Abstract
Background
Autism spectrum disorder (autism) describes a heterogeneous neurodevelopmental phenotype arising from the interplay of environmental and genetic factors in early life.
Methods
In a general population birth cohort, we employed a scoping approach to identify prospective associations between prenatal and birth factors and a subsequent autism diagnosis.
Results
Factors associated with increased likelihood of autism included those related to i) maternal health (maternal pre-pregnancy body mass index, pre-existing maternal mental health conditions, maternal use of selective serotonin reuptake inhibitors) ii) environmental exposures (maternal passive tobacco smoke exposure, and exposure to vinyl floors) iii) demographic factors (socioeconomic disadvantage). Factors associated with a decreased likelihood of autism included maternal dietary nutrition and supplementation (higher folic acid, magnesium, and iron, as well as adherence to the Australian Dietary Guidelines).
Conclusion
Our findings extend the evidence that autism may have a multifactorial origin in early life. Further studies should explore the composite effects of these prenatal and birth factors on autism outcomes via shared biological pathways, such as inflammation, and oxidative stress, in concert with genetic predisposition.
Impact
-
Autism spectrum disorder (autism) is a multifactorial condition. Here we report on multiple prenatal environmental, demographic, maternal and pregnancy factors that are associated with an increased likelihood of an autism diagnosis. For example, adherence to the Australian Dietary Guidelines during pregnancy is linked to a reduced likelihood of autism in the offspring, consistent with mounting evidence that prenatal nutrition impacts brain development. We examine how the multiple risk factors, identified by our comprehensive approach, may be linked to shared biological mechanisms. Future work should examine composite exposure measures acting through shared mechanisms as a more productive approach to understanding aetiology than focusing solely on individual exposures.
Similar content being viewed by others
Introduction
In this paper, we use the term ‘autism’ rather than ‘autism spectrum disorder’, in keeping with the preferences expressed by the autism community.1,2 Autism is a neurodevelopmental condition that influences how a person interacts and communicates with others and experiences the world around them.3 Autism diagnostic criteria require that the individual has persistent challenges in social interaction and communication, along with restricted, repetitive, and inflexible behaviours, interests, or activities that are atypical for the individual’s age and context.4 The World Health Organisation reports that the global prevalence of diagnosed autism is about 1 in 100 children worldwide. However, this estimate varies across studies and countries.5 In the Barwon Infant Study (BIS) cohort, the estimated prevalence of autism in the Barwon region, Victoria, Australia, was 74.8 per 1,000 children up to 11.5 years of age.6 Several countries are reporting an increase in autism prevalence over time. A multinational comparison study reported that the 10-year cumulative prevalence of autism (1990 to 2000) increased by 96% in Finland and by 354% in Denmark.7 In the US, the prevalence of autism diagnosis increased by 50% (from 18.5 per 1,000 to 27.6 per 1,000 children) between 2016 and 2020.8 Whether there has been a true increase in autism incidence remains controversial;9 nonetheless, the role of modern environmental factors requires careful evaluation.
The aetiology of autism is complex and likely heterogenous, involving multiple factors and gene-environment interactions, which dynamically influence risk and development over time. Twin studies indicate that genetic factors account for approximately 30–40% of autism risk.10 Multiple environmental factors impact gene expression via epigenetics and other mechanisms. Shared underlying biological mechanisms are not fully understood, but those of particular emerging interest include inflammation,11 mitochondrial dysfunction,12,13 and oxidative stress.14
In recent years, studies have focused on a range of prenatal and birth factors associated with autism.15,16,17 Reported prenatal factors linked to increased risk of autism include maternal age, mental health conditions, obesity, gestational diabetes, hypertension, infections during pregnancy, and maternal medication use such as selective serotonin reuptake inhibitors (SSRIs)16,17,18 and sodium valproate.19 Birth factors have also been linked to autism, including pregnancy and labour complications, delivery by caesarean section, breech presentation, preterm birth, foetal distress during birth, low birth weight, birth asphyxia.16
For many of these factors, findings vary between cohorts, possibly because autism is a multifactorial phenotype with a small individual effect size, and contributing factors can differ from population to population. Further, some studies lack power or have considered these factors in isolation.16 Focusing on a single or a small number of factors can limit the inference across a multiple risk factor range and can overlook how these factors may interact or contribute collectively to autism development.
In an Australian birth cohort, the BIS, we have demonstrated that the composite effect of multiple risk factors is important to consider, particularly if the risk factors are operating through a shared pathway.20,21 For example, oxidative stress, which occurs when the production of reactive oxygen species exceeds antioxidant and repair capacity, which is implicated in autism. We demonstrated that composite exposure to early life risk factors was associated with substantially higher odds of autism than that of any single exposure.22 Here, we aim to employ a scoping approach to examine the prospective associations between a wide range of prenatal and birth factors quantified in BIS and subsequent autism diagnosis, with the goal of identifying key factors of interest that may warrant attention for future causal analyses, including investigation of combined effects via shared biological pathways such as oxidative stress and inflammation. We extend our previous report on early autism symptom risk factors17 by examining a wide range of factors across various domains, such as demographic and household factors, maternal health and diet, environmental pollutants, and birth factors and autism diagnosis in children. We then aim to interpret the findings with consideration of the multifactorial aetiology of autism and consider the extent to which the risk factors here may be operating through common causal pathways. Using VOSviewer literature network analysis, we examined the extent to which the potential risk factors identified may be operating through common causal pathways.
Methods
Study design and participants
A population-derived birth cohort (mothers, n = 1064; infants, n = 1074, including 10 set of twins) was recruited using an unselected antenatal sampling frame in the Barwon region of Victoria, Australia. The recruitment for this longitudinal cohort was between June 2010 and June 2013. Eligibility criteria, population characteristics and measurement details have been previously described.23 The BIS cohort protocol was approved by the Barwon Health Human Research Ethics Committee (HREC 10/24) and the participating families provided written informed consent.
Exposure measures
Over 100 prenatal and birth factors were examined and listed in supplementary data (Box S1). The main domains examined were demographic and household factors, maternal health (including physical and mental) and diet, maternal exposure to environmental pollutants in pregnancy, and birth factors. Literature and data driven approaches were used to prioritise this final report. A data-adaptive approach is useful in low-knowledge settings where the list of important factors may be incomplete.24 We had an emphasis on prenatal dietary nutritional factors to reflect the quality and nutrient density of prenatal diet, given their known roles in brain development, including neurotransmitter signalling25 and neuroprotective effects,26 as well as autism development.27 During the in-clinic appointment at 28 weeks gestation, information on preconception and antenatal period was collected, including: demographic and household composition, antenatal health, nutrition and dietary patterns, vitamin intake, and supplementation. Additional information on 3rd trimester exposures such as fever, vitamin use and supplementation, alcohol use, smoking and passive tobacco exposure was self-reported retrospectively (at 4 weeks after birth) using a self-reported questionnaire. Information regarding medical conditions, including psychosocial conditions (anxiety, depression, bipolar or other), and delivery information was obtained from antenatal medical records. The selection of derived variables and categorisation were based on prior literature and is further described in detailed in Supplementary Methods. The scope of this study does not include genetic measures or other exposures measured from biological samples, such as urinary or blood chemicals, metabolomic and lipidomic measurements.
Literature-based evidence mapping and contextualisation
A literature search was conducted to review known associations between prenatal and birth factors and autism (Table S1). These factors were further contextualised in relation to shared biological pathways such as oxidative stress and inflammation (Table S1). We classified the strength of prior evidence using a ranking system based on the hierarchy of evidence (systematic reviews28,29 or large cohort studies.30) Findings from this study then informed a VOSviewer literature network analysis of clusters by keywords (Box S2).
Verified autism diagnosis
The process for obtaining a child’s verified autism diagnosis was previously described in detail.6,31 In brief, during a telephone interview in 2020–2022, 868 parents reported on their child’s autism status, with 80 parents reporting that their child had received a diagnosis or was under investigation for autism. Medical records were reviewed for 79/80 children; 64 children were confirmed to have a diagnosis of autism made by their treating paediatrician against the DSM-5 criteria. Twelve children were excluded because they were lost to follow-up, their diagnosis was still under investigation; they had an inconclusive diagnosis; or were not reported by their parent as diagnosed with autism but had a clinician-confirmed diagnosis at the time of verification completion (31 October 2023). Eight participants whose parents reported their child as under investigation for autism at the 2020–2022 review did not receive a subsequent diagnosis. Further, the verified autism diagnosis was compared to the Strengths and Difficulties Questionnaire (SDQ) scale available in BIS ages 4–10 with many subscales (Conduct Problems, Emotional Symptoms, Hyperactivity Score, Peer Problems, Prosocial Behaviour, Total Difficulties) being significantly associated (p<0.001). Detailed description of the diagnostic verification process including comparisons to other neurodevelopment outcomes is described elsewhere.6
Statistical analysis
Plots and summary statistics were used to assess the distribution of prenatal and birth factors. We calculated correlations between prenatal and birth factors and child’s autism. Visual representation of the correlations was generated using statistical package “corrplot” in R version 4.3.2 R Core Team, 2024 (Fig. 2, Fig. S1). Separate multiple logistic regression models were used to assess associations between each exposure and the binary autism outcome after adjusting for child’s sex and child’s age when autism status was first reported. These processing factors were applicable across all exposures.
Additionally, no adjustments for multiple comparisons were undertaken in this study based on the following rationale: First, factors are not independent of each other (Fig. 2, Fig. S1) and may be acting jointly. Secondly, for exploratory analysis, it is not necessary to adjust for p-value.32,33,34,35 Thirdly, it has been argued that corrections for multiple comparison may also reduce power, increasing the risk of type II error.7 Given the nature of this study, we prioritised detection, that is, avoiding type II errors rather than avoiding type I errors. The focus was on identifying potential associations for future research. Further, we followed suggestions by Althouse (2016) to (1) describe what was done in our study; (2) report effect sizes, confidence intervals, and p values without imposing a false dichotomy (e.g., interpreting p values <0.05 as ‘significant’); and (3) let readers judge the relative weight of the conclusions.32 Data was analysed using STATA version 17 (StataCorp LP). Further sparse bias was tested for variables with low cases (Supplementary Results).
Results
Cohort summary
Demographic and household composition
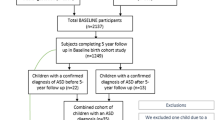
Participant demographics for the inception cohort are shown in Table S3 and study flowchart is shown in Fig. 1. In the analysis sample, the mean [SD] age of children at completion of the health screen was 9.0 [SD = 0.7] years (Table 1). The mean age at the completion of the autism verification was 11.5 [SD = 0.8] years. Of the 64 children diagnosed with autism, 43 were male (67.2%) and 21 were female (32.8%). In the analysis sample (n = 856), the average age of mothers and fathers at conception was 31.8 [SD = 4.5] years and 33.7 [SD = 5.5] years respectively (Table 1). Fifty-six per cent (n = 478) of mothers and 37.3% (n = 312) of fathers had a university education (undergraduate or postgraduate). A total of 74.1% (n = 628) of participants had all grandparents of European descent.
CBCL Child Behavior Checklist for ages 1.5–5 years, SDQ Strengths and Difficulties Questionnaire for ages 4–10.1 Due to no longer being resident in Barwon region (n = 12), <32 weeks of gestation (n = 8), serious illness in first few days of life (n = 7), major congenital disease (n = 5), stillbirth (n = 5), miscarriage (n = 2), or cord blood stored privately (n = 2).
Maternal health
The average maternal pre-pregnancy body mass index (BMI) was 25.4 (kg/m²) [SD = 5.5] (Table 1). Half of mothers (n = 428) reported any alcohol intake during pregnancy and 7% (n = 60) reported any smoking during pregnancy (Table 1). 4.4% (n = 27) of mothers scored high ( ≥13) on the Edinburgh Postnatal Depression Scale (EPDS) scale. 9.7% (n = 83) reported pre-existing psychosocial conditions, as extracted from antenatal medical records. Overall, 4.2% (n = 35) of mothers were taking SSRIs/SNRIs during pregnancy with 3.8% (n = 32) using SSRIs/SNRIs in 1st and 2nd trimesters and 2.8% (n = 24) using SSRIs/SNRIs in 3rd trimester. For dietary measures, the average Australian Recommended Food Score (ARFS) score was 35.4 [SD = 7.78] out of a maximum possible score of 74 (Table 2).
Environmental factors and pollutants
The average ambient air pollution at birth for Nitrogen Dioxide (NO2) was 5.5 Parts per billion (Ppb) [SD = 2.0] and for Particulate Matter <2.5μm (PM2.5) 7.7 µg/m3 [SD = 0.9] (Table 1). 10.6% (n = 89) of mothers reported ‘yes’ to exposure to passive tobacco smoke during the three months prior to conception or during the 1st and 2nd trimesters of pregnancy. For housing, 95.0% (n = 519) of the cohort lived in a house and 5% (n = 27) lived in an apartment, flat or unit. For additional information on household environment refer to Table S3.
Birth factors
The average gestational age at birth was 39.4 weeks [SD = 1.5] with 4.3% of births being preterm (32–37 weeks’ gestation vs rest) (Table 1). The average Apgar score at five minutes was 9 [SD = 0.8]. The median duration of labour was 5 h and 0.8% (n = 7) of infants required resuscitation at birth.
Prenatal and birth factors associated with autism diagnosis
Demographic and household composition
Several demographic and household composition factors were associated with child’s autism diagnosis by age 11.5 years (Table 1). Male children had higher odds of having autism compared to females (OR = 2.01; 95%CI = 1.17,3.4; P = 0.011). Infants of women with a university degree had 41% lower odds of being diagnosed with autism than infants without tertiary education (OR = 0.59; 95% CI = 0.35, 0.98; P = 0.043). Being a second or later-born child was associated with 58% lower odds of autism compared to first-born children (OR = 0.42; 95% CI = 0.22, 0.81; P = 0.01). Other factors such as paternal education, grandparents’ ancestry and maternal and paternal age were not associated with the child’s odds of autism (Table 1).
Maternal health
A higher maternal pre-pregnancy BMI was associated with increased odds of subsequent diagnosis of autism (OR = 1.06 per kg/m² increase; 95%CI = 1.02,1.11; P = 0.005). Any alcohol intake during the 3rd trimester was negatively associated (OR = 0.37; 95%CI = 0.17,0.81; P = 0.012) with offspring autism (Table S4). Any recreational marijuana use during pregnancy was associated with an increase in child’s autism (OR = 5.68; 95%CI = 1.46,22.62; P = 0.014). Maternal pre-existing psychosocial conditions were associated with increased odds of child’s autism (OR = 3.93; 95%CI = 2.10,7.39; P < 0.001). SSRI/SNRI use during 1st and 2nd trimester (OR = 6.43 95%CI = 2.76, 14.98; P < 0.001) and SSRI/SNRI use during 3rd trimester (OR = 9.01 95%CI = 3.70,21.9; P <0.01) was also associated with increased odds of the child having autism. Other maternal health measures such as preeclampsia, hypertension and gestational diabetes mellitus were not separately associated with autism. However, greater prenatal adversity (a combination of preeclampsia, hypertension, gestational diabetes, obesity and an EPDS score ≥1336) was associated with higher odds of autism (one of the nominated adversities vs none: OR = 2.04; 95%CI = 1.09,3.82; P = 0.025; two or more adversities vs none: OR = 2.36; 95%CI = 1.26,4.42; P = 0.008).
Maternal nutrition during pregnancy
Various indices of prenatal nutrition were associated with a reduced likelihood of subsequent autism in the offspring (Table 2). These included folate (OR = 0.69 per 100 µg/day increase; 95%CI = 0.49,0.92; P = 0.025), total choline (OR = 0.72 per 100 mg/day increase; 95%CI = 0.54,0.95; P = 0.019), magnesium (OR = 0.7 per 100 mg/day increase; 95%CI = 0.5,0.97; P = 0.032), niacin (OR = 0.01 per 100 mg/day increase; 95%CI = 0,0.66; P = 0.031), riboflavin (vitamin B2) (OR = 0.68, per g/day increase; 95%CI = 0.49,0.96; P = 0.029), thiamine (vitamin B1) (OR = 0.46 per g/day increase; 95%CI = 0.26,0.81; P = 0.007), iron (OR = 0.9 per mg/day increase; 95%CI = 0.84,0.97; P = 0.006), zinc (OR = 0.91 per mg/day increase; 95%CI = 0.84,0.99; P = 0.033), and phosphorus (OR = 0.93 per mg/day increase; 95%CI = 0.88,0.99; P = 0.031).
Overall dietary patterns were also examined; higher adherence to the Australian Dietary Guidelines represented by the ARFS was associated with lower odds (OR = 0.96; 95%CI = 0.93,0.99; P = 0.009) for autism in offspring (Table 2).
Additionally, supplementation during pregnancy was also examined (Table S5, Table S6). In a sensitivity analysis, we also adjusted for maternal energy intake (per kJ/day) (Table S7).
Environmental factors and pollutants
Maternal exposure to passive tobacco smoke 3 months prior to conception and/or during the 1st and/or 2nd trimesters was associated with higher odds of their child having autism (OR = 2.64; 95%CI = 1.38,5.05; P = 0.003) (Table 1). Vinyl flooring in the living area during pregnancy was associated with increased odds of autism (OR = 4.21; 95%CI = 1.45,12.21; P = 0.008). The estimated effect of gas heater use during pregnancy on the odds of autism was OR = 1.94; 95%CI = 0.95,3.94; P = 0.067. Exposure to other markers of environmental pollutants, such as pesticide use, ambient air pollution at birth and signs of mould/dampness, was not found to associate with autism (Table 1).
Birth factors
Preterm birth (32–37 weeks vs rest) (OR = 2.58; 95%CI = 1.02,6.51; P = 0.044) was associated with increased odds of child autism (Table 1). Other birth factors such as placental weight, gestational age at birth, Apgar score at 5 min, labour duration (hours) and delivery mode were not found to be associated with child’s autism (Table 1, Table S4).
Correlation of prenatal and birth factors
Prenatal and birth factors across various domains (maternal health and diet, maternal environmental pollutants, demographic and household, birth factors) were highly correlated (Fig. 2, Fig. S1). As an example, the wholefoods diet and the ARFS were positively correlated (R = 0.56, P <0.001). This highlights the co-occurrence patterns among many environmental factors.
A correlogram of demographic and household composition, maternal health and diet, maternal environmental pollutants and birth factors and autism diagnosis. Inter-domain correlations with >-0.23 and >0.23 and intra-domain correlations with magnitude >-0.07 and >0.08 respectively are displayed. Red lines represent positive correlations, and blue lines represent negative correlations. Pearson’s correlation was used for pairs of continuous and ordinal variables; point-biserial correlation was used for correlation between continuous and binary variables; phi coefficient was used for co1rrelation between two binary variables. DII Dietary inflammatory index, ARFS Australian recommended food score, BMI Body mass index, GDM gestational diabetes mellitus, SSRI Selective serotonin reuptake inhibitors, SNRI Serotonin and norepinephrine reuptake inhibitors, PWR Placenta-to-birth weight ratio, M maternal, C child, SEIFA Socioeconomic Indexes For Area, PA Prenatal adversity.
Qualitative integration of current findings with existing literature on prenatal and birth factors
Many of the findings that we report above have prior evidence of association with autism, thereby strengthening their relevance. For example, there is evidence from large reviews that maternal BMI, maternal alcohol use, maternal smoking and outdoor air pollution during pregnancy are associated with childhood neurodevelopmental delays and autism28,29,37,38 (Table S1).
Interrelated risk factors may act through shared pathways. To support this, we draw on existing literature and emphasise the need for future research to examine these pathways when investigating the causality of such multifaceted relationships. Table S2 summarises current literature evidence on common mechanisms of action, particularly oxidative stress and inflammation, through which these exposures may act. Additionally, Fig. 3 outlines the literature network overlap of oxidative stress and inflammation in key exposures such as socioeconomic status, maternal mental health, obesity, diet and nutrition and environmental pollutants. Socioeconomic status, maternal mental health and diet had the most literature related to oxidative stress and inflammation.
Discussion
We examined prospective associations between a wide range of prenatal and birth factors and the likelihood of the child receiving an autism diagnosis by a paediatrician against DSM-5 criteria, with the goal of identifying possible early life factors acting through unifying pathways to increase the likelihood of autism. This approach revealed important patterns that will inform future investigations. Lower socioeconomic position correlated with many factors as well as with increased likelihood of autism. Pre-existing maternal mental health conditions and SSRI/SNRIs use during pregnancy were associated with a substantially increased risk of autism. There are a variety of potential causal and non-causal explanations for this finding, which, given the public health significance, clearly warrant further investigation. Passive tobacco smoke before conception and 1st and 2nd trimesters and exposure to vinyl floors in the living room during pregnancy were positively associated with the infant subsequently being diagnosed with autism. Conversely, many prenatal nutritional indices were inversely associated with autism. Furthermore, higher adherence to the Australian Dietary Guidelines, as indicated by higher ARFS, was associated with a reduced risk of child autism. These results are consistent with evidence that pregnancy is a critical period for early life programming that can have lifelong effects.39
Maternal health
Maternal physical and mental health has been associated with an increased likelihood of autism in children. Conditions such as obesity, gestational diabetes, and preeclampsia have been shown to impact foetal brain development,40,41,42 as well as being associated with increased likelihood of autism.43,44 In our study there was no evidence that preeclampsia, hypertension and gestational diabetes mellitus were individually associated with autism, possibly due to low power. However, exploring combined factors such as a previously established metric of ‘prenatal adversity’, which includes each of these pregnancy complications and has been linked to inflammation,36 was associated with a greater likelihood of the child being diagnosed with autism. Our approach is consistent with the argument that combination effects or prenatal comorbid exposures may act synergistically to exacerbate the risk of neurodevelopmental conditions.45
Maternal mental health has previously been associated with offspring autism. Lu et al. (2022) found that depression during pregnancy was associated with an 75% increase in the risk for autism diagnosis in the child.46 Similarly, the Environmental Influences on Child Health Outcomes program combined 33 prenatal/paediatric cohorts (n = 3994) and reported prenatal depression was associated with an increase in moderate to severe autism-related traits by 64%.47 Further to this, the use of SSRIs during pregnancy has been associated with an increased likelihood of offspring autism in the literature.48,49 A large record linkage study reported that such an association did not persist after adjustment for factors such as socioeconomic factors, psychiatric history before birth and perinatal factor—gestational age.50 In our setting, Pham et al. reported associations between SSRI use during pregnancy and perinatal factors such as low Apgar scores and need for resuscitation at birth. Perinatal factors may represent downstream consequences of exposure; therefore, routine adjustment should not be made without considering these issues.51 Maternal SSRI use may be a consequence of underlying maternal psychosocial conditions.18,52 In addition inflammation during pregnancy may play an antecedent role in the development of both (i) psychiatric conditions such as depression that are a trigger to SSRIs use53,54 as well as (ii) offspring autism.55 Alternatively, maternal SSRI/SNRIs use during pregnancy may be causally linked to child autism via pathways such as oxidative stress.55 Therefore, careful evaluation of causality via cohort studies with investigation of mediation pathways and accompanying experimental work, is a priority.
Maternal nutrition and supplementation
Prenatal nutrition is emerging as a key determinant of offspring neurodevelopment.56 Multiple prenatal dietary micronutrients and minerals have been shown to be associated with reduced likelihood of a child’s autism including folate, choline, zinc, and iron.57,58,59,60 Studies from the Childhood Autism Risks from Genetics and Environment case (CHARGE) and In the Markers of Autism Risk in Babies: Learning Early Signs (MARBLES) cohorts found that prenatal vitamin use during early pregnancy was associated with a significantly reduced risk (40–50%) of having an autistic child.61,62 However, it is important to note that many prenatal multivitamins differ in dosage and nutrient composition, leading to measurement misclassification, which may contribute to null results reported in some studies.63
From a dietary pattern perspective, eating in alignment with the Australian Dietary Guidelines during pregnancy reduced likelihood of child autism, suggesting that food variety and nutrient density are important during pregnancy. A recent study examining dietary patterns reported a significant reduction in the likelihood of child’s autism across two large cohorts, after high adherence to prenatal healthy dietary pattern.64 Therefore, the prenatal dietary patterns and their association with an increased likelihood of autism needs further evaluation.
Moreover, a review has been conducted examining diet as a potential modifying factor of other environmental factors in autism.26 In fact, 10 out of the 12 studies identified prenatal dietary factors as a significant modifier attenuating the associations between adverse environmental exposure and autism. This review emphasised the importance of understanding the mechanisms of combined environmental exposures on neurodevelopmental outcomes.26
Prenatal pollution and environmental chemicals
Prenatal environmental pollutants have been reported to increase likelihood of autism in the offspring. Previously, studies have linked the mother’s urinary metabolome, including nicotine metabolites and exposure to second-hand tobacco smoke, with increased autism in the offspring.65,66 A study, including a meta-analysis of over 200,000 participants, reported that marijuana use during pregnancy increased the likelihood of autism in offspring.67,68,69 Ambient air pollution has also been linked to autism70,71 as shown previously to be associated with autism symptoms at age two in this study.17 We found that vinyl flooring was associated with a child’s autism diagnosis in part due to exposure to pollution in the home. Previous studies have reported a similar association for vinyl flooring implicating chemicals such as phthalates.72,73 These chemicals are neurotoxic and are associated with an increased likelihood of autism74; however, further research is needed to strengthen the link between the neurotoxic chemicals and the underlying mechanisms in autism.
Pollutant exposure measures derived from biosamples, such as maternal exposure to the endocrine-disrupting plastic-associated chemicals bisphenols and phthalates have been previously studied in the BIS cohort Childhood Autism Risks from Genetics and Environment case (CHARGE)22,75 and were outside of the scope of this current study.
Socioeconomic disadvantage
Our findings complement and extend previous research linking greater socioeconomic disadvantage with autism diagnosis in the offspring.76,77,78 Previous research from different countries, including Japan, Taiwan, Denmark and Sweden, found that low socioeconomic disadvantage is associated with a higher likelihood of autism, as we observed,77,79,80 whereas other studies have not.78,81,82 Socioeconomically disadvantaged groups are at a heightened likelihood of autism, potentially due to a range of contributing factors. They often face greater challenges during pregnancy, including complications such as preeclampsia and gestational diabetes.83 Additionally, higher rates of smoking, obesity, and poor dietary habits are prevalent among these groups, which can further exacerbate this risk.84 We reported in 2024 that, in BIS, maternal diet mediated the association between socioeconomic adversity and poorer offspring cognition by 22% and suggested that modifiable maternal diet may partially explain the association between socioeconomic adversity and child neurocognitive vulnerability.85 From a public health perspective, addressing modifiable factors such as diet, pollutant exposure, and obesity – if causal - should be integral to the development and implementation of public health policies.
Strengths and weaknesses
The strengths of this study include the unselected antenatal sampling frame, the high retention rate (84% to the 2020–2022 child health screen), and that we were able to review the medical records of 79 of the 80 (99%) children with parent-reported autism to identify the 64 children with a diagnosis of autism made by a paediatrician against the DSM 5 criteria. Further, we have assembled and examined a uniquely comprehensive set of environmental factors, on the premise that multiple factors will likely operate via unifying biological pathways. We have used both prior knowledge and data-driven approaches to identify and examine over 100 early life factors relevant to changes in the likelihood of autism in the offspring. Many factors correlated, consistent with multiple environmental factors operating via unifying biological pathways to autism development (Fig. 2, Fig. S1). We did not correct for multiple hypothesis testing because these exposures are unlikely acting independently and could increase the type II error in this exploratory study.7 Additionally, due to the nature of this approach including wide range of factors examined, fully adjusted multivariate analysis was not performed including potential confounding factors. The results here warrant future consideration including the exploration of shard common pathways across several exposures of interest, and of course, replication. In BIS, considering multiple exposures simultaneously has highlighted their convergence on shared biological pathways. For example, Ponsonby et al. showed that prenatal exposure to plastics, maternal smoking during pregnancy and no fish oil supplementation have been linked to altered oxidative stress which is further linked to autism symptoms in children.22 Additionally, Tanner et al. applied environmental mixtures analysis incorporating several of the potential autism risk factors identified in this study, and reported an overall mixture association with epigenetic changes in a key gene previously linked with autism in males.21,31 We acknowledge possible misclassification when deriving daily dietary nutrient measures from the food frequency questionnaire and environmental pollutants such as pesticide use during pregnancy, particularly as most of these measures are self-reported. While we have not examined confounders relevant to health service presentation and delivery, standardised criteria were applied across clinical services. Gene-environment interactions modulate neurodevelopment, therefore, these prenatal and birth factors may interact differently with an individual’s genotype.22 Finally, we look at autism as a single neurodevelopmental phenotype and in isolation of potentially co-existing neurodevelopmental factors. Further studies are required that take into consideration co-existing neurodevelopmental diagnoses such as Attention-Deficit/Hyperactivity Disorder. Finally, the BIS cohort is relatively homogeneous with respect to education level, socioeconomic status, and ethnicity. As a result, further work needs done to generalise our findings to other populations.
Future directions on proposed mechanisms of interest
Our findings reinforce that environmental factors associated with autism are multifactorial. Many of these environmental features or exposures may act by common pathways to promote pro-inflammatory and pro-oxidative profiles.22,53 We examined some of the features in this study in the context of inflammation and oxidative stress in literature, which is depicted in Table S1. These processes also impact on mitochondria dysfunction which is increasingly recognised to be an autism feature.12,13 This scoping study was designed to identify possible early life factors altering subsequent autism risk. Future work could be conducted using several approaches. Firstly, exposures that have been identified as potential autism risk factors and have established linked to the same biological pathway, such as inflammation, could be combined into composite scores. For example, we have previously reported that the following are associated with prenatal inflammation: maternal obesity, higher parity, pre-existing psychosocial conditions and passive smoking in pregnancy.53,85 Thus, these factors should be considered as possible co-exposures in an inflammatory pathway. Secondly, future research should employ multivariable analyses integrating directed acyclic graphs to identify and adjust for confounders, enabling in-depth examination of key exposures and shared biological pathways.27 For example, for SSRI use during pregnancy, one would need to evaluate the indication for SSRI use (such as depression), replicate these results in an external cohort and understand the putative mechanistic impacts, including epigenetics, lipidomics and mitochondrial pathways to fully understand the causal relationships. In our future work, we will study composite exposure associated with autism and use multivariable models to test biological mediation, as well as considering genetic predisposition, as outlined in our work regarding modern approaches to causal inference in highly dimensioned studies.24,85,86
In conclusion, our findings highlight that the prenatal period is critical for early life programming of autism. A diverse range of prenatal and birth factors, many of which are modifiable, may contribute to the aetiology of autism through shared mechanistic pathways. If multiple modifiable environmental factors influence child development through shared mechanistic pathways, future research could improve our understanding of neurodevelopmental outcomes by examining the combined effects of these factors. The focus should be on identifying unifying, targetable mechanisms and developing composite exposure measures, rather than studying single exposures in isolation.
Data availability
Access to BIS data including all data used in this paper can be requested through the BIS Steering Committee by contacting the corresponding author. Requests to access cohort data are considered on scientific and ethical grounds and, if approved, provided under collaborative research agreements. Deidentified cohort data can be provided in Stata or CSV format. Additional project information, including cohort data description and access procedures, is available at the cohort study’s website http://www.barwoninfantstudy.org.au.
References
Kenny, L. et al. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 20, 442–462 (2015).
Pellicano, E. & den Houting, J. Annual Research Review: Shifting from ‘normal science’ to neurodiversity in autism science. Journal of Child Psychology and Psychiatry 63, 381–396 (2022).
Pellicano, E. et al. A capabilities approach to understanding and supporting autistic adulthood. Nature Reviews Psychology 1, 624–639 (2022).
World Health Organization. International Classification of Diseases 11th Revision (ICD-11) World Health Organization2022.
World Health Organization. Autism 2023 [Available from: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders.
Love C. et al. Cumulative Incidence of Autism Spectrum Disorder with Longitudinal Evaluation of Neurocognitive and Behavioral Functioning 2024.
Atladottir, H. O. et al. The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur Child Adolesc Psychiatry 24, 173–183 (2015).
Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder 2023 [Available from: https://www.cdc.gov/autism/data-research/?CDC_AAref_Val=https://www.cdc.gov/ncbddd/autism/data.html.
Pearson, H. Autism is on the rise: what’s really behind the increase?. Nature 644, 860–863 (2025).
Ramaswami, G. & Geschwind, D. H. Genetics of autism spectrum disorder. Handb Clin Neurol 147, 321–329 (2018).
Siniscalco, D., Schultz, S., Brigida, A. L. & Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals (Basel) 11, 56 (2018).
Frye, R. E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin Pediatr Neurol 35, 100829 (2020).
Khaliulin, I., Hamoudi, W. & Amal, H. The multifaceted role of mitochondria in autism spectrum disorder. Molecular Psychiatry 30, 629–650 (2025).
Liu, X. et al. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front Psychiatry 13, 813304 (2022).
Yuan, J., Zhao, Y., Lan, X., Zhang, Y. & Zhang, R. Prenatal, perinatal and parental risk factors for autism spectrum disorder in China: a case- control study. BMC Psychiatry 24, 219 (2024).
Hisle-Gorman, E. et al. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatric Research 84, 190–198 (2018).
Pham, C. et al. Early life environmental factors associated with autism spectrum disorder symptoms in children at age 2 years: A birth cohort study. Autism 26, 1864–1881 (2022).
Love, C. et al. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Medicine 22, 393 (2024).
Jentink, J. et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med 362, 2185–2193 (2010).
Aleksandrova, K., Koelman, L. & Rodrigues, C. E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biology 42, 101869 (2021).
Tanner S. et al. Prenatal Environmental Determinants of Aromatase Brain-Promoter Methylation in Cord Blood: Chemical, Airborne, Pharmacological, and Nutritional Factors. bioRxiv. 2025:2025.07.24.666701.
Ponsonby, A.-L. et al. Prenatal phthalate exposure, oxidative stress-related genetic vulnerability and early life neurodevelopment: A birth cohort study. NeuroToxicology 80, 20–28 (2020).
Vuillermin, P. et al. Cohort Profile: The Barwon Infant Study. Int J Epidemiol 44, 1148–1160 (2015).
Ponsonby, A.-L. Reflection on modern methods: building causal evidence within high-dimensional molecular epidemiological studies of moderate size. International Journal of Epidemiology 50, 1016–1029 (2021).
Gasmi, A. et al. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules 28, 210 (2022).
Bragg, M. et al. Prenatal Diet as a Modifier of Environmental Risk Factors for Autism and Related Neurodevelopmental Outcomes. Curr Environ Health Rep 9, 324–338 (2022).
Zhong, C., Tessing, J., Lee, B. K. & Lyall, K. Maternal Dietary Factors and the Risk of Autism Spectrum Disorders: A Systematic Review of Existing Evidence. Autism Res 13, 1634–1658 (2020).
Chen, D. et al. The correlation between prenatal maternal active smoking and neurodevelopmental disorders in children: a systematic review and meta-analysis. BMC Public Health 23, 611 (2023).
Dutheil, F. et al. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environmental Pollution 278, 116856 (2021).
Suarez, E. A. et al. Association of Antidepressant Use During Pregnancy With Risk of Neurodevelopmental Disorders in Children. JAMA Intern Med 182, 1149–1160 (2022).
Symeonides, C. et al. Male autism spectrum disorder is linked to brain aromatase disruption by prenatal BPA in multimodal investigations and 10HDA ameliorates the related mouse phenotype. Nature Communications 15, 6367 (2024).
Althouse, A. D. Adjust for Multiple Comparisons? It’s Not That Simple. The Annals of Thoracic Surgery 101, 1644–1645 (2016).
Rubin, M. Do p Values Lose Their Meaning in Exploratory Analyses? It Depends How You Define the Familywise Error Rate. Review of General Psychology 21, 269–275 (2017).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Ranstam, J. Hypothesis-generating and confirmatory studies, Bonferroni correction, and pre-specification of trial endpoints. Acta Orthop 90, 297 (2019).
Wang H. et al. Prenatal environmental adversity and child neurodevelopmental delay: the role of maternal low-grade systemic inflammation and maternal anti-inflammatory diet. Eur Child Adolesc Psychiatry. 2023.
Sanchez, C. E. et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev 19, 464–484 (2018).
Chu, J. T. W. et al. Impact of prenatal alcohol exposure on neurodevelopmental outcomes: a systematic review. Health Psychol Behav Med 10, 973–1002 (2022).
Frye, R. E. et al. Physiological mediators of prenatal environmental influences in autism spectrum disorder. Bioessays 43, e2000307 (2021).
Testo, A., McBride, C., Bernstein, I. M. & Dumas, J. Preeclampsia and its relationship to pathological brain aging. Front Physiol 13, 979547 (2022).
Van Dam, J. M. et al. Reduced Cortical Excitability, Neuroplasticity, and Salivary Cortisol in 11-13-Year-Old Children Born to Women with Gestational Diabetes Mellitus. EBioMedicine 31, 143–149 (2018).
Na, X. et al. Maternal Obesity during Pregnancy is Associated with Lower Cortical Thickness in the Neonate Brain. AJNR Am J Neuroradiol 42, 2238–2244 (2021).
Li M.et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137.
Xiang, A. H. et al. Association of maternal diabetes with autism in offspring. Jama 313, 1425–1434 (2015).
Smith, B. L. Improving translational relevance: The need for combined exposure models for studying prenatal adversity. Brain, Behavior, & Immunity - Health. 16, 100294 (2021).
Lu, J., Wang, Z., Liang, Y. & Yao, P. Rethinking autism: the impact of maternal risk factors on autism development. Am J Transl Res 14, 1136–1145 (2022).
Avalos, L. A. et al. Prenatal depression and risk of child autism-related traits among participants in the Environmental influences on Child Health Outcomes program. Autism Res 16, 1825–1835 (2023).
Croen, L. A. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 68, 1104–1112 (2011).
Morales, D. R., Slattery, J., Evans, S. & Kurz, X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Medicine 16, 6 (2018).
Li, J. et al. A Nationwide Study on the Risk of Autism After Prenatal Stress Exposure to Maternal Bereavement. Pediatrics 123, 1102–1107 (2009).
Beversdorf, D. Q., Stevens, H. E., Margolis, K. G. & Van de Water, J. Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Curr Pharm Des 25, 4331–4343 (2020).
Lee, J. & Chang, S. M. Confounding by Indication in Studies of Selective Serotonin Reuptake Inhibitors. Psychiatry Investig 19, 873–883 (2022).
Sominsky, L. et al. Pre-pregnancy obesity is associated with greater systemic inflammation and increased risk of antenatal depression. Brain Behav Immun 113, 189–202 (2023).
Zeng, Y. et al. Inflammatory Biomarkers and Risk of Psychiatric Disorders. JAMA Psychiatry 81, 1118 (2024).
Bhat, R. S., Alonazi, M., Al-Daihan, S. & El-Ansary, A. Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain. Metabolites 13, 310 (2023).
Maitin-Shepard, M. et al. Food, nutrition, and autism: from soil to fork. The American Journal of Clinical Nutrition 120, 240–256 (2024).
Derbyshire, E. & Maes, M. The Role of Choline in Neurodevelopmental Disorders-A Narrative Review Focusing on ASC, ADHD and Dyslexia. Nutrients 15, 2876 (2023).
Liu, X., Zou, M., Sun, C., Wu, L. & Chen, W. X. Prenatal Folic Acid Supplements and Offspring’s Autism Spectrum Disorder: A Meta-analysis and Meta-regression. J Autism Dev Disord 52, 522–539 (2022).
Vyas, Y., Lee, K., Jung, Y. & Montgomery, J. M. Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspring Shank3-knockout mice. Molecular Brain 13, 110 (2020).
Schmidt, R. J., Tancredi, D. J., Krakowiak, P., Hansen, R. L. & Ozonoff, S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol 180, 890–900 (2014).
Schmidt, R. J. et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22, 476–485 (2011).
Schmidt, R. J., Iosif, A. M., Guerrero Angel, E. & Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 76, 391–398 (2019).
Zhong, C., Tessing, J., Lee, B. K. & Lyall, K. Maternal Dietary Factors and the Risk of Autism Spectrum Disorders: A Systematic Review of Existing Evidence. Autism Research 13, 1634–1658 (2020).
Friel, C. et al. Healthy Prenatal Dietary Pattern and Offspring Autism. JAMA Netw Open 7, e2422815 (2024).
Szapuova, Z. J. et al. Association between Environmental Tobacco Smoke Exposure and Adaptive Behavior in Individuals with Autism Spectrum Disorder. Toxics 10, 189 (2022).
Kim, B. et al. Prenatal exposure to paternal smoking and likelihood for autism spectrum disorder. Autism 25, 1946–1959 (2021).
Corsi, D. J. et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med 26, 1536–1540 (2020).
Wood, A. G. et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia 56, 1047–1055 (2015).
Tadesse, A. W., Dachew, B. A., Ayano, G., Betts, K. & Alati, R. Prenatal cannabis use and the risk of attention deficit hyperactivity disorder and autism spectrum disorder in offspring: A systematic review and meta-analysis. Journal of Psychiatric Research 171, 142–151 (2024).
Flanagan, E. et al. Exposure to local, source-specific ambient air pollution during pregnancy and autism in children: a cohort study from southern Sweden. Scientific Reports 13, 3848 (2023).
Román, P. et al. Exposure to Environmental Pesticides and the Risk of Autism Spectrum Disorders: A Population-Based Case-Control Study. Medicina (Kaunas) 60, 479 (2024).
Philippat, C. et al. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ Health 14, 56 (2015).
Larsson, M., Weiss, B., Janson, S., Sundell, J. & Bornehag, C.-G. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. NeuroToxicology 30, 822–831 (2009).
Gao, H. et al. Associating prenatal phthalate exposure with childhood autistic traits: investigating potential adverse outcome pathways and the modifying effects of maternal vitamin D. Eco-Environment & Health 3, 425–435 (2024).
Eisner, A. et al. Cord blood immune profile: Associations with higher prenatal plastic chemical levels. Environ Pollut 315, 120332 (2022).
Lam, P. H., Chiang, J. J., Chen, E. & Miller, G. E. Race, socioeconomic status, and low-grade inflammatory biomarkers across the lifecourse: A pooled analysis of seven studies. Psychoneuroendocrinology 123, 104917 (2021).
Rai, D. et al. Parental Socioeconomic Status and Risk of Offspring Autism Spectrum Disorders in a Swedish Population-Based Study. Journal of the American Academy of Child & Adolescent Psychiatry 51, 467–76.e6 (2012).
Yu, T., Lien, Y. J., Liang, F. W. & Kuo, P. L. Parental Socioeconomic Status and Autism Spectrum Disorder in Offspring: A Population-Based Cohort Study in Taiwan. Am J Epidemiol 190, 807–816 (2021).
Shahid Khan, M., Alamgir Kabir, M. & Mohammad Tareq, S. Socio-economic status and autism spectrum disorder: A case-control study in Bangladesh. Preventive Medicine Reports 38, 102614 (2024).
Fujiwara, T. Socioeconomic Status and the Risk of Suspected Autism Spectrum Disorders Among 18-Month-Old Toddlers in Japan: A Population-Based Study. Journal of Autism and Developmental Disorders 44, 1323–1331 (2014).
Thomas, P. et al. The association of autism diagnosis with socioeconomic status. Autism 16, 201–213 (2012).
Durkin, M. S. et al. Autism Spectrum Disorder Among US Children (2002-2010): Socioeconomic, Racial, and Ethnic Disparities. Am J Public Health 107, 1818–1826 (2017).
Kim, M. K. et al. Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. International Journal for Equity in Health 17, 2 (2018).
Lohse, T., Rohrmann, S., Bopp, M. & Faeh, D. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PLoS One 11, e0148563 (2016).
Gogos, A. et al. Socioeconomic adversity, maternal nutrition, and the prenatal programming of offspring cognition and language at two years of age through maternal inflammation. Brain Behav Immun 122, 471–482 (2024).
Thomson, S. et al. Increased maternal non-oxidative energy metabolism mediates association between prenatal di-(2-ethylhexyl) phthalate (DEHP) exposure and offspring autism spectrum disorder symptoms in early life: A birth cohort study. Environment international 171, 107678 (2023).
Acknowledgements
The authors thank the BIS participants for the generous contribution they have made to this project. The authors also thank current and past staff for their efforts in recruiting and maintaining the cohort and in obtaining and processing the data and biospecimens. The other members of the BIS Investigator Group are Martin O’Hely, Toby Mansell and Sarath Ranganathan. We thank John Carlin, Amy Loughman, Terry Dwyer, and Katie Allen for their past work as BIS investigators. The establishment work and infrastructure for BIS were provided by the Murdoch Children’s Research Institute, Deakin University and Barwon Health. Subsequent funding was secured from the National Health and Medical Research Council of Australia (NHMRC), Minderoo Foundation, The Shepherd Foundation, The Jack Brockhoff Foundation, Scobie & Claire McKinnon Trust, Shane O’Brien Memorial Asthma Foundation, Our Women Our Children’s Fundraising Committee Barwon Health, Rotary Club of Geelong, Australian Food Allergy Foundation, GMHBA limited, Vanguard Investments Australia Ltd, Percy Baxter Charitable Trust, Perpetual Trustees, Gwenyth Raymond Trust, William and Vera Ellen Houston Memorial Trust. The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant. In-kind support was provided by the Cotton On Foundation and CreativeForce. The study sponsors were not involved in the collection, analysis, and interpretation of data, writing of the report, or the decision to submit the report for publication. Research at Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. L Holland is supported by The Florey Institute of Neuroscience and Mental Health graduate research scholarship, funded by the MRFF Preventative and Public Health Research grant [AP1200719]. This work was also sup-ported by NHMRC, Australia Investigator Grants [APP1197234 to AL Ponsonby; APP1175744 to D Burgner]. During the preparation of manuscript, the first author used ChatGPT to improve spelling, grammar, and general editing only. After using this tool, first author personally reviewed ChatGPT suggestions to ensure any changes generated by the tool were appropriate.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualisation: L.H., K.D., L.S., W.M., L.H., R.S. C.S., D.B., P.S., P.V., A.L.P. Methodology: L.H., K.D., S.T., L.S., W.M., L.H., R.S. C.S., D.B., P.S., P.V., A.L.P. Data collection: L.H., L.S., C.L., R.S., C.S., D.B., P.S., P.V., ALP. Data analysis: L.H., S.T., A.L.P. Visualisation: L.H., K.D., S.T., R.S., A.L.P., Funding acquisition: L.S., R.S., D.B., P.S., P.V., A.L.P. Writing – original draft: L.H., A.L.P. Writing - review and editing: L.H., K.D., S.T., L.S., W.M., C.L., L.H., R.S., C.S., D.B., P.S., P.V., A.L.P. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
The BIS protocol was approved by the Barwon Health Human Research Ethics Committee (HREC 10/24) and the participating families provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holland, L., Drummond, K., Thomson, S. et al. Prenatal and birth factors associated with child autism diagnosis: a birth cohort perspective. Pediatr Res (2026). https://doi.org/10.1038/s41390-025-04747-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04747-x