Abstract
Background
Despite advancements in treatment, metastatic prostate cancer remains a lethal disease. As prostate cancer becomes resistant to standard of care treatments like androgen receptor pathway inhibitors (ARPIs) and chemotherapy, cell surface tumor antigens and receptors become increasingly heterogeneous and diverse, dependent on androgen receptor dependency with relevance for both diagnostic positron emission tomography (PET) imaging and cell surface targeting therapeutics. Our review aims to describe emerging theranostic targets and agents in cell surface imaging and therapies.
Methods
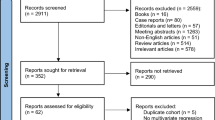
A literature search was carried out in March 2025, on Pubmed, as well as Clinicaltrials.gov to determine cell surface targets with viable trials for imaging and/or therapeutic agents. Keyword searches included “Prostate Cancer” AND “CRPC” AND “Cell Surface Targets.”
Results
Among the literature, 13 novel targets with robust supporting literature were found. Targets were subsequently divided into targets of interest in AR-positive and AR-negative (NEPC and/or double negative) mCRPC. Ongoing and completed trials for imaging and/or therapeutics leveraging these targets was described.
Conclusion
Numerous prostate cancer cell surface markers are emerging as theranostic targets. For patients ineligible for or developing progression following PSMA-targeting therapies, extending cell surface targeting therapeutics, whether they are ADCs, cellular therapies, or RPTs, is increasingly vital.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–103.
Morris MJ, Castellano D, Herrmann K, de Bono JS, Shore ND, Chi KN, et al. 177)Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. Lancet. 2024;404:1227–39.
Vlachostergios PJ, Niaz MJ, Sun M, Mosallaie SA, Thomas C, Christos PJ, et al. Prostate-specific membrane antigen uptake and survival in metastatic castration-resistant prostate cancer. Front Oncol. 2021;11:630589.
Murthy V, Allen-Auerbach M, Lam R, Owen D, Czernin J, Calais J. PSMA-negative lesion progression under (177)Lu-PSMA radioligand therapy. J Nucl Med. 2023;64:1502–3.
Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7-H3 as a therapeutic target in advanced prostate cancer. Eur Urol. 2023;83:224–38.
Zhang T, Agarwal A, Almquist RG, Runyambo D, Park S, Bronson E, et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Biomark Res. 2021;9:14.
Thang SP, Violet J, Sandhu S, Iravani A, Akhurst T, Kong G, et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for (177)Lu-labelled PSMA radioligand therapy. Eur Urol Oncol. 2019;2:670–6.
Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022;23:1389–97.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9–20.
Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024;390:875–88.
Sharifi MN, Shi Y, Chrostek MR, Callahan SC, Shang T, Berg TJ, et al. Clinical cell-surface targets in metastatic and primary solid cancers. JCI Insight. 2024;9:e183674.
Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci USA. 2018;115:E4473–E82.
Antonarakis ES, Park SH, Goh JC, Shin SJ, Lee JL, Mehra N, et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol. 2023;41:3839–50.
Brady L, Kriner M, Coleman I, Morrissey C, Roudier M, True LD, et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat Commun. 2021;12:1426.
Ocana A, Amir E, Pandiella A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 2020;22:15.
Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J Nucl Med. 2006;47:1007–15.
Shen M, Liu S, Stoyanova T. The role of Trop2 in prostate cancer: an oncogene, biomarker, and therapeutic target. Am J Clin Exp Urol. 2021;9:73–87.
Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19:589–608.
Poty S, Francesconi LC, McDevitt MR, Morris MJ, Lewis JS. alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J Nucl Med. 2018;59:878–84.
Fu Z, Gao C, Wu T, Wang L, Li S, Zhang Y, et al. Peripheral neuropathy associated with monomethyl auristatin E-based antibody-drug conjugates. iScience. 2023;26:107778.
Buteau JP, Kostos L, Alipour R, Jackson P, McInstosh L, Emmerson B, et al. Clinical trial protocol for VIOLET: a single-center, phase I/II trial evaluation of radioligand treatment in patients with metastatic castration-resistant prostate cancer with [(161)Tb]Tb-PSMA-I&T. J Nucl Med. 2024;65:1231–8.
Gualberto A. Brentuximab Vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies. Expert Opin Investig Drugs. 2012;21:205–16.
Li K, Guo J, Guo H, Zhang Q, Huang H, Zhou L, et al. Updated results from a phase I/II study of CBP-1018, a bi-ligand–drug conjugate (Bi-XDC) as late line therapy for patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2025;43:161.
Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359–71.
Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28:724–34.
Dorff TB, Blanchard MS, Adkins LN, Luebbert L, Leggett N, Shishido SN, et al. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2024;30:1636–44.
Zhang B, Yang M, Zhang W, Liu N, Wang D, Jing L, et al. Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside. Cell Death Dis. 2024;15:50.
Paredes-Moscosso SR, Nathwani AC. 10 years of BiTE immunotherapy: an overview with a focus on pancreatic cancer. Front Oncol. 2024;14:1429330.
Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017;32:474–89 e6.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11.
Antonarakis ES, Chandhasin C, Osbourne E, Luo J, Sadar MD, Perabo F. Targeting the N-terminal domain of the androgen receptor: a new approach for the treatment of advanced prostate cancer. Oncologist. 2016;21:1427–35.
Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–8.
Nolan-Stevaux O. AMG 509: A novel, humanized, half-Life extended, bispecific STEAP1 × CD3 T cell recruiting XmAb® 2+1 antibody. Cancer Res. 2020;80:DDT02–3.
Ylitalo EB, Thysell E, Jernberg E, Lundholm M, Crnalic S, Egevad L, et al. Subgroups of Castration-resistant Prostate Cancer Bone Metastases Defined Through an Inverse Relationship Between Androgen Receptor Activity and Immune Response. Eur Urol. 2017;71:776–87.
Burnell SEA, Spencer-Harty S, Howarth S, Bodger O, Kynaston H, Morgan C, et al. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PLoS One. 2019;14:e0220456.
Carrasquillo JA, Fine BM, Pandit-Taskar N, Larson SM, Fleming SE, Fox JJ, et al. Imaging patients with metastatic castration-resistant prostate cancer using (89)Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med. 2019;60:1517–23.
Kelly WK, Danila DC, Lin CC, Lee JL, Matsubara N, Ward PJ, et al. Xaluritamig, a STEAP1 x CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 2024;14:76–89.
Miller CD, Lozada JR, Zorko NA, Elliott A, Makovec A, Radovich M, et al. Pan-cancer interrogation of B7-H3 (CD276) as an actionable therapeutic target across human malignancies. Cancer Res Commun. 2024;4:1369–79.
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900.
Xia L, Wu Y, Ren Y, Wang Z, Zhou N, Zhou W, et al. A whole-body imaging technique for tumor-specific diagnostics and screening of B7H3-targeted therapies. J Clin Invest. 2025;135:e186388.
de Bono J, Helissey C, Fizazi K, Maroto Rey JP, Roubaud G, Antonarakis ES, et al. TAMARACK: randomized phase II trial of the B7-H3 targeting antibody drug conjugate (ADC) vobramitamab duocarmazine (vobra duo) in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2024;35:S996–S7.
Patel M, Dai T, Koyoma T, Falchook GS, Friedman CF, Piha-Paul SA, et al. Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with advanced solid tumors: Updated clinical and biomarker results from a phase I/II study. Ann Oncol. 2023;34:S481–S2.
Wang X, Singh, J, Kobayashi, M, Maejima, T, Wada, N, Nishimura, M, et al. Evaluation of immunomodulatory effects of ifinatamab deruxtecan (I-DXd) in the IDeate-Pantumor01 phase 1/2 study in patients with advanced solid tumors. J Immunotherapy Cancer. 2024;12.
Shenderov E, De Marzo AM, Lotan TL, Wang H, Chan S, Lim SJ, et al. Neoadjuvant enoblituzumab in localized prostate cancer: a single-arm, phase 2 trial. Nat Med. 2023;29:888–97.
Hekim C, Leinonen J, Narvanen A, Koistinen H, Zhu L, Koivunen E, et al. Novel peptide inhibitors of human kallikrein 2. J Biol Chem. 2006;281:12555–60.
Shen F, Kelly WK, Pandit-Thaskar N, McDevitt T, Smith R, Menard K, et al. Preclinical characterization of human Kallikrein 2 (hK2) as a novel target for the treatment of prostate cancer. J Clin Oncol. 2024;42:202.
Pandit-Taskar N, O’Donoghue JA, Chetty D, Max S, Wanik D, Ilovich O, et al. A phase 0 study to assess the biodistribution and pharmacokinetics of a radiolabeled antibody targeting human kallikrein 2 in participants with metastatic castration-resistant prostate cancer. J Nucl Med. 2024;65:1051–6.
Morris M, Wong JYC, Nordquist L, Zelig Szmulewitz R, Agarwal N, Attiyehm EF, et al. A phase 1 study of JNJ-69086420 (JNJ-6420), an actinium-225 (225Ac) -labeled antibody targeting human kallikrein 2 (hK2), for metastatic castration-resistant prostate cancer (mCRPC). Jou. Rna Clin Oncol. 2024;42:5010.
Stein MN, Vinceneux A, Robbrecht D, Doger B, Autio KA, Schweizer MT, et al. Pasritamig, a first-in-class, bispecific T-cell engager targeting human kallikrein 2, in metastatic castration-resistant prostate cancer: a phase I study. J Clin Oncol. 2025;43:2515–26.
Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16:3533–8.
Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–96.
Stein MN, Dumbrava EE, Teply BA, Gergis US, Guiterrez ME, Reshef R, et al. PSCA-targeted BPX-601 CAR T cells with pharmacological activation by rimiducid in metastatic pancreatic and prostate cancer: a phase 1 dose escalation trial. Nat Commun. 2024;15:10743.
Xie J, Molck C, Paquet-Fifield S, Butler L, Australian Prostate Cancer B, Sloan E, et al. High expression of TROP2 characterizes different cell subpopulations in androgen-sensitive and androgen-independent prostate cancer cells. Oncotarget. 2016;7:44492–504.
Ajkunic A, Sayar E, Roudier MP, Patel RA, Coleman IM, De Sarkar N, et al. Assessment of TROP2, CEACAM5 and DLL3 in metastatic prostate cancer: Expression landscape and molecular correlates. NPJ Precis Oncol. 2024;8:104.
Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021;384:1529–41.
Powles T, Tagawa S, Vulsteke C, Gross-Goupil M, Park SH, Necchi A, et al. Sacituzumab govitecan in advanced urothelial carcinoma: TROPiCS-04, a phase III randomized trial. Ann Oncol. 2025;36:561–71.
Lang J, Tagawa ST, Slovin S, Sperger SM, Schehr JL, Stahfield C, et al. Final clinical and liquid biopsy (LBx) results of phase II trial of sacituzumab govitecan (SG) in patients (Pts) with metastatic castration resistant prostate cancer (mCRPC) progressing on androgen receptor signaling inhibitors (ARSI). Ann Oncol. 2024;35:S993–S4.
Tang F, Xu D, Wang S, Wong CK, Martinez-Fundichely A, Lee CJ, et al. Chromatin profiles classify castration-resistant prostate cancers suggesting therapeutic targets. Science. 2022;376:eabe1505.
Pitzen SP, Rudenick AN, Qiu Y, Zhang W, Munro SA, McCluskey BM, et al. Comparative transcriptomics reveals a mixed basal, club, and hillock epithelial cell identity in castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2025;122:e2415308122.
Zaffuto E, Pompe R, Zanaty M, Bondarenko HD, Leyh-Bannurah SR, Moschini M, et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin Genitourin Cancer. 2017;15:e793–e800.
Puca L, Gavyert K, Sailer V, Conteduca V, Dardenne E, Sigouros M, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med. 2019;11:eaav0891.
Tendler S, Dunphy MP, Agee M, O’Donoghue J, Aly RG, Choudhury NJ, et al. Imaging with [(89)Zr]Zr-DFO-SC16.56 anti-DLL3 antibody in patients with high-grade neuroendocrine tumours of the lung and prostate: a phase 1/2, first-in-human trial. Lancet Oncol. 2024;25:1015–24.
Ahn MJ, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389:2063–75.
Aggarwal R, Rottey S, Bernard-Tessier A, Mellado-Gonzalez B, Kosaka R, Stadler WM, et al. Phase 1b study of tarlatamab in de novo or treatment-emergent neuroendocrine prostate cancer (NEPC). J Clin Oncol. 2024;42:5012.
Beltran H, Johnson ML, Jain P, Schenk EL, Sanborn RE, Thompson JR, et al. Updated results from a phase 1/2 study of HPN328, a tri-specific, half-life (T1/2) extended DLL3-targeting T-cell engager in patients (pts) with small cell lung cancer (SCLC) and other neuroendocrine cancers (NEC). J Clin Oncol. 2024;42:8090.
Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–58.
Mansfield AS, Hong DS, Hann CL, Farago AF, Beltran H, Waqar SN, et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3-expressing advanced solid tumors. NPJ Precis Oncol. 2021;5:74.
Korsen JA, Gutierrez JA, Tully KM, Carter LM, Samuels ZV, Khitrov S, et al. Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer. Proc Natl Acad Sci USA. 2022;119:e2203820119.
Tran TT, Tran QH, Nguyen QT, Le MT, Trinh DT, Thai KM. Identification of potential interleukin-8 inhibitors acting on the interactive site between chemokine and CXCR2 receptor: A computational approach. PLoS One. 2022;17:e0264385.
Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci Transl Med. 2019;11:eaax0428.
Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543:728–32.
Spatz P, Chen X, Reichau K, Huber ME, Muhlig S, Matsusaka Y, et al. Development and Initial Characterization of the First (18)F-CXCR2-Targeting Radiotracer for PET Imaging of Neutrophils. J Med Chem. 2024;67:6327–43.
Su Y, Liu Y, Behrens CR, Bidlingmaier S, Lee NK, Aggarwal R, et al. Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight. 2018;3:e121497.
Kunz N, Kemper C. Complementing Anticancer Therapy: Antibody-Drug Conjugates Targeting CD46 as Prostate Cancer Treatment. J Clin Oncol. 2025;43:1835–8.
Bidkar AP, Peter R, Wadhwa A, Bobba KN, Bidlingmaier S, Meher N, et al. Effective treatment of disseminated prostate cancer using CD46-targeted 225Ac therapy. Clin Cancer Res. 2025;31:2963–77.
Li J, Huang T, Hua J, Wang Q, Su Y, Chen P, et al. CD46 targeted (212)Pb alpha particle radioimmunotherapy for prostate cancer treatment. J Exp Clin Cancer Res. 2023;42:61.
Aggarwal R, Vuky J, VanderWeele D, Rettig M, Heath EI, Quigley D, et al. Phase I, first-in-human study of FOR46 (FG-3246), an immune-modulating antibody-drug conjugate targeting CD46, in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2025;43:1824–34.
Shakhnazaryan N, Curry N, Rastogi M, Avins D, Pandey S, de Kouchkovsky I, et al. A phase 1b dose escalation study of FOR46, a novel antibody-drug conjugate targeting a tumor-specific epitope of CD46, in combination with enzalutamide (Enza) in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2024;42:5066.
DeLucia DC, Cardillo TM, Ang L, Labrecque MP, Zhang A, Hopkins JE, et al. Regulation of CEACAM5 and therapeutic efficacy of an anti-CEACAM5-SN38 antibody-drug conjugate in neuroendocrine prostate cancer. Clin Cancer Res. 2021;27:759–74.
Besse B, Lo Russo G, Lena H, Nadal E, Cousin S, Kowalski D, et al. Tusamitamab ravtansine vs docetaxel in previously treated advanced nonsquamous NSCLC: results from phase 3 CARMEN-LC03 trial. J Thorac Oncol. 2024;19:S25–S6.
Dotan E, Cohen SJ, Starodub AN, Lieu CH, Messersmith WA, Simpson PS, et al. phase I/II trial of labetuzumab govitecan (anti-CEACAM5/SN-38 antibody-drug conjugate) in patients with refractory or relapsing metastatic colorectal cancer. J Clin Oncol. 2017;35:3338–46.
Baek DS, Kim YJ, Vergara S, Conard A, Adams C, Calero G, et al. A highly-specific fully-human antibody and CAR-T cells targeting CD66e/CEACAM5 are cytotoxic for CD66e-expressing cancer cells in vitro and in vivo. Cancer Lett. 2022;525:97–107.
Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharm Rev. 2008;60:1–42.
Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–8.
Qiao J, Grabowska MM, Forestier-Roman IS, Mirosevich J, Case TC, Chung DH, et al. Activation of GRP/GRP-R signaling contributes to castration-resistant prostate cancer progression. Oncotarget. 2016;7:61955–69.
Belge Bilgin G, Bilgin C, Orscelik A, Burkett BJ, Thorpe MP, Johnson DR, et al. Detection rate of gastrin-releasing peptide receptor (GRPr) targeted tracers for positron emission tomography (PET) imaging in primary prostate cancer: a systematic review and meta-analysis. Ann Nucl Med. 2024;38:865–76.
Duan H, Moradi F, Davidzon GA, Liang T, Song H, Loening AM, et al. 68)Ga-RM2 PET-MRI versus MRI alone for evaluation of patients with biochemical recurrence of prostate cancer: a single-centre, single-arm, phase 2/3 imaging trial. Lancet Oncol. 2024;25:501–8.
Li S, Nguyen A, Counter W, John NC, De Leon J, Hruby G, et al. Utility of (64)Cu-Sarcophagine-Bombesin PET/CT in Men with Biochemically Recurrent Prostate Cancer and Negative or Equivocal Findings on (68)Ga-PSMA-11 PET/CT. J Nucl Med. 2024;65:1371–5.
Gonzalez-Rueda S, Garcia-Perez O, Luna-Gutierrez M, Ocampo-Garcia B, Santos-Cuevas C, Ramirez-Nava G, et al. Theranostic potential of the iPSMA-bombesin radioligand in patients with metastatic prostate cancer: a pilot study. Pharmaceutics. 2024;16.
Butler W, Xu L, Zhou Y, Cheng Q, Hauck JS, He Y, et al. Oncofetal protein glypican-3 is a biomarker and critical regulator of function for neuroendocrine cells in prostate cancer. J Pathol. 2023;260:43–55.
Lin F, Clift, R, Salvador, K, Guest, M, Kim, D, Horton, S, et al. Novel GPC3-targeted radiopharmaceutical therapy for hepatocellular carcinoma and prostate cancer: Preclinical characterization in rodents and non-human primates. J Nuclear Med. 2024;65:241022.
Madera L, Hernandez Rojas A, Colombo R, Wu A, Piscitelli CL, Urosev D, et al. ZW251, a novel glypican-3-targeting antibody drug conjugate bearing a topoisomerase 1 inhibitor payload. Cancer Res. 2023;83:2658.
Kesch C, Yirga L, Dendl K, Handke A, Darr C, Krafft U, et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur J Nucl Med Mol Imaging. 2021;49:385–9.
Wang X, Zhang X, Zhang X, Guan L, Gao X, Xu L, et al. Design, preclinical evaluation, and first-in-human PET study of [(68)Ga]Ga-PSFA-01: a PSMA/FAP heterobivalent tracer. Eur J Nucl Med Mol Imaging. 2025;52:1166–76.
Zhao N, Chopra S, Trepka K, Wang YH, Sakhamuri S, Hooshdaran N, et al. CUB domain-containing protein 1 (CDCP1) is a target for radioligand therapy in castration-resistant prostate cancer, including PSMA null disease. Clin Cancer Res. 2022;28:3066–75.
Gafita A, Rauscher I, Weber M, Hadaschik B, Wang H, Armstrong WR, et al. Novel framework for treatment response evaluation using PSMA PET/CT in patients with metastatic castration-resistant prostate cancer (RECIP 1.0): an international multicenter study. J Nucl Med. 2022;63:1651–8.
Powderly J, Cote, G, Flaherty, K, Szmulewitz, RZ, Ribas, A, Weber, J, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunotherapy of Cancer. 2015;3:08.
Beheshti M, Taimen P, Kemppainen J, Jambor I, Muller A, Loidl W, et al. Value of (68)Ga-labeled bombesin antagonist (RM2) in the detection of primary prostate cancer comparing with [(18)F]fluoromethylcholine PET-CT and multiparametric MRI-a phase I/II study. Eur Radio. 2023;33:472–82.
Darson MF, Pacelli A, Roche P, Rittenhouse HG, Wolfert RL, Young CY, et al. Human glandular kallikrein 2 (hK2) expression in prostatic intraepithelial neoplasia and adenocarcinoma: a novel prostate cancer marker. Urology. 1997;49:857–62.
Sharma SK, Pourat J, Abdel-Atti D, Carlin SD, Piersigilli A, Bankovich AJ, et al. Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res. 2017;77:3931–41.
Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67.
Zhou J, Fan X, Chen N, Zhou F, Dong J, Nie Y, et al. Identification of CEACAM5 as a biomarker for prewarning and prognosis in gastric cancer. J Histochem Cytochem. 2015;63:922–30.
Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–66.
Author information
Authors and Affiliations
Contributions
BA: Reviewed the literature, drafted multiple sections of the manuscript, and designed figures and tables. JM: Reviewed the literature, drafted multiple sections of the manuscript, and designed tables. AA: Developed the original concept and provided critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
BA: None. Jane McKenzie: None. AA. • Research support (to Duke) from the NIH/NCI, PCF/Movember, DOD, Astellas, Pfizer, Bayer, Janssen, BMS, AstraZeneca, Merck, Pathos, Amgen, Novartis. • Consulting or advising relationships with Astellas, Pfizer, Bayer, Janssen, BMS, AstraZeneca, Merck, Forma, Celgene, Myovant, Exelixis, GoodRx, Novartis, Medscape, MJH, Z Alpha, Telix.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashraf, B., McKenzie, J. & Armstrong, A.J. Beyond PSMA: theranostic cell surface targets in metastatic prostate cancer. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01037-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01037-9