Abstract
Background/Objectives
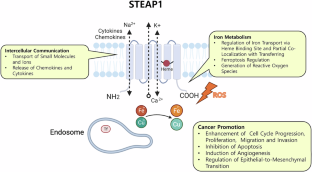
Six transmembrane epithelial antigen of prostate 1 (STEAP1), a cell surface protein, is highly expressed in prostate cancer and is known to be associated with disease progression and poor prognosis. Based on its specificity for prostate cancer, significant progress has been made over the past decade to capitalize on STEAP1 as a diagnostic and treatment target, and its potential future role in prostate cancer care is of considerable interest.
Subjects/Methods
This review evaluates the current and emerging strategies targeting STEAP1, integrating findings from preclinical studies and clinical trials.
Results
This review discusses STEAP1-based diagnostics, including molecular imaging (89Zr-DFO-MSTP2109A) and liquid biopsy methods, as well as therapeutics, such as STEAP1 antibodies, antibody-drug conjugates (DSPT3086S, ADRX-0405, ABBV-969, and DXC008), chimeric antigen receptor T-cell therapy (STEAP1 CAR-T), bispecific T-cell engagers (Xaluritamig/AMG 509, BC261), and cancer vaccines.
Conclusions
STEAP1 represents a promising diagnostic and therapeutic target in prostate cancer, and its potential role in shaping future management of the disease warrants continued investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2.2026. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed 23 Sep 2025).
Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–8.
Gomes IM, Arinto P, Lopes C, Santos CR, Maia CJ. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason score. Urol Oncol. 2014;32:53.e23–9.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Nakamura H, Arihara Y, Takada K. Targeting STEAP1 as an anticancer strategy. Front Oncol. 2023;13:1285661.
Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023;14:2041.
Ihlaseh-Catalano SM, Drigo SA, de Jesus CM, Domingues MA, Trindade Filho JC, de Camargo JL, et al. STEAP1 protein overexpression is an independent marker for biochemical recurrence in prostate carcinoma. Histopathology. 2013;63:678–85.
Danila DC, Szmulewitz RZ, Vaishampayan UN, Higano CS, Gilbert HN, Brunstein F, et al. Phase I study of DSTP3086S, an antibody-drug conjugate targeting six-transmembrane epithelial antigen of prostate 1, in metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:3518–27.
Nolan-Stevaux O, Darpo B, Prawira A, Aggarwal R, Williams K, Liao R, et al. AMG 509 (Xaluritamig), an anti-STEAP1 XmAb 2+1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer. Cancer Discov. 2024;14:90–103.
Gomes IM, Maia CJ, Santos CR. STEAP proteins: from structure to applications in cancer therapy. Mol Cancer Res. 2012;10:573–87.
Yamamoto T, Tamai Y, Tanaka M, Yamazaki Y, Nakano M, Kanai Y, et al. Six-transmembrane epithelial antigen of the prostate-1 plays a role for in vivo tumor growth via intercellular communication. Exp Cell Res. 2013;319:2617–26.
Grunewald TGP, Bach H, Cossarizza A, Matsumoto I. The STEAP protein family: versatile oxidoreductases and targets for cancer immunotherapy with overlapping and distinct cellular functions. Biol Cell. 2012;104:641–57.
Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–94.
Chen WJ, Lin J, Luo L, Chen M, Liu Y, Zhao Z, et al. Regulatory roles of six-transmembrane epithelial antigen of the prostate family members in the occurrence and development of malignant tumors. Front Cell Dev Biol. 2021;9:752426.
Oosterheert W, Marchese S, Mattevi A. Structure, function and mechanism of six-transmembrane epithelial antigen of the prostate (STEAP) enzymes: insights into a transmembrane oxidoreductase family related to NADPH oxidases. In: Structure and function of membrane proteins. Springer; 2023. pp. 521–34. https://doi.org/10.1007/978-3-031-23752-2_31.
Oosterheert W, Gros P. Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J Biol Chem. 2020;295:9502–12.
Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–55.
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40.
Grunewald TGP, Diebold I, Esposito I, Plehm S, Hauer K, Thiel U, et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol Cancer Res. 2012;10:52–65.
Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–36.
Nakamura H, Morimoto Y, Komatsu T, Li B, Takano N, Arihara Y, et al. Six-transmembrane epithelial antigen of the prostate 1 protects against increased oxidative stress via a nuclear erythroid 2-related factor pathway in colorectal cancer. Cancer Gene Ther. 2019;26:313–22.
Pan Y, Li Y, Guo L, Zhao Y, Zhao X. [Influence of expression of six transmembrane epithelial antigen of the prostate-1 on intracellular reactive oxygen species level and cell growth: an in vitro experiment]. Zhonghua Yi Xue Za Zhi. 2008;88:641–4.
Huo SF, Zhu WT, Wu H, Jian B, Zhao ZY, Feng JY, et al. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci Rep. 2020;40:BSR20193169.
Zhu J, Xu S, Gao W, Dong J, Xu B, Qin Y, et al. The molecular mechanisms of regulating oxidative stress-induced ferroptosis and therapeutic strategy in tumors. Oxid Med Cell Longev. 2020;2020:8810785.
Zou X, Peng H, Zhou A, Gong X, Song X, Wu W, et al. Inhibition of STEAP1 ameliorates inflammation and ferroptosis of acute lung injury caused by sepsis in LPS-induced human pulmonary microvascular endothelial cells. Mol Biol Rep. 2023;50:5667–74.
Liang J, Gao Z, Wang Y, Liu X, Wei B, Fan L, et al. Ferroptosis landscape in prostate cancer from molecular and metabolic perspective. Cell Death Discov. 2023;9:128.
Cao PHA, Skovbakke SL, Christensen JK, Boström PJ, Rørth M, Nordentoft I, et al. Unlocking ferroptosis in prostate cancer – the road to novel therapies and imaging markers. Nat Rev Urol. 2024;21:615–37.
Rocha SM, Santos FM, Socorro S, Passarinha LA, Maia CJ. Proteomic analysis of STEAP1 knockdown in human LNCaP prostate cancer cells. Biochim Biophys Acta Mol Cell Res. 2023;1870:119522.
Jiao Z, Wang S, Xu Z, Ren F, Li Y, Liu X, et al. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem Cell Biol. 2020;154:215–30.
Gomes IM, Rocha SM, Gaspar C, Alho I, Gouveia AM, Oliveira MJ, et al. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol. 2018;35:40.
Rocha SM, Alho I, Gaspar C, Arinto P, Maia CJ, Gomes IM, et al. STEAP1 regulation and its influence modulating the response of LNCaP prostate cancer cells to bicalutamide, enzalutamide and apalutamide. Mol Med Rep. 2023;27:52.
Lin TY, Park JA, Long A, Guo HF, Cheung NKV. Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. J Immunother Cancer. 2021;9:e003114.
Rocha SM, Socorro S, Passarinha LA, Maia CJ. Comprehensive landscape of STEAP family members expression in human cancers: Unraveling the potential usefulness in clinical practice using integrated bioinformatics analysis. Data. 2022;7:64.
Rocha SM, Gouveia MJ, Alho I, Passarinha LA, Maia CJ, Gomes IM, et al. STEAP1 knockdown decreases the sensitivity of prostate cancer cells to paclitaxel, docetaxel and cabazitaxel. Int J Mol Sci. 2023;24:6643.
Bizzaro CL, D’Agostino S, Chen X, Shahid Z, Li M, Almeida L, et al. Exploring STEAP1 expression in prostate cancer cells in response to androgen deprivation and in small extracellular vesicles. Mol Cancer Res. 2025. https://doi.org/10.1158/1541-7786.MCR-24-0903.
Carrasquillo JA, Fine BM, Pandit-Taskar N, Larson SM, Fleming M, Smith-Jones PM, et al. Imaging patients with metastatic castration-resistant prostate cancer using (89)Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med. 2019;60:1517–23.
Khanna K, Salmond N, Lynn KS, Leong HS, Williams KC. Clinical significance of STEAP1 extracellular vesicles in prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:802–11.
Poon AC, Tang Y, Li C, Wu S. Nanoscale flow cytometry of patient plasma for the detection of prostate cancer-associated extracellular vesicles. STEM Fellowsh J. 2017;3:1–2.
Sheng M, Lee JH, Kim S, Patel S, Wong C, Chan J, et al. Abstract 61: a novel immunotherapy for metastatic prostate cancer: a monoclonal antibody that can bind both STEAP1 and STEAP2. Cancer Res. 2024;84:61–61.
OncologyPipeline. Vandortuzumab vedotin discontinued after Phase I trial in 2016. 2023. https://www.oncologypipeline.com/apexonco/esmo-2023-amgens-steap1-project-spurs-cautious-optimism.
Yu J, Chen L, Zhou P, Li D, Wang J, Huang Y, et al. The anti-STEAP1/PSMA ADC, DXC008: assessment of anti-tumor efficacy, pharmacokinetic, and toxicity properties. J Clin Oncol. 2025;43:e17161.
Zhang L, Zhou X, Pan Y, Li M, Huang H, Wang Y, et al. The role of STEAP1 in prostate cancer: implications for diagnosis and therapeutic strategies. Biomedicines. 2025;13:794
A Phase I, open-label, multicenter, first-in-human, dose escalation and expansion clinical study to evaluate the safety, tolerability, pharmacokinetic profiles and preliminary efficacy of DXC008 in patients with prostate cancer and other solid tumors (such as Ewing sarcoma). 2025. ClinicalTrials.gov identifier: NCT06926283. https://clinicaltrials.gov/study/NCT06926283 (accessed 23 Sep 2025).
Kelly WK, Aggarwal R, Petrylak DP, Tagawa ST, Vogelzang NJ, Yu EY, et al. Xaluritamig, a STEAP1 × CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 2024;14:76–89.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69.
Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28:724–34.
Stein MN, Frank L, Petrylak DP, Heath EI, Yu EY, George DJ, et al. Immunotherapeutic trial in men with biochemical recurrence after definitive local therapy for prostate cancer: a clinical trial in progress. J Immunother Cancer. 2023;11:A755.
Prime-boost immunotherapeutic trial in men with biochemical recurrence after definitive local therapy for prostate cancer. 2025. ClinicalTrials.gov identifier: NCT05617040. https://www.clinicaltrials.gov/study/NCT05617040 (accessed 23 Sep 2025).
Author information
Authors and Affiliations
Contributions
JYH drafted the sections on the introduction, clinical implications, diagnostics, and therapeutics, and prepared the tables and figures, as well as formatted the overall manuscript. MS wrote the sections on molecular mechanisms and biological roles and contributed to figure preparation. DK provided critical revision of the entire manuscript and drafted the conclusion. HSL contributed to the molecular mechanisms and roles section and reviewed the manuscript. UE, as principal investigator, supervised the project and contributed to critical revision, overall review, and guidance.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heo, J.Y., Sheng, M., Khalaf, D. et al. STEAP1-targeted strategies in advanced prostate cancer: a review on therapeutic and diagnostic implications. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01038-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01038-8
