Abstract
Background
Staging patients with high-risk prostate cancer (HRPCa) with conventional imaging of computed tomography (CT) and bone scintigraphy (BS) is suboptimal. Therefore, we aimed to compare the accuracy of whole-body magnetic resonance imaging (WBMRI) with conventional imaging to stage patients with HRPCa.
Methods
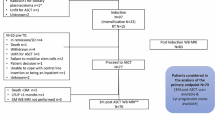
We prospectively enrolled patients with newly diagnosed HRPCa (prostate‐specific antigen ≥20 ng/ml and/or Grade Group ≥4). Patients underwent BS, CT of the abdomen and pelvis, and WBMRI within 30 days of evaluation. The primary endpoint was the diagnostic performances of detecting metastatic disease to the lymph nodes and bone for WBMRI and conventional imaging. The reference standard was defined by histopathology or by all available clinical information at 6 months of follow-up. To compare diagnostic tests, Exact McNemar’s test and area under the curve (AUC) of the receiver operating characteristics curves were utilized.
Results
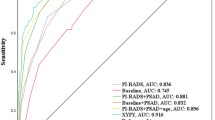
Among 92 patients enrolled, 15 (16.3%) and 8 (8.7%) patients were found to have lymphatic and bone metastases, respectively. The sensitivity, specificity, and accuracy of WBMRI in detecting lymphatic metastases were 0.60 (95% confidence interval 0.32–0.84), 0.84 (0.74–0.92), and 0.80 (0.71–0.88), respectively, while CT were 0.20 (0.04–0.48), 0.92 (0.84–0.97), and 0.80 (0.71–0.88). The sensitivity, specificity, and accuracy of WBMRI to detect bone metastases were 0.25 (0.03–0.65), 0.94 (0.87–0.98), and 0.88 (0.80–0.94), respectively, while CT and BS were 0.12 (0–0.53), 0.94 (0.87–0.98), and 0.87 (0.78–0.93). For evaluating lymphatic metastases, WBMRI demonstrated a higher sensitivity (p = 0.031) and discrimination compared to CT (0.72 versus 0.56, p = 0.019).
Conclusions
For staging patients with HRPCa, WBMRI outperforms CT in the detection of lymphatic metastases and performs as well as CT and BS in the detection of bone metastases. Further studies are needed to assess the cost effectiveness of WBMRI and the utility of combined PSMA PET and WBMRI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Eastham JA, Auffenberg GB, Barocas DA, Chou R, Crispino T, Davis JW, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J Urol. 2022;208:10–18.
EAU Guidelines. Edn. presented at the EAU Annual Congress Milan. Arnhem, the Netherlands; EAU Guidelines Office: 2023.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2016;69:428–35.
Ham WS, Chalfin HJ, Feng Z, Trock BJ, Epstein JI, Cheung C, et al. New Prostate Cancer Grading System Predicts Long-term Survival Following Surgery for Gleason Score 8-10 Prostate Cancer. Eur Urol. 2017;71:907–12.
Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. 2012;61:1132–8.
Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skelet Radio. 2014;43:1503–13.
Bjurlin MA, Turkbey B, Rosenkrantz AB, Gaur S, Choyke PL, Taneja SS. Imaging the High-risk Prostate Cancer Patient: Current and Future Approaches to Staging. Urology. 2018;116:3–12.
Van Nieuwenhove S, Van Damme J, Padhani AR, Vandecaveye V, Tombal B, Wuts J, et al. Whole-body magnetic resonance imaging for prostate cancer assessment: Current status and future directions. J Magn Reson Imaging. 2022;55:653–80.
Cruz IAN, Fayad LM, Ahlawat S, Lederman HM, Nico MAC, Ormond Filho AG, et al. Whole-Body MRI in Musculoskeletal Oncology: A Comprehensive Review with Recommendations. Radio Imaging Cancer. 2023;5:e220107.
Barkhausen J, Quick HH, Lauenstein T, Goyen M, Ruehm SG, Laub G, et al. Whole-body MR imaging in 30 seconds with real-time true FISP and a continuously rolling table platform: feasibility study. Radiology. 2001;220:252–6.
Gruber B, Froeling M, Leiner T, Klomp DWJ. RF coils: A practical guide for nonphysicists. J Magn Reson Imaging. 2018;48:590–604.
Lauenstein TC, Goehde SC, Herborn CU, Goyen M, Oberhoff C, Debatin JF, et al. Whole-body MR imaging: evaluation of patients for metastases. Radiology. 2004;233:139–48.
de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol. 2016;70:233–45.
Pasoglou V, Larbi A, Collette L, Annet L, Jamar F, Machiels JP, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? Prostate. 2014;74:469–77.
Anttinen M, Ettala O, Malaspina S, Jambor I, Sandell M, Kajander S, et al. A Prospective Comparison of (18)F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur Urol Oncol. 2021;4:635–44.
Johnston EW, Latifoltojar A, Sidhu HS, Ramachandran N, Sokolska M, Bainbridge A, et al. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate- and high-risk prostate cancer: diagnostic accuracy and interobserver agreement for nodal and metastatic staging. Eur Radio. 2019;29:3159–69.
Lecouvet FE, El Mouedden J, Collette L, Coche E, Danse E, Jamar F, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62:68–75.
Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Logager VB, Madsen C, et al. (68)Ga-PSMA-PET/CT in comparison with (18)F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29:1221–30.
Larbi A, Omoumi P, Pasoglou V, Michoux N, Triqueneaux P, Tombal B, et al. Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. Eur Radio. 2019;29:4503–13.
Saylor B. PSMA uptake, access appear high for US-based urologists. Urology Times. 2023. https://www.urologytimes.com/view/psma-uptake-access-appearhigh-for-us-based-urologists.
Manafi-Farid R, Ranjbar S, Jamshidi Araghi Z, Pilz J, Schweighofer-Zwink G, Pirich C, et al. Molecular Imaging in Primary Staging of Prostate Cancer Patients: Current Aspects and Future Trends. Cancers. 2021;13:5360.
Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol. 2016;69:393–6.
Costelloe CM, Madewell JE, Kundra V, Harrell RK, Bassett RL Jr, Ma J. Conspicuity of bone metastases on fast Dixon-based multisequence whole-body MRI: clinical utility per sequence. Magn Reson Imaging. 2013;31:669–75.
Ma J, Costelloe CM, Madewell JE, Hortobagyi GN, Green MC, Cao G, et al. Fast dixon-based multisequence and multiplanar MRI for whole-body detection of cancer metastases. J Magn Reson Imaging. 2009;29:1154–62.
Asa S, Ozgur E, Uslu-Besli L, Ince B, Sager S, Demirdag C, et al. Hybrid Ga-68 prostate-specific membrane antigen PET/MRI in the detection of skeletal metastasis in patients with newly diagnosed prostate cancer: Contribution of each part to the diagnostic performance. Nucl Med Commun. 2023;44:65–73.
Thalgott M, Duwel C, Rauscher I, Heck MM, Haller B, Gafita A, et al. One-Stop-Shop Whole-Body (68)Ga-PSMA-11 PET/MRI Compared with Clinical Nomograms for Preoperative T and N Staging of High-Risk Prostate Cancer. J Nucl Med. 2018;59:1850–6.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004;171:2122–7.
Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, et al. Diagnostic Performance and Safety of Positron Emission Tomography with (18)F-rhPSMA-7.3 in Patients with Newly Diagnosed Unfavourable Intermediate- to Very-high-risk Prostate Cancer: Results from a Phase 3, Prospective, Multicentre Study (LIGHTHOUSE). Eur Urol. 2023;84:361–70.
Van Damme J, Tombal B, Collette L, Van Nieuwenhove S, Pasoglou V, Gerard T, et al. Comparison of (68)Ga-Prostate Specific Membrane Antigen (PSMA) Positron Emission Tomography Computed Tomography (PET-CT) and Whole-Body Magnetic Resonance Imaging (WB-MRI) with Diffusion Sequences (DWI) in the Staging of Advanced Prostate Cancer. Cancers. 2021;13:5286.
Kaufmann S, Kruck S, Gatidis S, Hepp T, Thaiss WM, Hennenlotter J, et al. Simultaneous whole-body PET/MRI with integrated multiparametric MRI for primary staging of high-risk prostate cancer. World J Urol. 2020;38:2513–21.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to all the following: AMF was involved with the design of the work, analysis and interpretation of the data, drafting the manuscript, reviewing the manuscript, and final approval. BFC was involved with the conception and design of the work, acquisition of data, reviewing the manuscript, and final approval. CWS was involved with the acquisition of the data, reviewing the manuscript, and final approval. JS was involved with the analysis and interpretation of the data, reviewing the manuscript, and final approval. AQ was involved with the acquisition of the data, reviewing the manuscript, and final approval. VK was involved with the acquisition of the data, reviewing the manuscript, and final approval. PGC was involved with the acquisition of the data, reviewing the manuscript, and final approval. DAK was involved with the acquisition of the data, reviewing the manuscript, and final approval. GCR was involved with the acquisition of the data, reviewing the manuscript, and final approval. DSS was involved with the acquisition of the data, reviewing the manuscript, and final approval. JM was involved with the acquisition of the data, reviewing the manuscript, and final approval. TKB was involved with the conception and design of the work, acquisition of the data, interpretation of the data, reviewing the manuscript, and final approval.
Corresponding author
Ethics declarations
Competing interests
JM reports IP licensing to GE Healthcare and Siemens Healthineers. The remaining authors do not report any relevant conflicts of interest.
Ethics approval
All procedures performed in studies involving human participants were approved by the University of Texas MD Anderson Cancer Center Institutional Review Board and with the 1964 Helsinski declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, A.M., Chapin, B.F., Shi, C.W. et al. Whole-body magnetic resonance imaging for staging patients with high-risk prostate cancer. Prostate Cancer Prostatic Dis 28, 761–766 (2025). https://doi.org/10.1038/s41391-024-00893-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00893-1