Abstract
Background
ARTO trial was a phase II randomized trial suggesting the benefit of a concomitant treatment strategy including Abiraterone acetate plus predisone (AAP) and stereotactic body radiotherapy (SBRT) in oligometastatic castrate resistant prostate cancer (omCRPC). The object of the current analysis is to explore whether the benefit provided by SBRT to AAP is maintained at later stages of disease after oligoprogression
Methods
Patients enrolled in ARTO trial in whom a first progression event was reported were divided in two groups according to the treatment approach received, regardless of the initial randomization. After first progression event, Patients in Group A received SBRT on oligoprogressive disease, while patients in group B received second line systemic treatment. Palliative RT was not considered for the purpose of this analysis. Progression-Free survival (PFS) 1 and 2 were defined as time between AAP start and first progression event and time between first and second progression event, death or last follow up, (whichever came first), respectively. Cox regression analysis was performed to compare PFS1 + PFS2 in patients in group A vs Group B. Kaplan–Meier analysis was performed to compare overall survival between the two groups
Results
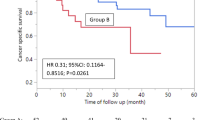
Median PFS1 + PFS2 was 45.9 months vs. not reached in group A (n = 43) vs Group B (n = 20), respectively (HR 0.63, 95% CI 0.17–2.33, p value 0.489), no significant difference was detected. Median OS was not reached in neither of the two arms of treatment, with a non-significant trend in favour of the experimental arm (HR 0.50, 95% CI 0.14–1.78, p = 0.284)
Conclusions
Results from the present analysis show that SBRT after progression may be a viable and feasible option for omCRPC after progression if compared to second line systemic therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, GF, upon reasonable request.
References
Francolini G, Allegra AG, Detti B, Di Cataldo V, Caini S, Bruni A, et al. Stereotactic body radiation therapy and abiraterone acetate for patients affected by oligometastatic castrate-resistant prostate cancer: a randomized phase II trial (ARTO). J Clin Oncol. 2023;41:5561–8.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl J Med. 2020;382:2091–102.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration- resistant prostate cancer. N. Engl J Med. 2021;385:1091–103.
Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, et al. Apalutamide in patients with metastatic castration- sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN Study. J Clin Oncol. 2021;39:2294–303.
Armstrong AJ, Azad AA, Iguchi T, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40:1616–22.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:686–700.
Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Fléchon A, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399:1695–707.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone- sensitive prostate cancer. N. Engl J Med. 2022;386:1132–42.
Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer- metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185:178–215.
Triggiani L, Alongi F, Buglione M, Detti B, Santoni R, Bruni A, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116:1520–5.
Triggiani L, Mazzola R, Magrini SM, Ingrosso G, Borghetti P, Trippa F, et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World J Urol. 2019;37:2631–7.
Francolini G, Detti B, Di Cataldo V, Garlatti P, Aquilano M, Allegra A, et al. Study protocol and preliminary results from a mono- centric cohort within a trial testing stereotactic body radiotherapy and abiraterone (ARTO- NCT03449719). Radiol Med. 2022;127:912–8.
Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, McDermott R, Hervonen P, Ginman C, et al. 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14:117–24.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl J Med. 2019;381:121–31.
Francolini G, Porreca A, Facchini G, Santini D, Bruni A, Simoni N, et al. PERSIAN trial (NCT05717660): an ongoing randomized trial testing androgen deprivation therapy, apalutamide and stereotactic body radiotherapy. An alternative “triplet” for oligometastatic hormone sensitive prostate cancer patients. Med Oncol. 2023;41:39. Published 2023 Dec 29.
Saad F, Clarke NW, Oya M, Shore N, Procopio G, Guedes JD, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1094–108.
Chi KN, Rathkopf D, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41:3339–51.
Abida W, Campbell D, Patnaik A, Bryce AH, Shapiro J, Bambury RM, et al. Rucaparib for the Treatment of Metastatic Castration- resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol. 2023;84:321–30.
Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl J Med. 2020;383:2345–57.
Deek MP, Van der Eecken K, Sutera P, Deek RA, Fonteyne V, Mendes AA, et al. Long-term outcomes and genetic predictors of response to metastasis-directed therapy versus observation in oligometastatic prostate cancer: analysis of STOMP and ORIOLE trials. J Clin Oncol. 2022;40:3377–82.
de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl J Med. 2019;381:2506–18.
Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–9.
Tang C, Sherry AD, Haymaker C, Bathala T, Liu S, Fellman B, et al. Addition of metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: the EXTEND phase 2 randomized clinical trial. JAMA Oncol. 2023;9:825–34.
Acknowledgements
ARTO trial was conducted with a partial unconditional support grant by Janssen Cilag SpA. The authors thank the Fondazione Radioterapia Oncologica (FRO), individuals, and other organizations that have provided support for this scientific research. More specifically, the authors would like to deeply thank Conad, Fondazione Cassa di Risparmio di Pistoia e Pescia, Fabiani, Rinascente, Giorgio Tesi Group, IFCA, Permira.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
GF, NB, VdC, PG, MA, SC contributed to the study conception and design, AB, GI, RmD, LT, MAu, LTr, SP, GT,FA,GM, BAjF,AL,CF, FAl contributed to the material preparation, data collection and analysis, GS, DG, PBo, ML, GFr, LB, ID, IM, RV, LL contributed to the draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors decalare no competing interests.
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Area Vasta Centro (approval no. 12855_spe, October 9, 2018). Informed consent was obtained from all individual participants included in the study. Every human participant provided an informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Francolini, G., Bertini, N., Di Cataldo, V. et al. Impact of stereotactic body radiotherapy after progression in castrate resistant prostate cancer patients undergoing first line abiraterone treatment. A subgroup analysis from ARTO trial (NCT03449719). Prostate Cancer Prostatic Dis 28, 908–912 (2025). https://doi.org/10.1038/s41391-025-00950-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-025-00950-3
This article is cited by
-
Best of 2025 in prostate cancer and prostatic diseases
Prostate Cancer and Prostatic Diseases (2026)
-
Whack-a-met: serial SABR for repeated oligoprogression in metastatic CRPC
Prostate Cancer and Prostatic Diseases (2025)