is not always feasible for very small populations, especially in cases where target populations are as small as one individual.102 For such instances, an “N-of-1” approach has shown increasing potential as a model for developing individualized, genetically targeted therapies used by only one or very few people with a specific rare condition. These N-of-1 genetic therapies are usually designed to restore a protein’s function or reduce the expression of a dysfunctional protein, targeting the root cause of the disease.
Individualized outcome assessments and biomarkers that can reliably and objectively measure clinical impacts relevant to the patient and their underlying condition should similarly be considered when designing N-of-1 trials.103 However, drug trials of the CNS have met repeated failures due to insufficient modulation of the intended target, highlighting the need for additional research focused on identifying CNS biomarkers.104 Establishing dose-dependent CNS effects requires one or more quantitative CNS measures of a drug’s effect on brain function to allow effective interpretation of clinical results informing mechanisms and future dose selection in efficacy trials. Due in part to the difficulty of identifying precise, biologically relevant markers, many drugs that have met preclinical success fail to translate to clinical outcomes. Furthermore, regulatory hurdles and ethical considerations have limited the number of successful clinical trials103,105 combined with the small patient populations for rare diseases which requires novel flexible clinical trial design approaches, for example, adaptive trials that allow modifications on the study design based on accumulating data.106
Failed clinical trials for neurobehavioral endpoints
Fragile X syndrome (FXS) is a NDD caused by the full silencing of the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene and is the most commonly known genetic cause of ASD and inherited intellectual disabilities.107 Silencing of FMR1, which normally encodes the Fragile X Messenger Ribonucleoprotein (FMRP), leads to subsequent loss FMRP function which regulates the synthesis of multiple proteins involved in synaptic functioning.104 Fmr1-knockout mice have been used in preclinical studies to evaluate the efficacy of two primary targets: group 1 metabotropic glutamate receptors (mGluRs) and gamma amino-butyric acid (GABA) receptors.108 Despite preclinical results demonstrating rescue of functional, morphological, and behavioral deficits in rodents,104 several clinical trials of two mGluR5-targeting therapies in adults with FXS failed to show statistically significant differences from placebo utilizing efficacy outcomes based on primary caregiver-rated measures.109
Questions have emerged about whether these clinical trials utilized the optimal outcome measures. Notably, phenotypes addressed in Fmr1-knockout mice such as rate of protein synthesis, audiogenic seizures, and spine morphology have not been explored clinically due to inaccessible or unattainable readouts, and other utilized preclinical readouts such as self-grooming are not reliable proxies of human symptoms.108 Additional measures that can be broadly applied to both preclinical models and clinically as part of therapy trials, such as EEG signatures, may be needed to improve translation.110
The RASopathies are a group of disorders characterized by germline mutations in one or more genes encoding a component of the RAS/MAPK pathway, resulting in increased signaling.111 Among the RASopathies, neurofibromatosis type 1 (NF1) and Noonan syndrome (NS) have been explored in both preclinical and clinical trials. Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, inhibit the synthesis of substrates needed for prenylation which is required for activation of RAS at the cellular level, serving as the focus of many NF1 and NS trials.112 The efficacy of statins has been reported in both NF1 mice, for improved synaptic plasticity and cognitive function, as well as NS mice for the treatment of memory and learning deficits. Translating these preclinical findings to the clinic required several randomized placebo-controlled trials exploring the efficacy of statins for improving cognitive function that were conducted but saw minimal success.113 Both lovastatin, the first commercially available statin, and simvastatin, a second-generation statin, showed potential benefits in smaller trials for NF1, but failed to show efficacy in primary outcome measures in three large randomized controlled trials evaluating cognition and behavior in children with NF1.112 Clinical results for recently completed NS trials utilizing statins have yet to be released at the time of writing.
PTEN hamartoma tumor syndrome (PHTS) characterizes a group of inherited syndromes caused by loss of function germline PTEN mutations, resulting in its loss of function as a tumor suppressor.114 Loss of PTEN function upregulates the PI3K/AKT/MTOR signaling pathway, providing a potential avenue for pharmacological inhibition at different points of the pathway. Neurobehavioral symptoms in PHTS have been suggested to respond to treatment through mTOR inhibitors as reported in preclinical studies investigating the rescue of neuronal hypertrophy and autistic-like behavioral abnormalities in mice with Pten deletions.115 However, clinical studies examining the efficacy and safety of mTOR inhibitors on behavior and cognition have been limited. A phase-2, double-blinded, placebo-controlled study investigating the mTOR inhibitor everolimus on a variety of behavioral and cognitive endpoints in humans with PHTS was conducted but failed to demonstrate intervention-related improvements in primary outcome measures.115 Promise for future opportunities were highlighted as secondary efficacy endpoints moved towards the direction of improvement.
Lessons learned
As previously mentioned, a common factor inhibiting the success of therapeutics in clinical trials for NDDs results from the difficulty for drugs to permeate the blood-brain barrier. While protecting the brain from most blood-borne substances, the BBB excludes approximately 98% of small molecule drugs and all macromolecular therapeutics from accessing the brain.69 When considering efficacy criterion for therapies in patient cohorts, the ability to penetrate the BBB and generate dose-dependent effects on the CNS is necessary and, likewise, challenging to discern with certainty in preclinical studies utilizing animal models.
Recent findings on the mechanistic similarities between genes associated in autism and cancer predisposition may provide insights addressing disparate challenges with similar therapeutic approaches.116 Elevated frequencies of heterozygous pathogenic variants in genes associated with both NDDs and somatic cancers, including PTEN, provide a framework to suggest that these recurrent mutations serve as a key component in the shared ASD and cancer etiology.117
Findings from clinical trials and cancer have highlighted the need for combination therapies to address and overcome the emergence of single drug resistance. For cancer to respond, different and combined medications are needed to target multiple gene changes underlying that cancer. The same approach has been attributed to the various gene variants underlying rare genetic NDDs and their corresponding mechanisms in the brain.36,118 A therapeutic approach incorporating combinations of medications targeting the same signaling pathway or combining medications with behavioral interventions may be necessary in cases where a therapeutic intervention leads to compensatory feedback loops, thus insuring targeting of a signal along the entirety of its pathway.119 Many medications have similarly shown promise in their early stages of intake but lose efficacy over time. In such cases, combination medications may serve as a solution.120
Impact and ethical considerations of rare disease therapeutics on common neurodevelopmental phenotypes
Individuals with NDDs face several challenges to their physical health. Co-occurring medical conditions like seizures, sleep disorders, gastrointestinal, immunological, musculoskeletal, and psychiatric disproportionately impact neurodiverse individuals.121,122 Given the high prevalence of NDDs, most individuals with neurodevelopmental phenotypes likely represent varying degrees of polygenic risk. This genomic complexity makes small molecules and gene therapies developed for rare genetic syndromes of limited clinical utility within larger groups. This genetic heterogeneity also marks a major challenge in developing targeted interventions for co-occurring medical concerns. Therefore, unique approaches will be required to translate the vast efforts in place developed for rare diseases to impact those with polygenic risk genomic profiles. This is particularly relevant as the promise of polygenic risk scores in predicting disease risk and opportunities for therapy appears to be complex, specifically for common medical conditions like cardiovascular disease.123 The development of therapies with applicability to multiple genetic syndromes or groups of disorders based on shared epistatic interactions or similar mechanistic impacts on cellular/systems functions may serve as a window for broader therapeutic applicability. A few examples are emerging of these approaches. For example, therapeutics approved for the treatment of Rett syndrome may have broader applicability to other genetic syndromes based on its effect on neuronal synaptic and inflammation.81 Similarly, small molecules like MTOR inhibitors may have broader applicability across many genetic etiologies that all intersect in downstream hyperactivation of this pathway and due to its broad effects in modulating synaptic growth, plasticity and behavior.
Ethical challenges are also emerging surrounding the use of gene therapies in NDDs. The potential use of these therapies for cognitive enhancement and prenatal eugenics, coupled with off target effects, genetic mosaicism, germline transmission risks, and challenges with informed consent and barriers to access due to costs remain unaddressed challenges as the demand for therapeutic interventions increases with the expansion of novel genetic conditions. Coupled with the technical uncertainties of the specific gene therapy approach utilized and the need for long-term surveillance of clinical outcomes, the financial burden to the clinical and research infrastructure remain unsolved challenges.124,125,126,127 The challenge of broad therapeutic applicability in the larger population of individuals affected by NDDs also raises ethical questions about target of these therapies. Harnessing the innate plasticity of the nervous system to promote changes in behavioral circuits and networks through therapeutics could open opportunities for misuse. As the field of neurogenetics accelerates the pace of translating basic discoveries into therapeutic interventions, community conversation has emerged surrounding their potential impact on altering intellectual capacity and behavioral diversity. For many, the challenges these therapies face with access to the CNS facilitates refocusing their efficacy to addressing their impacts on treating co-occurring diagnoses associated with significant morbidity and quality of life rather than on a cure for their cognitive and behavioral phenotypes. These conversations occur in parallel with initiatives that seek a rigorous ethics-informed approach to the participation of research studies that use genomics information.128 Integrating community involvement as these advances translate to the clinic will be essential to ensure that the needs of those most impacted by health disparities associated with neurodevelopmental disabilities are met.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Genovese, A. & Butler, M. G. Clinical assessment, genetics, and treatment approaches in Autism Spectrum Disorder (ASD). Int. J. Mol. Sci. 21, 4726 (2020).
Genovese, A. & Butler, M. G. The autism spectrum: behavioral, psychiatric and genetic associations. Genes 14, 677 (2023).
Maenner, M. J. Prevalence and characteristics of autism spectrum disorder among children aged 8 Years — autism and developmental disabilities monitoring network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 72, 1–14 (2023).
Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 49, 1319–1325 (2017).
Thapar, A. & Rutter, M. Genetic advances in autism. J. Autism Dev. Disord. 51, 4321–4332 (2021).
Jenner, L., Richards, C., Howard, R. & Moss, J. Heterogeneity of autism characteristics in genetic syndromes: key considerations for assessment and support. Curr. Dev. Disord. Rep. 10, 132–146 (2023).
Bruno, L. P. et al. New candidates for autism/intellectual disability identified by whole-exome sequencing. Int. J. Mol. Sci. 22, 13439 (2021).
AlMutiri, R., Malta, M., Shevell, M. I. & Srour, M. Evaluation of individuals with non-syndromic global developmental delay and intellectual disability. Children 10, 414 (2023).
Liao, P., Vajdic, C., Trollor, J. & Reppermund, S. Prevalence and incidence of physical health conditions in people with intellectual disability – a systematic review. PLoS ONE 16, e0256294 (2021).
Srivastava, S. et al. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet. Med. 21, 2413–2421 (2019).
Stafford, C. F. & Sanchez-Lara, P. A. Impact of genetic and genomic testing on the clinical management of patients with autism spectrum disorder. Genes 13, 585 (2022).
Chen, G. T. & Geschwind, D. H. Challenges and opportunities for precision medicine in neurodevelopmental disorders. Adv. Drug Deliv. Rev. 191, 114564 (2022).
Savatt, J. M. & Myers, S. M. Genetic testing in neurodevelopmental disorders. Front. Pediatr. 9, 526779 (2021).
Pandey, R. et al. A meta-analysis of diagnostic yield and clinical utility of genome and exome sequencing in pediatric rare and undiagnosed genetic diseases. Genet. Med. 27, 101398 (2025).
Manickam, K. et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 23, 2029–2037 (2021).
Chung, C. W. T. et al. Early detection of tuberous sclerosis complex: an opportunity for improved neurodevelopmental outcome. Pediatr. Neurol. 76, 20–26 (2017).
Tao, V. Q. et al. The clinical impact of chromosomal microarray on paediatric care in Hong Kong. PLoS ONE 9, e109629 (2014).
Copeland, H., Kivuva, E., Firth, H. V. & Wright, C. F. Systematic assessment of outcomes following a genetic diagnosis identified through a large-scale research study into developmental disorders. Genet. Med. 23, 1058–1064 (2021).
Butler, M. G., Moreno-De-Luca, D. & Persico, A. M. Actionable genomics in clinical practice: paradigmatic case reports of clinical and therapeutic strategies based upon genetic testing. Genes 13, 323 (2022).
Kehrer-Sawatzki, H., Bäzner, U., Krämer, J., Lewerenz, J. & Pfeiffer, C. The NF1 microdeletion syndrome: early genetic diagnosis facilitates the management of a clinically defined disease. J. Dtsch. Dermatol. Ges. 20, 273–277 (2022).
Ní Ghrálaigh, F., Gallagher, L. & Lopez, L. M. Autism spectrum disorder genomics: the progress and potential of genomic technologies. Genomics 112, 5136–5142 (2020).
Betancur, C. & Buxbaum, J. D. Gene constraint and genotype-phenotype correlations in neurodevelopmental disorders. Curr. Opin. Genet. Dev. 65, 69–75 (2020).
Kim, S.-W. & An, J.-Y. Advancing precision diagnosis in autism: Insights from large-scale genomic studies. Mol. Cells 100248 https://doi.org/10.1016/j.mocell.2025.100248 (2025).
Durmaz, A. A. et al. Evolution of genetic techniques: past, present, and beyond. BioMed. Res. Int. 2015, 461524 (2015).
Verkerk, A. J. M. H. et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991).
Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorder. Nature 466, 368–372 (2010).
Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science 316, 445–449 (2007).
Sanders, S. J. et al. Multiple recurrent de novo copy number variations (CNVs), including duplications of the 7q11.23 Williams-Beuren syndrome region, are strongly associated with autism. Neuron 70, 863–885 (2011).
Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351 (2016).
Fernández-Marmiesse, A., Gouveia, S. & Couce, M. L. NGS technologies as a turning point in rare disease research, diagnosis and treatment. Curr. Med. Chem. 25, 404–432 (2018).
Wang, T. et al. The Human Pangenome Project: a global resource to map genomic diversity. Nature 604, 437–446 (2022).
Sullivan, J. A., Schoch, K., Spillmann, R. C. & Shashi, V. Exome/genome sequencing in undiagnosed syndromes. Annu. Rev. Med. 74, 489–502 (2023).
Gudmundsson, S. et al. Variant interpretation using population databases: Lessons from gnomAD. Hum. Mutat 43, 1012–1030 (2022).
Posey, J. E. et al. Insights into genetics, human biology and disease gleaned from family based genomic studies. Genet. Med. 21, 798–812 (2019).
Turner, T. N. et al. Genomic patterns of de novo mutation in simplex autism. Cell 171, 710–722.e12 (2017).
Sahin, M. & Sur, M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science 350, aab3897 (2015).
Chawner, S. et al. A genetics-first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Am. J. Psychiatry 178, 77–86 (2021).
Srivastava, S. et al. Survey of the landscape of society practice guidelines for genetic testing of neurodevelopmental disorders. Ann. Neurol. 96, 900–913 (2024).
D’Gama, A. M. Somatic mosaicism and autism spectrum disorder. Genes 12, 1699 (2021).
Baranova, J. et al. Autism spectrum disorder: signaling pathways and prospective therapeutic targets. Cell. Mol. Neurobiol. 41, 619–649 (2021).
Leblond, C. S. et al. A genetic bridge between medicine and neurodiversity for autism. Annu. Rev. Genet. 58, 487–512 (2024).
Vuattoux, D. et al. Cancer care of children, adolescents and adults with autism spectrum disorders: key information and strategies for oncology teams. Front. Oncol. 10, 595734 (2021).
Miller, H. L. et al. Motor problems in autism: Co-occurrence or feature?. Dev. Med. Child Neurol. 66, 16–22 (2024).
Wong, N. R. et al. Clinical factors associated with genetic diagnosis in suspected neurogenetic disorders in a tertiary care clinic. Genet. Med. 27, 101252 (2025).
Specchio, N., Di Micco, V., Trivisano, M., Ferretti, A. & Curatolo, P. The epilepsy–autism spectrum disorder phenotype in the era of molecular genetics and precision therapy. Epilepsia 63, 6–21 (2022).
Curatolo, P., Scheper, M., Emberti Gialloreti, L., Specchio, N. & Aronica, E. Is tuberous sclerosis complex-associated autism a preventable and treatable disorder?. World J. Pediatr. 20, 40–53 (2024).
Faienza, M. F. et al. Cardiac phenotype and gene mutations in RASopathies. Genes 15, 1015 (2024).
Yi, J.-S., Perla, S. & Bennett, A. M. An assessment of the therapeutic landscape for the treatment of heart disease in the RASopathies. Cardiovasc. Drugs Ther. 37, 1193–1204 (2023).
Chaput, D. & Andelfinger, G. MEK inhibition for RASopathy-associated hypertrophic cardiomyopathy: clinical application of a basic concept. Can. J. Cardiol. 40, 789–799 (2024).
Rosser, T., Panigrahy, A. & McClintock, W. The diverse clinical manifestations of tuberous sclerosis complex: a review. Semin. Pediatr. Neurol. 13, 27–36 (2006).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17, 405–424 (2015).
Landry, L. G., Ali, N., Williams, D. R., Rehm, H. L. & Bonham, V. L. Lack of diversity in genomic databases is a barrier to translating precision medicine research into practice. Health Aff. 37, 780–785 (2018).
Iroegbu, J. D., Ijomone, O. K., Femi-Akinlosotu, O. M. & Ijomone, O. M. ERK/MAPK signalling in the developing brain: Perturbations and consequences. Neurosci. Biobehav. Rev. 131, 792–805 (2021).
Jiang, C.-C. et al. Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Signal. Transduct. Target. Ther. 7, 229 (2022).
Besterman, A. D. et al. Genomics-informed neuropsychiatric care for neurodevelopmental disorders: Results from a multidisciplinary clinic. Genet. Med. 101333 https://doi.org/10.1016/j.gim.2024.101333 (2024).
Barton, K. S. et al. Pathways from Autism Spectrum Disorder (ASD) Diagnosis to Genetic Testing. Genet. Med. 20, 737 (2017).
Zion, T. N. et al. Insurance denials and diagnostic rates in a pediatric genomic research cohort. Genet. Med. 25, 100020 (2023).
Best, S., Vidic, N., An, K., Collins, F. & White, S. M. A systematic review of geographical inequities for accessing clinical genomic and genetic services for non-cancer related rare disease. Eur. J. Hum. Genet. 30, 645–652 (2022).
Pinzón-Espinosa, J. et al. Barriers to genetic testing in clinical psychiatry and ways to overcome them: from clinicians’ attitudes to sociocultural differences between patients across the globe. Transl. Psychiatry 12, 442 (2022).
ACMG Board of Directors Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet. Med. 17, 505–507 (2015).
Malinowski, J. et al. Systematic evidence-based review: outcomes from exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability. Genet. Med. 22, 986–1004 (2020).
Biesecker, B. Genetic counseling and the central tenets of practice. Cold Spring Harb. Perspect. Med. 10, a038968 (2020).
Kristoffersson, U. & Johansson-Soller, M. Pregnancy planning and genetic testing: exploring advantages, and challenges. Genes 15, 1205 (2024).
Vogt, L., Quiroz, V. & Ebrahimi-Fakhari, D. Emerging therapies for childhood-onset movement disorders. Curr. Opin. Pediatr. 36, 331 (2024).
Gavin, P. The importance of natural histories for rare diseases. Expert Opin. Orphan Drugs 3, 855–857 (2015).
Pena, S. A. et al. Gene therapy for neurological disorders: challenges and recent advancements. J. Drug Target. 28, 111–128 (2020).
Gao, J. et al. Gene therapy for CNS disorders: modalities, delivery and translational challenges. Nat. Rev. Neurosci. 25, 553–572 (2024).
Jiao, Y. et al. Drug delivery across the blood–brain barrier: a new strategy for the treatment of neurological diseases. Pharmaceutics 16, 1611 (2024).
Wu, D. et al. The blood–brain barrier: structure, regulation and drug delivery. Signal Transduct. Target. Ther. 8, 217 (2023).
Pedder, J. H. et al. Crossing the blood–brain barrier: emerging therapeutic strategies for neurological disease. Lancet Neurol. 24, 246–260 (2025).
Hersh, D. S. et al. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 22, 1177–1193 (2016).
Parellada, M. et al. In search of biomarkers to guide interventions in autism spectrum disorder: a systematic review. Am. J. Psychiatry 180, 23–40 (2023).
Goodspeed, K. et al. Electroencephalographic (EEG) Biomarkers in Genetic Neurodevelopmental Disorders. J. Child Neurol. 38, 466–477 (2023).
Shen, L. et al. Biomarkers in autism spectrum disorders: Current progress. Clin. Chim. Acta 502, 41–54 (2020).
Okoye, C. et al. Early diagnosis of autism spectrum disorder: a review and analysis of the risks and benefits. Cureus 15, e43226 (2023).
Mulraney, M. et al. International consensus on standard outcome measures for neurodevelopmental disorders: a consensus statement. JAMA Netw. Open 7, e2416760 (2024).
Budimirovic, D. B. et al. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J. Neurodev. Disord. 9, 14 (2017).
Copping, N. A., McTighe, S. M., Fink, K. D. & Silverman, J. L. Emerging gene and small molecule therapies for the neurodevelopmental disorder angelman syndrome. Neurotherapeutics 18, 1535–1547 (2021).
Gold, W. A. et al. Rett syndrome. Nat. Rev. Dis. Prim. 10, 1–17 (2024).
Camillo, L., Pozzi, M., Bernardo, P., Pisano, S. & Nobile, M. Profile of trofinetide in the treatment of rett syndrome: design, development and potential place in therapy. Drug Des. Dev. Ther. 18, 5023–5040 (2024).
Hudu, S. A. et al. Trofinetide for Rett Syndrome: highlights on the development and related inventions of the first USFDA-approved treatment for rare pediatric unmet medical need. J. Clin. Med. 12, 5114 (2023).
Keam, S. J. Trofinetide: first approval. Drugs 83, 819–824 (2023).
Schwantje, M., Verhagen, L. M., van Hasselt, P. M. & Fuchs, S. A. Glucose transporter type 1 deficiency syndrome and the ketogenic diet. J. Inherit. Metab. Dis. 43, 216–222 (2020).
Kolic, I. et al. GLUT1 deficiency syndrome—early treatment maintains cognitive development? (Literature Review and Case Report). Genes 12, 1379 (2021).
Falsaperla, R. et al. Is ketogenic diet a ‘precision medicine’? Recent developments and future challenges. Eur. J. Paediatr. Neurol. 48, 13–16 (2024).
Heussinger, N. et al. 10 patients, 10 years – Long term follow-up of cardiovascular risk factors in Glut1 deficiency treated with ketogenic diet therapies: a prospective, multicenter case series. Clin. Nutr. 37, 2246–2251 (2018).
Jiang, C. & Zhang, Y. Current updates on arrhythmia within Timothy syndrome: genetics, mechanisms and therapeutics. Expert Rev. Mol. Med. 25, e17 (2023).
Rinaldi, B. et al. Smith-Magenis syndrome—clinical review, biological background and related disorders. Genes 13, 335 (2022).
Kaplan, K. A., Elsea, S. H. & Potocki, L. Management of sleep disturbances associated with Smith-Magenis syndrome. CNS Drugs 34, 723–730 (2020).
Polymeropoulos, C. M. et al. Tasimelteon safely and effectively improves sleep in Smith–Magenis syndrome: a double-blind randomized trial followed by an open-label extension. Genet. Med. 23, 2426–2432 (2021).
Hong, D. & Iakoucheva, L. M. Therapeutic strategies for autism: targeting three levels of the central dogma of molecular biology. Transl. Psychiatry 13, 58 (2023).
Keary, C. J. & McDougle, C. J. Current and emerging treatment options for Angelman syndrome. Expert Rev. Neurother. 23, 835–844 (2023).
Nitschke, S. & Minassian, B. A. Antisense oligonucleotide therapy for progressive myoclonus epilepsies. in Jasper’s Basic Mechanisms of the Epilepsies (eds. Noebels, J. L., Avoli, M., Rogawski, M. A., Vezzani, A. & Delgado-Escueta, A. V.) (Oxford University Press, New York, 2024).
Quilón, P. G. et al. Antisense oligonucleotides as a precision therapy for developmental and epileptic encephalopathies. CNS Neurosci. Ther. 30, e70050 (2024).
Gross, A. M. Using real world data to support regulatory approval of drugs in rare diseases: a review of opportunities, limitations & a case example. Curr. Probl. Cancer 45, 100769 (2021).
Hechtelt Jonker, A. et al. Boosting delivery of rare disease therapies: the IRDiRC Orphan Drug Development Guidebook. Nat. Rev. Drug Discov. 19, 495–496 (2020).
Klein Haneveld, M. J. et al. Improving care for rare genetic neurodevelopmental disorders: a systematic review and critical appraisal of clinical practice guidelines using AGREE II. Genet. Med. 26, 101071 (2024).
Orphan Drug Act of 1983. Pub L. No. 97–414, 96 Stat. 2049.
Meekings, K. N., Williams, C. S. M. & Arrowsmith, J. E. Orphan drug development: an economically viable strategy for biopharma R&D. Drug Discov. Today 17, 660–664 (2012).
Waxman H. A. The history and development of the Orphan Drug Act. in Orphan Diseases and Orphan Drugs (eds Scheinberg I. H., Walsh J. M.) (Manchester University Press, 1986).
Kesselheim, A. S., Tan, Y. T. & Avorn, J. The roles of academia, rare diseases, and repurposing in the development of the most transformative drugs. Health Aff. 34, 286–293 (2015).
Jonker, A. H. et al. The state-of-the-art of N-of-1 therapies and the IRDiRC N-of-1 development roadmap. Nat. Rev. Drug Discov. 24, 40–56 (2025).
Kim-McManus, O. et al. A framework for N-of-1 trials of individualized gene-targeted therapies for genetic diseases. Nat. Commun. 15, 9802 (2024).
Grabb, M. C. & Potter, W. Z. CNS trial failures: using the fragile X syndrome-mGluR5 drug target to highlight the complexities of translating preclinical discoveries into human trials. J. Clin. Psychopharmacol. 42, 234–237 (2022).
Defelippe, V. M. et al. Toward responsible clinical n-of-1 strategies for rare diseases. Drug Discov. Today 28, 103688 (2023).
Chow, S.-C. & Chang, M. Adaptive design methods in clinical trials – a review. Orphanet J. Rare Dis. 3, 11 (2008).
Protic, D. & Hagerman, R. State-of-the-art therapies for fragile X syndrome. Dev. Med. Child Neurol. 66, 863–871 (2024).
Berry-Kravis, E. M. et al. Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat. Rev. Drug Discov. 17, 280–299 (2018).
Protic, D. D. et al. Fragile X syndrome: from molecular aspect to clinical treatment. Int. J. Mol. Sci. 23, 1935 (2022).
Kenny, A., Wright, D. & Stanfield, A. C. EEG as a translational biomarker and outcome measure in fragile X syndrome. Transl. Psychiatry 12, 34 (2022).
Hebron, K. E., Hernandez, E. R. & Yohe, M. E. The RASopathies: from pathogenetics to therapeutics. Dis. Model. Mech. 15, dmm049107 (2022).
Saint-Laurent, C., Mazeyrie, L., Yart, A. & Edouard, T. Novel therapeutic perspectives in Noonan syndrome and RASopathies. Eur. J. Pediatr. 183, 1011–1019 (2024).
Ottenhoff, M. J., Krab, L. C. & Elgersma, Y. Considerations for clinical therapeutic development of statins for neurodevelopmental disorders. eNeuro 7, ENEURO.0392-19.2020 (2020).
Haddadi, N., Travis, G., Nassif, N. T., Simpson, A. M. & Marsh, D. J. Toward systems pathology for PTEN diagnostics. Cold Spring Harb. Perspect. Med. 10, a037127 (2020).
Srivastava, S. et al. A randomized controlled trial of everolimus for neurocognitive symptoms in PTEN hamartoma tumor syndrome. Hum. Mol. Genet. 31, 3393–3404 (2022).
Crawley, J. N., Heyer, W.-D. & LaSalle, J. M. Autism and cancer share risk genes, pathways and drug targets. Trends Genet. TIG 32, 139–146 (2016).
Gabrielli, A. P., Manzardo, A. M. & Butler, M. G. GeneAnalytics pathways and profiling of shared autism and cancer genes. Int. J. Mol. Sci. 20, 1166 (2019).
Yavuz, B. R. et al. Neurodevelopmental disorders and cancer networks share pathways, but differ in mechanisms, signaling strength, and outcome. NPJ Genom. Med. 8, 37 (2023).
Mintz Hemed, N. & Melosh, N. A. An integrated perspective for the diagnosis and therapy of neurodevelopmental disorders – From an engineering point of view. Adv. Drug Deliv. Rev. 194, 114723 (2023).
Keppler-Noreuil, K. M., Parker, V. E. R., Darling, T. N. & Martinez-Agosto, J. A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. C. Semin. Med. Genet. 172, 402–421 (2016).
Micai, M. et al. Prevalence of co-occurring conditions in children and adults with autism spectrum disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 155, 105436 (2023).
O’Nions, E. et al. Diagnosis of common health conditions among autistic adults in the UK: evidence from a matched cohort study. Lancet Reg. Health Eur. 41, 100907 (2024).
Abramowitz, S. A. et al. Evaluating performance and agreement of coronary heart disease polygenic risk scores. JAMA 333, 60–70 (2025).
Shellhaas, R. A., deVeber, G. & Bonkowsky, J. L. Gene-targeted therapies in pediatric neurology: challenges and opportunities in diagnosis and delivery. Pediatr. Neurol. 125, 53–57 (2021).
Halley, M. C., Halverson, C. M. E., Tabor, H. K. & Goldenberg, A. J. Rare disease, advocacy and justice: intersecting disparities in research and clinical care. Am. J. Bioeth. 23, 17–26 (2023).
Major, R. M. & Juengst, E. T. Prenatal gene editing for neurodevelopmental diseases: ethical considerations. Am. J. Hum. Genet. 112, 201–214 (2025).
Djordjevic, D., McFadyen, A. & Anderson, J. A. Ethical challenges and opportunities in the development and approval of novel therapeutics for rare diseases. J. Med. Access 7, 27550834231177507 (2023).
Natri, H. M. et al. Ethical challenges in autism genomics: recommendations for researchers. Eur. J. Med. Genet. 66, 104810 (2023).
Acknowledgements
We acknowledge the Autism Intervention Research Network on Physical Health (AIR-P) for supporting this project.
Funding
This project is supported by the health resources and services administration (HRSA) of the U.S. Department of health and human services (HHS) under the autism intervention research network on physical health (AIR-P) grant, UT2MC39440. The information, content and/or conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. J.A.M.A. was supported by The Developmental Synaptopathies Consortium (U54NS092090), which is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN).
Author information
Authors and Affiliations
Contributions
A.A.A. and J.A.M.A. conceived the review content, wrote the manuscript, and are responsible for its contents. A.A.A. completed literature review, table, and figure. J.A.M.A. completed table content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assadourian, A.A., Martinez-Agosto, J.A. Precision diagnostic and therapeutic interventions in rare genetic neurodevelopmental disorders. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04611-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04611-y