Abstract
Background
The 4K Score is a blood-based test that estimates the risk of clinically significant prostate cancer (Grade Group ≥2, GG2 + ) by combining four kallikrein markers with clinical variables. However, benign prostatic hyperplasia (BPH) can elevate PSA levels, potentially leading to risk overestimation in men with large prostates. We developed a novel metric, 4K Density (4K Score divided by prostate volume), to adjust for prostate size and improve risk stratification.
Methods
We retrospectively reviewed 3150 men who underwent 4K Score testing at the University of Miami Desai Sethi Urology Institute from 2014 to 2024. After excluding those without a prostate biopsy or MRI within six months of the 4K Score, 1983 men remained. Statistical analysis using SAS v9.4 included logistic regression, receiver operating characteristic (ROC) analysis, and Youden’s Index to determine optimal cutoffs for GG2+ detection. The performance of 4K Density was compared to the 4Kscore and PSA Density in predicting GG2+ cancer.
Results
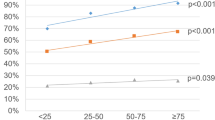
Among the 1983 men, 661 (33%) had GG2+ cancer. 4K Density was significantly higher in men with GG2+ cancer compared to those without (median 0.93 vs. 0.25, p < .0001). In multivariable analysis, 4K Density was the strongest independent predictor (OR 3.51, 95% CI 3.64–4.66), outperforming 4Kscore and PSA density. 4K Density also had the highest AUC (0.81, (95%CI)), compared to 4Kscore (0.76, 95 %CI, <0.0001) and PSA density (0.76, 95% CI, <0.0001). At an optimized cutoff of 0.56, 4K Density achieved 89.9% NPV and 48.5% PPV for detecting GG2+ cancer.
Conclusions
4K Density is a novel, volume-adjusted biomarker that improves detection of clinically significant prostate cancer and outperforms PSA density and the 4Kscore test. It may be helpful in larger prostates, where confounding from BPH is present. Prospective validation is warranted to confirm its clinical utility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Generalized SAS 9.4 code used for statistical analyses in this study is available from the corresponding author upon reasonable request. Due to patient privacy considerations, individual-level data cannot be shared.
References
Centers for Disease Control and Prevention. Prostate cancer incidence by stage at diagnosis. United States Cancer Statistics [Internet]. 2024 [cited 2025 Apr 21]. Available from: https://www.cdc.gov/united-states-cancer-statistics/publications/prostate-cancer.html
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16. https://doi.org/10.1056/NEJM198710083171501.
National Cancer Institute. Prostate-specific antigen (PSA) test [Internet]. 2022 [updated 2022 Mar 1; cited 2025 Apr 21]. Available from: https://www.cancer.gov/types/prostate/psa-fact-sheet
Black MH, Diamandis EP. The diagnostic and prognostic utility of prostate-specific antigen for diseases of the breast. Breast Cancer Res Treat. 2000;59:1–14. https://doi.org/10.1023/A:1006380306781.
Vaccarella S, Li M, Bray F, Kvale R, Serraino D, Lorenzoni V, et al. Prostate cancer incidence and mortality in Europe and implications for screening activities: population based study. BMJ. 2024;377:e077738. https://doi.org/10.1136/bmj-2021-077738.
Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464–70. https://doi.org/10.1016/j.eururo.2014.10.021.
Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199:1459–63. https://doi.org/10.1016/j.juro.2017.11.113.
4Kscore. Detect your risk of aggressive prostate cancer | 4Kscore® test [Internet]. 2022 [cited 2025 Mar 12]. Available from: https://4kscore.com
Punnen S, Pavan N, Parekh DJ. Finding the wolf in sheep’s clothing: the 4Kscore is a novel blood test that can accurately identify the risk of aggressive prostate cancer. Rev Urol. 2015;17:3–13.
Pinsky PF, Kramer BS, Crawford ED, Grubb RL, Urban DA, Andriole GL, et al. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006;68:352–6. https://doi.org/10.1016/j.urology.2006.02.026.
Paterson NR, Lavallée LT, Nguyen LN, Witiuk K, Ross J, Mallick R, et al. Prostate volume estimations using magnetic resonance imaging and transrectal ultrasound compared to radical prostatectomy specimens. Can Urol Assoc J. 2016;10:264–8. https://doi.org/10.5489/cuaj.3236.
Choe S, Patel HD, Lanzotti N, Okabe Y, Rac G, Shea SM, et al. MRI vs. transrectal ultrasound to estimate prostate volume and PSAD: impact on prostate cancer detection. Urology. 2023;171:172–8. https://doi.org/10.1016/j.urology.2022.11.008.
Stephan C, Jung K, Lein M, Rochow H, Friedersdorff F, Maxeiner A. PHI density prospectively improves prostate cancer detection. World J Urol. 2021;39:3273–9. https://doi.org/10.1007/s00345-020-03585-2.
Tosoian JJ, Druskin SC, Andreas D, Mullane P, Chappidi M, Joo S, et al. Prostate Health Index density improves detection of clinically significant prostate cancer. BJU Int. 2017;120:793–8. https://doi.org/10.1111/bju.13762.
Benson MC, Whang IS, Olsson CA, McMahon DJ, Cooner WH. The use of prostate-specific antigen density to enhance the predictive value of intermediate levels of serum prostate-specific antigen. J Urol. 1992;147:817–21. https://doi.org/10.1016/S0022-5347(17)37394-9.
Britton CJ, Andrews JR, Arafa A, Kim Y, Latuche LR, Schulte PJ, et al. Prostate extracellular vesicles and prognostic biomarkers of clinically significant prostate cancer: a prospective single-institution pilot study. Prostate. 2025;85:594–602. https://doi.org/10.1002/pros.24861.
Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–4. https://doi.org/10.1016/j.juro.2011.06.057.
Food and Drug Administration. Summary of safety and effectiveness data (SSED) [Internet]. [cited 2025 May 2]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf19/P190022B.pdf
Omri N, Kamil M, Alexander K, Edmond S, Ariel Z, David K, et al. Association between PSA density and pathologically significant prostate cancer: the impact of prostate volume. Prostate. 2020;80:1444–9. https://doi.org/10.1002/pros.24078.
Pickersgill NA, Vetter JM, Andriole GL, Shetty AS, Fowler KJ, Mintz AJ, et al. Accuracy and variability of prostate multiparametric magnetic resonance imaging interpretation using the Prostate Imaging Reporting and Data System: a blinded comparison of radiologists. Eur Urol Focus. 2020;6:267–72. https://doi.org/10.1016/j.euf.2018.10.008.
Garcia-Reyes K, Passoni NM, Palmeri ML, Kauffman CR, Choudhury KR, Polascik TJ, et al. Detection of prostate cancer with multiparametric MRI: effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging. 2015;40:134–42. https://doi.org/10.1007/s00261-014-0197-7.
Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst. 2015;107:djv095 https://doi.org/10.1093/jnci/djv095.
Borque-Fernando Á, Rubio-Briones J, Esteban LM, Dong Y, Calatrava A, Gomez-Ferrer Á, et al. Role of the 4Kscore test as a predictor of reclassification in prostate cancer active surveillance. Prostate Cancer Prostatic Dis. 2019;22:84–90. https://doi.org/10.1038/s41391-018-0074-5.
Hougen HY, Reis IM, Han S, Prakash NS, Thomas J, Stoyanova R, et al. Evaluating 4Kscore’s role in predicting progression on active surveillance for prostate cancer independently of clinical information and PIRADS score. Prostate Cancer Prostatic Dis. 2025;28:180–6. https://doi.org/10.1038/s41391-024-00898-w.
Lin DW, Newcomb LF, Brown MD, Sjoberg DD, Dong Y, Brooks JD, et al. Evaluating the four kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary Prostate Active Surveillance Study. Eur Urol. 2017;72:448–54. https://doi.org/10.1016/j.eururo.2016.11.017.
Acknowledgements
We thank the patients included in this retrospective study for their invaluable contribution. We also acknowledge the University of Miami Desai Sethi Urology Institute for providing access to clinical data and OPKO Health for support with 4Kscore test data. Statistical support was provided by the Department of Urology at the Desai Sethi Urology Institute. This work was not funded.
Author information
Authors and Affiliations
Contributions
TG: Conceptualization, Investigation, Manuscript writing, Data curation. ON: Manuscript writing, investigation. JP: Investigation, data curation. TF: data curation, formal analysis. GR: Investigation, data curation. AW: Statistical analysis JR: Manuscript writing, data curation. KZ: Manuscript writing and review, Data curation. EC: Methodology, writing and review, Data curation. PF: Manuscript writing and review, Data curation. AK: Manuscript writing and review, Data curation. BN: Conceptualization, manuscript writing and review. CR: Conceptualization, manuscript writing and review. MG: Supervision, conceptualization, manuscript writing and review. DP: Supervision, Conceptualization, manuscript writing and review. SP: Conceptualization, Project administration, supervision, manuscript review and editing. *All Authors reviewed and approved the final manuscript
Corresponding author
Ethics declarations
Competing interests
SP is a consultant for OPKO Health, the provider of the 4Kscore test. All other authors (TG, ON, J.P, TF, GR, AW. JR, KZ, EC, PF, AK, BN, CR, MG, DP) declare no competing financial or non-financial interests.
Ethics approval
This study was approved by the University of Miami Institutional Review Board (IRB) under IRB#20140785. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guerard, T., Porto, J.G., Fekete, T. et al. 4K density: Adjusting the 4Kscore for prostate volume to improve risk stratification of clinically significant prostate cancer in men undergoing prostate biopsy. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01043-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01043-x