Abstract
Background
Extraprostatic extension (EPE) in prostate cancer (PCa) has implications for nerve-sparing approaches. mpMRI-based nomograms show modest accuracy, highlighting the need for improved predictive models. This study evaluates 18F-DCFPyL prostate-specific membrane antigen (PSMA) positron emission tomography/ computed tomography (PET/CT) for predicting side-specific EPE using maximum standardized uptake value (SUVmax).
Methods
This single-center cohort study included patients undergoing RALP by a single surgeon (AKT) from January 2022 to September 2024. Baseline variables included demographics, PSA, biopsy, MRI, and PSMA parameters (SUVmax, EPE, SVI). The primary endpoint was side-specific EPE on final pathology. Univariable and multivariable logistic regression identified significant predictors. A nomogram was built based on this. To evaluate model performance, a 1000-iteration bootstrap approach was used to compare (1) the institutional MRI-only 2018 model, (2) an MRI + PSMA Fixed Model, and (3) a retrained MRI + PSMA Model built on each bootstrap sample.
Results
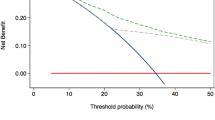
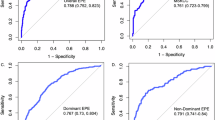
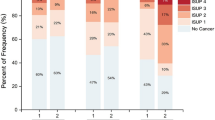
Three hundred fifty-five patients were analyzed. EPE was detected in 18.9% by MRI and 5.4% by PSMA PET. Median intraprostatic SUVmax was 11.30. EPE-positive sides were more likely to have MRI/PSMA-detected EPE (p < 0.001), PIRADS 5 lesions (p < 0.001), aggressive biopsy GGG (p < 0.001), higher positive cores (p < 0.001), and greater percent tumor involvement (p < 0.001). Median SUVmax was significantly higher in the EPE group (9.1 vs. 5.4; p < 0.001). Multivariable analysis identified PSA, MRI-detected EPE, GGG, tumor involvement percentage, and SUVmax ≥13 as significant predictors. The PSMA + MRI Fixed Model outperformed the MRI-only model (median AUC: 0.7542 vs. 0.7350) with p < 0.001. Calibration plots showed strong agreement between predicted and observed probabilities, and decision curve analysis demonstrated greater net clinical benefit across relevant thresholds.
Conclusion
We developed a nomogram integrating PSMA PET with MRI and clinicopathological variables, outperforming our institutional model. PSMA uptake strongly predicts side-specific EPE, which can enhance preoperative assessment and improve postoperative functional outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Ball MW, Partin AW, Epstein JI. Extent of extraprostatic extension independently influences biochemical recurrence-free survival: evidence for further pT3 subclassification. Urology. 2015;85:161–4.
Eisenberg MS, Karnes RJ, Kaushik D, Rangel L, Bergstralh EJ, Boorjian SA. Risk stratification of patients with extraprostatic extension and negative lymph nodes at radical prostatectomy: identifying optimal candidates for adjuvant therapy. J Urol. 2013;190:1735–41.
Tewari A, Peabody JO, Fischer M, Sarle R, Vallancien G, Delmas V, et al. An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur Urol. 2003;43:444–54.
Eastham JA, Auffenberg GB, Barocas DA, Chou R, Crispino T, Davis JW, et al. Clinically localized prostate cancer: AUA/ASTRO Guideline, Part II: principles of active surveillance, principles of surgery, and follow-up. Journal Urol. 2022;208:19–25.
Zhu M, Gao J, Han F, Yin L, Zhang L, Yang Y, et al. Diagnostic performance of prediction models for extraprostatic extension in prostate cancer: a systematic review and meta-analysis. Insights Imaging. 2023;14:140.
Fang B, Zhu Y. Applying prediction models in clinical practice: the importance of fine details. Prostate Cancer Prostatic Dis. 2024;27:584.
O’Keefe DS, Bacich DJ, Huang SS, Heston WDW. A perspective on the evolving story of PSMA biology, PSMA-based imaging, and endoradiotherapeutic strategies. J Nucl Med. 2018;59:1007–13.
Voter AF, Werner RA, Pienta KJ, Gorin MA, Pomper MG, Solnes LB, et al. Piflufolastat F-18 (18 F-DCFPyL) for PSMA PET imaging in prostate cancer. Expert Rev Anticancer Ther. 2022;22:681–94.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023;50:1466–86.
Hupe MC, Philippi C, Roth D, Kümpers C, Ribbat-Idel J, Becker F, et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front Oncol. 2018;8:623.
Martini A, Gupta A, Lewis SC, Cumarasamy S, Haines KG, Briganti A, et al. Development and internal validation of a side-specific, multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension of prostate cancer. BJU Int. 2018;122:1025–33.
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–51.
Mehralivand S, Shih JH, Harmon S, Smith C, Bloom J, Czarniecki M, et al. A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology. 2019;290:709–19.
Srigley JR. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med. 2006;130:303–17.
Magi-Galluzzi C, Evans AJ, Delahunt B, Epstein JI, Griffiths DF, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Modern Pathol. 2011;24:26–38.
Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011;108:984–92.
Epstein JI, Carmichael MJ, Pizovt G, Walsh PC. Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term followup. Journal Urol. 1993;150:135–41.
Ditonno F, Bologna E, Licari LC, Franco A, Cannoletta D, Checcucci E, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) during robot-assisted radical prostatectomy: a systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis. 2024. Online ahead of print.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–9.
von Klot CAJ, Merseburger AS, Böker A, Schmuck S, Ross TL, Bengel FM, et al. 68Ga-PSMA PET/CT imaging predicting intraprostatic tumor extent, extracapsular extension and seminal vesicle invasion prior to radical prostatectomy in patients with prostate cancer. Nucl Med Mol Imaging. 2017;51:314–22.
Sonni I, Felker ER, Lenis AT, Sisk AE, Bahri S, Allen-Auerbach M, et al. Head-to-head comparison of 68 Ga-PSMA-11 PET/CT and mpMRI with a histopathology gold standard in the detection, intraprostatic localization, and determination of local extension of primary prostate cancer: results from a prospective single-center imaging trial. Journal Nucl Med. 2022;63:847–54.
Mookerji N, Pfanner T, Hui A, Huang G, Albers P, Mittal R, et al. Fluorine-18 prostate-specific membrane antigen-1007 PET/CT vs multiparametric mri for locoregional staging of prostate cancer. JAMA Oncol. 2024;10:1097–103.
Eissa A, Elsherbiny A, Zoeir A, Sandri M, Pirola G, Puliatti S, et al. Reliability of the different versions of Partin tables in predicting extraprostatic extension of prostate cancer: a systematic review and meta-analysis. Minerva Urologica e Nefrologica. 2019;71:457–78.
Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42.
Martini A, Wever L, Soeterik TFW, Rakauskas A, Fankhauser CD, Grogg JB, et al. An updated model for predicting side-specific extraprostatic extension in the era of MRI-targeted biopsy. Prostate Cancer Prostatic Dis. 2024;27:520–4.
Heetman JG, van der Hoeven EJRJ, Rajwa P, Zattoni F, Kesch C, Shariat S, et al. External validation of nomograms, including MRI features for the prediction of side-specific extraprostatic extension. Prostate Cancer Prostatic Dis. 2024;27:492–9.
Heetman JG, Soeterik TFW, Wever L, Meyer AR, Nuininga JE, van Soest RJ, et al. A side-specific nomogram for extraprostatic extension may reduce the positive surgical margin rate in radical prostatectomy. World J Urol. 2022;40:2919–24.
Soeterik TFW, Heetman JG, Hermsen R, Wever L, Lavalaye J, Vinken M, et al. The added value of prostate-specific membrane antigen positron emission tomography/computed tomography to magnetic resonance imaging for local staging of prostate cancer in patients undergoing radical prostatectomy. Eur Urol Oncol. 2025;8:731–8.
Ditonno F, Franco A, Manfredi C, Veccia A, Valerio M, Bukavina L, et al. Novel non-MRI imaging techniques for primary diagnosis of prostate cancer: micro-ultrasound, contrast-enhanced ultrasound, elastography, multiparametric ultrasound, and PSMA PET/CT. Prostate Cancer Prostatic Dis. 2024;27:29–36.
Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18 F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021;206:52–61.
Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase iii, multicenter study. Clinical Cancer Res. 2021;27:3674–82.
Heilinger J, Weindler J, Roth KS, Krapf P, Schomäcker K, Dietlein M, et al. Threshold for defining PSMA-positivity prior to 177Lu-PSMA therapy: a comparison of [68Ga]Ga-PSMA-11 and [18F]F-DCFPyL in metastatic prostate cancer. EJNMMI Res. 2023;13:83.
Evangelista L, Maurer T, van der Poel H, Alongi F, Kunikowska J, Laudicella R, et al. [68Ga]Ga-PSMA versus [18F]PSMA positron emission tomography/computed tomography in the staging of primary and recurrent prostate cancer. a systematic review of the literature. Eur Urol Oncol. 2022;5:273–82.
Jiang Z, Guo J, Hu L, Yang S, Meng B, Tang Q. Diagnostic performance of 18 F-DCFPyL PET vs. 68 Ga-PSMA PET/CT in patients with suspected prostate cancer: A systemic review and meta-analysis. Oncol Lett. 2024;27:188.
EAU Guidelines Office, Arnhem, The Netherlands. http://uroweb.org/guidelines/compilations-of-all-guidelines/ [Internet]. EAU Guidelines. Edn. presented at the EAU Annual Congress Madrid 2025. ISBN 978-94-92671-29-5.
Author information
Authors and Affiliations
Contributions
NT and AT conceived and designed the study. NT, AMa, AMe, YA, SG, HS, BH, SB, CK and LO contributed to data collection. AMa, RB, BK, and HJ performed statistical analysis. NT, HJod, and KK conducted the literature review and contributed to manuscript drafting. VW and ML provided clinical interpretation and critical input. AT supervised the project and contributed to manuscript revision. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations, including the principles outlined in the Declaration of Helsinki. Ethical approval for this study was obtained from the Institutional Review Board (IRB) of the Mount Sinai Hospital (Reference Number: MSH IRB 25-00146). The requirement for informed consent was waived by the IRB due to the retrospective nature of the study and the use of de-identified data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tillu, N., Maheshwari, A., Kolanukuduru, K. et al. Predicting side-specific extraprostatic extension in prostate cancer using an 18F-DCFPyL PSMA-PET/CT–based nomogram. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01001-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01001-7
This article is cited by
-
Deep learning algorithm assisting diagnosis of prostate cancer extracapsular extension based on [18F]PSMA-1007 PET/CT and multiparametric MRI: A multicenter study
Prostate Cancer and Prostatic Diseases (2025)