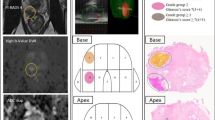
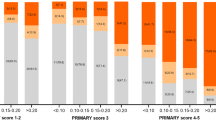
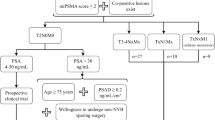
Abstract
Background
Prostate biopsy is the most common approach for diagnosing prostate cancer (PCa); however, it has inherent limitations, such as the invasive procedure, postoperative complications, and false negative results. We aimed to provide a noninvasive diagnostic strategy for patients with highly suspected PCa and to evaluate the feasibility of performing biopsy-spared radical prostatectomy.
Methods
This prospective study included a total of 57 patients between November 10, 2022, and December 1, 2023. All 57 patients underwent radical prostatectomy without prior prostate biopsy based on a noninvasive diagnostic strategy consisting of a diagnostic prediction model [comprised of the prostate imaging-reporting and data system (PI-RADS) score and prostate-specific antigen density (PSAD)] and the 18F-prostate-specific membrane antigen (PSMA)-1007 positron emission tomography (PET)/computed tomography (CT) examination. The primary endpoint was the positive predictive value (PPV) of clinically significant PCa [the International Society of Urological Pathology (ISUP) grade ≥2, Gleason score ≥3 + 4]. The secondary endpoints were a PPV of any-grade PCa (ISUP grade ≥ 1, Gleason score ≥3 + 3) and high-grade PCa (ISUP grade ≥3, Gleason score ≥4 + 3), and the false positive rate of the diagnostic strategy.
Results
Of the 371 screened patients with clinically suspected PCa, 57 patients fulfilled the criteria and consented to participate in this study. The median PSAD level was 0.56 (0.42–0.82) ng/mL2; 13 (22.8%) patients were identified as having a PI-RADS score of 4, and 44 (77.2%) patients with a PI-RADS score of 5. The median SUVmax of 18F-PSMA-1007 PET/CT was 21.6 (15.8–33.0). For the 57 enrolled patients who received radical prostatectomy directly, the PPV of clinically significant PCa was 98.2% [56/57, 95% confidence interval (CI): 90.6–100%]. Only 1.8% (1/57, 95% CI: 0.0–9.4%) of patients were diagnosed with clinically insignificant PCa (ISUP grade = 1, Gleason score = 3 + 3). The PPV of any-grade PCa and high-grade PCa were 100% and 73.7% (42/57, 95% CI: 60.3–84.5%), respectively. No one had a false positive result.
Conclusions
We proposed a noninvasive diagnostic strategy consisting sequentially of a diagnostic prediction model and the 18F-PSMA-1007 PET/CT examination for diagnosing PCa. Despite some limitations, our initial findings suggest the potential feasibility of radical prostatectomy without prior prostate biopsy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The study protocol and statistical analysis plan (English version) has been published. The anonymized individual patients’ data were also provided as supplementary material in this study. All data are available upon reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71:353–65.
Liu C, Liu T, Zhang Z, Zhang N, Du P, Yang Y, et al. (68)ga-psma pet/ct combined with pet/ultrasound-guided prostate biopsy can diagnose clinically significant prostate cancer in men with previous negative biopsy results. J Nucl Med. 2020;61:1314–9.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric mri and trus biopsy in prostate cancer (promis): A paired validating confirmatory study. Lancet. 2017;389:815–22.
Xing NZ, Wang MS, Fu Q, Yang FY, Li CL, Li YJ, et al. Feasibility of prostatectomy without prostate biopsy in the era of new imaging technology and minimally invasive techniques. World J Clin Cases. 2019;7:1403–9.
Meissner VH, Rauscher I, Schwamborn K, Neumann J, Miller G, Weber W, et al. Radical prostatectomy without prior biopsy following multiparametric magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography. Eur Urol. 2022;82:156–60.
Wang C, Yuan L, Shen D, Zhang B, Wu B, Zhang P, et al. Combination of pi-rads score and psad can improve the diagnostic accuracy of prostate cancer and reduce unnecessary prostate biopsies. Front Oncol. 2022;12:1024204.
Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer HP, Wieczorek K, et al. The value of psa density in combination with pi-rads™ for the accuracy of prostate cancer prediction. J Urol. 2017;198:575–82.
Washino S, Okochi T, Saito K, Konishi T, Hirai M, Kobayashi Y, et al. Combination of prostate imaging reporting and data system (pi-rads) score and prostate-specific antigen (psa) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017;119:225–33.
Wang CM, Yuan L, Liu XH, Chen SQ, Wang HF, Dong QF, et al. Developing a diagnostic model for predicting prostate cancer: A retrospective study based on chinese multicenter clinical data. Asian J Androl. 2023;26:34–40.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (primary): A prospective multicentre study. Eur Urol. 2021;80:682–9.
Tang W, Tang Y, Qi L, Zhang Y, Tang G, Gao X, et al. Benign prostatic hyperplasia-related false-positive of prostate-specific membrane antigen-positron emission tomography in the diagnosis of prostate cancer: The achilles’ heel of biopsy-free radical prostatectomy? J Urol. 2023;210:845–55.
Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head-to-head comparison of (68)ga-psma-11 with (18)f-psma-1007 pet/ct in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med. 2020;61:527–32.
Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual comparison of (18)f-psma-1007 pet/ct, multiparametric mri, and radical prostatectomy specimens in patients with primary prostate cancer: A retrospective, proof-of-concept study. J Nucl Med. 2017;58:1805–10.
Wang C, Dong Q, Liu X, Ni M, Xie Q, Xiao J, et al. Protocol for snotob study: radical prostatectomy without prostate biopsy following (18)f-psma-1007 pet/ct based on a diagnostic model: A single-centre, single-arm, open-label study. BMJ Open. 2023;13:e073983.
Bossuyt, Reitsma PM, Bruns DE JB, Gatsonis CA, Glasziou PP, Irwig L, et al. Stard 2015: an updated list of essential items for reporting diagnostic accuracy studies. Bmj. 2015;351:h5527.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–51.
Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-psma: the eanm standardized reporting guidelines v1.0 for psma-pet. Eur J Nucl Med Mol Imaging. 2021;48:1626–38.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 international society of urological pathology (isup) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2023;388:1547–58.
Briganti A, Fossati N, Catto JWF, Cornford P, Montorsi F, Mottet N, et al. Active surveillance for low-risk prostate cancer: The european association of urology position in 2018. Eur Urol. 2018;74:357–68.
Beatrici E, Labban M, Stone BV, Filipas DK, Reis LO, Lughezzani G, et al. Uncovering the changing treatment landscape for low-risk prostate cancer in the USA from 2010 to 2020: Insights from the national cancer data base. Eur Urol. 2023;84:527–30.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. Eau-eanm-estro-esur-siog guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, et al. What are we missing? False-negative cancers at multiparametric mr imaging of the prostate. Radiology. 2018;286:186–95.
Sathianathen NJ, Omer A, Harriss E, Davies L, Kasivisvanathan V, Punwani S, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–14.
Mazzone E, Stabile A, Pellegrino F, Basile G, Cignoli D, Cirulli GO, et al. Positive predictive value of prostate imaging reporting and data system version 2 for the detection of clinically significant prostate cancer: A systematic review and meta-analysis. Eur Urol Oncol. 2021;4:697–713.
Yilmaz EC, Shih JH, Belue MJ, Harmon SA, Phelps TE, Garcia C, et al. Prospective evaluation of pi-rads version 2.1 for prostate cancer detection and investigation of multiparametric mri-derived markers. Radiology. 2023;307:e221309.
Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, et al. Psma ligands for pet imaging of prostate cancer. J Nucl Med. 2017;58:1545–52.
Wagaskar VG, Mittal A, Sobotka S, Ratnani P, Lantz A, Falagario UG, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of retzius and sparing the pouch of douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol. 2021;80:213–21.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. Eau-eanm-estro-esur-isup-siog guidelines on prostate cancer-2024 update. Part i: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2024;86:148–63.
Olah C, Mairinger F, Wessolly M, Joniau S, Spahn M, Kruithof-de Julio M, et al. Enhancing risk stratification models in localized prostate cancer by novel validated tissue biomarkers. Prostate Cancer Prostatic Dis. 2024. https://doi.org/10.1038/s41391-024-00918-9
Prestagiacomo LE, Tradigo G, Aracri F, Gabriele C, Rota MA, Alba S, et al. Data-independent acquisition mass spectrometry of eps-urine coupled to machine learning: A predictive model for prostate cancer. ACS Omega. 2023;8:6244–52.
Nyberg T, Brook MN, Ficorella L, Lee A, Dennis J, Yang X, et al. Canrisk-prostate: a comprehensive, externally validated risk model for the prediction of future prostate cancer. J Clin Oncol. 2023;41:1092–104.
Acknowledgements
We thank all participants, their families and all staff for their contributions to this study. In particularly, we express our gratitude to Chao Gao, MD (Department of Cardiology, Xijing Hospital, Xi’an, China); Zhe Wang, MD (Department of Pathology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China); and Zhihua Zhang, PhD (Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, China). We sincerely appreciate assistance from Medical Research Center of Anhui Provincial Hospital in providing convenient conditions.
Funding
This study was supported by the Key Research and Development Program of Anhui Province (202204295107020003), the Distinguished Young Scholars Fund of Anhui Province (2022AH020078), the Key health Project of Anhui Province (AHWJ2022a037), and Major Joint Project of New Medicine of USTC (YD9110002018).
Author information
Authors and Affiliations
Contributions
Conception and design: JX, TT, QX and CW. Data acquisition: CW, LY, MN, YG and YM. Data analysis and interpretation: CW, LY, MN, YG and YL. Critical revision of the manuscript for scientific and factual content: JX, TT, QX, YL and DZ. Drafting the manuscript: CW, TT, MN, LY and YG. Statistical analysis: CW and XL. Supervision: JX, TT, QX, YL and DZ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of USTC (2022KY-142 and 2023KY-083). The implementation of the clinical trial strictly abided by the Declaration of Helsinki. Written informed consent was obtained from all the enrolled patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Xie, Q., Yuan, L. et al. Radical prostatectomy without prostate biopsy based on a noninvasive diagnostic strategy: a prospective single-center study. Prostate Cancer Prostatic Dis 28, 496–502 (2025). https://doi.org/10.1038/s41391-024-00931-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00931-y