Abstract
Background
It is estimated that by 2040 there will be 1,017,712 new cases of prostate cancer worldwide. Androgen deprivation therapy (ADT) is widely used as a treatment option for all disease stages. ADT, and the resulting decline in androgen levels, may indirectly affect gut microbiota. Factors affecting gut microbiota are wide-ranging; however, literature is scarce on the effects of ADT on gut microbiota and metabolome profiles in patients with prostate cancer.
Methods
To study the changes of gut microbiome by ADT, this 24-week observational study investigated the relationship between testosterone levels and changes in gut microbiota in Japanese patients with prostate cancer undergoing ADT. Sequential faecal samples were collected 1 and 2 weeks before ADT, and 1, 4, 12, and 24 weeks after ADT. Blood samples were collected at almost the same times. Bacterial 16 S rRNA gene-based microbiome analyses and capillary electrophoresis-time-of-flight mass spectrometry-based metabolome analyses were performed.
Results
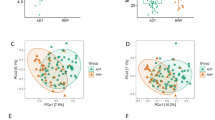
In total, 23 patients completed the study. The α- and ß-diversity of gut microbiota decreased significantly at 24 weeks after ADT (p = 0.017, p < 0.001, respectively). Relative abundances of Proteobacteria, Gammaproteobacteria, Pseudomonadales, Pseudomonas, and concentrations of urea, lactate, butyrate, 2-hydroxyisobutyrate and S-adenosylmethionine changed significantly after ADT (p < 0.05). There was a significant positive correlation between the abundance of Proteobacteria, a known indicator of dysbiosis, and the concentration of lactate (R = 0.49, p < 0.01).
Conclusions
The decline in testosterone levels resulted in detrimental changes in gut microbiota. This dysbiosis may contribute to an increase in frailty and an increased risk of adverse outcomes in patients with prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data collected for this study are available on request. Please note that the data for participant number 1 was collected during the study after deidentification; these data included gut microbiota genome analyses and clinical information, but no end of study data are available for this participant. The 16 S rRNA gut microbiota gene sequences have been deposited in the DDBJ database (http://getentry.ddbj.nig.ac.jp/) under accession number DRA009707. The clinical data are available on permission of the corresponding authors (sfukuda@sfc.keio.ac.jp and shorie@juntendo.ac.jp).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–24.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today. Lyon, France: International Agency for Research on Cancer. World Health Organization. https://gco.iarc.fr/today (accessed 22 March 2021).
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89.
Panigrahi GK, Praharai PP, Kittaka H, Mridha AR, Black OM, Singh R, et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med. 2019;8:1110–23.
Ministry of Health, Labour and Welfare Cancer and Disease Control Division. Cancer Incidence of Japan. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/gan/gan_toroku.html (accessed 22 March 2021).
Sawada N. Risk and preventive factors for prostate cancer in Japan: The Japan Public Health Center-based prospective (JPHC) study. J Epidemiol. 2017;27:2–7.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
American Cancer Society: Hormone therapy for prostate cancer. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/hormone-therapy.html (accessed 22 March 2021).
Sugimura R, Kawahara T, Uemura H. Geriatric 8 screening of frailty in patients with prostate cancer. Lett Editor Int J Urol 2020;27:1161–3.
Hinotsu S, Namiki M, Ozono S, Akaza H. NCCN Asia Consensus Statement prostate cancer. Jpn J Clin Oncol. 2018;48:964–5.
Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Mens Health. 2020;38:48–60.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 2016;14:e1002533.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–12.
Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8.
Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–22.
Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17:322–32.
Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21:539–48.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60.
Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. https://doi.org/10.1080/10618600.1996.10474713
Goda T, Watanabe K, Kobayashi J, Nagai Y, Ohara N, Takahashi D. A case of hyperammonemia with obstructive urinary tract infection by urease-producing bacteria. Rinsho Shinkeigaku. 2017;57:130–3. https://doi.org/10.5692/clinicalneurol.cn-00100.
Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503.
Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD, et al. An investigation Into physical frailty as a link Between the gut microbiome and cognitive health. Front Aging Neurosci. 2018;10:398.
Zhang L, Liao J, Chen Q, Chen M, Kuang Y, Chen L, et al. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res. 2020;32:2001–11.
Coelho T, Paúl C, Gobbens RJJ, Fernandes L. Determinants of frailty: the added value of assessing medication. Front Aging Neurosci. 2015;7:56. https://doi.org/10.3389/fnagi.2015.00056
Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? a conceptual review. Cancer. 2007;110:2604–13.
Saad F, Röhrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology 2017;63:144–56.
Lam T, Poljak A, McLean M, Bahl N, Ho KKY, Birzniece V. Testosterone prevents protein loss via the hepatic urea cycle in human. Eur J Endocrinol. 2017;176:489–96.
Mora D, Arioli S. Microbial urease in health and disease. PLOS Pathog. 2014;10:e1004472.
Gao J, Cahill CM, Huang X, Roffman JL, Lamon-Fava S, Fava M, et al. S-adenosyl methionine and transmethylation pathways in neuropsychiatric diseases Throughout life. Neurotherapeutics 2018;15:156–75.
Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, et al. Possible role of S-adenosylmethionine, S-adenosylhomocysteine, and polyunsaturated fatty acids in predementia syndromes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:467–70.
Linnebank M, Popp J, Smulders Y, Smith D, Semmler A, Farkas M, et al. S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurodegener Dis. 2010;7:373–8.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol. 2016;34:566–71.
Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–7.
Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99:201–12.
Gillis CC, Hughes ER, Spiga L, Winter MG, Zhu W, Furtado de Carvalho T, et al. Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe. 2018;23:570.
Li JKM, Wang LL, Wong CYP, Chiu PKF, Teoh JYC, Kwok HSW, et al. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2021;24:1063–72.
Acknowledgements
We are grateful to the study participants. We would like to thank Yuka Ohara, Mitsuko Komatsu, Noriko Kagata, Noriko Fukuda, and Naoki Tanigawa for their technical support. Medical writing support, in accordance with GPP guidelines, was provided by Mediwrite Asia Inc Pte Ltd and included proofreading and editing services. This work was supported in part by JSPS KAKENHI (18H04805 to SF), AMED-CREST (JP20gm1010009 to SF), JST ERATO (JPMJER1902 to SF), the Takeda Science Foundation (to SF), the Food Science Institute Foundation (to SF), and the Programme for the Advancement of Research in Core Projects under Keio University’s Longevity Initiative (to SF).
Author information
Authors and Affiliations
Contributions
SF and SH designed the project. AK, TT, and CI performed the main part of microbiome and metabolome analysis. AK, TT, CI, and WA wrote the draft of manuscript. SF supervised the project and wrote the paper. NO, GN, AH, HK, TC, FS, MN, SI, and SM contributed to experimental design and data analysis. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles of Declaration of Helsinki. The samples and clinical information used in this study were obtained by written informed consent and with the approval of the institutional review boards of Juntendo University school of Medicine (Approval number: 14-117). This study was also registered in the UMIN clinical registry (UMIN000021161).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kure, A., Tsukimi, T., Ishii, C. et al. Gut environment changes due to androgen deprivation therapy in patients with prostate cancer. Prostate Cancer Prostatic Dis 26, 323–330 (2023). https://doi.org/10.1038/s41391-022-00536-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-022-00536-3
This article is cited by
-
The role of the human microbiome in prostate cancer: a systematic review from diagnosis to treatment
Prostate Cancer and Prostatic Diseases (2025)
-
Quality of life and supportive care needs in prostate cancer: the impact of treatment received and care service utilization among Māori and non-Māori patients in New Zealand
Supportive Care in Cancer (2025)
-
Gut microbiota in patients with prostate cancer: a systematic review and meta-analysis
BMC Cancer (2024)
-
Changes in the gut microbial profile during long-term androgen deprivation therapy for prostate cancer
Prostate Cancer and Prostatic Diseases (2024)