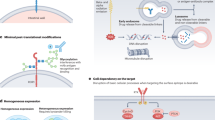
Abstract
Background
The aim of this study is to better characterize the expression profiles of well-established tumor-associated antigen (TAA) for antibody-drug conjugates (ADC) in primary lesion (PL) and bone metastatic lesion(BML) of prostate cancer (PCa).
Methods
Mass spectrometry (MS) and immunohistochemistry (IHC) were used to comprehensively compare the expression of HER2, NECTIN4, TROP2, PSMA, TF, STEAP1 and B7H3 in the matched cohort (n = 27 pairs), which included matched PL and BML samples from the same patients. IHC was then used to validate the expression of these TAAs in another independent unmatched cohort, including PL (n = 100) and BML (n = 49). IHC assessment included traditional semi-quantitative evaluation, computer-assisted H-score assessment and normalized membrane ratio (NMR) analysis.
Results
B7H3, STEAP1 and PSMA consistently exhibited high and stable expression rate in matched PL and BML, whereas the expression levels of the other TAAs may fluctuate between the two status. In the unmatched cohort, the expression levels of TROP2, TF, PSMA, and B7H3 were significantly lower, while the expression levels of HER2 and STEAP1 were significantly higher in BML than in PL (all p < 0.05). Overall, STEAP1, B7H3 and PSMA exhibited high expression rates in BML, with STEAP1 and B7H3 depicting relatively homogeneous high membranous expression patterns. The co-expression of these TAAs was frequently observed. In the dual-TAA combination analysis, any pairwise combination among B7H3, STEAP1, and PSMA exhibited relatively high expression coverage(å 85%) for BML.
Conclusions
B7H3, STEAP1, and PSMA might be the predominant targets in both PL and BML. Our findings reveal the dynamic and heterogeneous nature of TAA expression in PCa and may provide insights for integrating ADC-based targeted therapies into the existing treatment landscape for PCa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files. No custom code was developed.
References
Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021;519:20–9.
Yanagisawa T, Rajwa P, Thibault C, Gandaglia G, Mori K, Kawada T, et al. Androgen receptor signaling inhibitors in addition to docetaxel with androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur Urol. 2022;82:584–98.
Messina C, Giunta EF, Signori A, Rebuzzi SE, Banna GL, Maniam A, et al. Combining PARP inhibitors and androgen receptor signalling inhibitors in metastatic prostate cancer: a quantitative synthesis and meta-analysis. Eur Urol Oncol. 2024;7:179–88.
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7:93.
Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72:165–82.
Bosi C, Bartha A, Galbardi B, Notini G, Naldini MM, Licata L, et al. Pan-cancer analysis of antibody-drug conjugate targets and putative predictors of treatment response. Eur J Cancer. 2023;195:113379.
Lopez de Sa A, Diaz-Tejeiro C, Poyatos-Racionero E, Nieto-Jimenez C, Paniagua-Herranz L, Sanvicente A, et al. Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J Hematol Oncol. 2023;16:118.
Esapa B, Jiang J, Cheung A, Chenoweth A, Thurston DE, Karagiannis SN. Target antigen attributes and their contributions to clinically approved antibody-drug conjugates (ADCs) in haematopoietic and solid cancers. Cancers. 2023;15:1845.
Cimadamore A, Cheng M, Santoni M, Lopez-Beltran A, Battelli N, Massari F, et al. New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol. 2018;8:653.
Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, DeLaCruz A, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147–54.
Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–8.
Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, et al. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7-H3 for solid cancer. Mol Cancer Ther. 2020;19:2235–44.
Cimadamore A, Boixareu C, Sharp A, Beltran H, de Bono JS. Novel therapeutic strategies for metastatic prostate cancer care. Eur Urol. 2025;88:437–48.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20.
Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–56.
Nie L, Wu G, Culley DE, Scholten JC, Zhang W. Integrative analysis of transcriptomic and proteomic data: challenges, solutions and applications. Crit Rev Biotechnol. 2007;27:63–75.
Thyparambil SP, Liao W-L, Heaton R, Strasbaugh A, Melkie MA, Ling X. Proteomic profiling of antibody-drug conjugate (ADC) biomarkers in pancreatic cancer. J Clin Oncol. 2023;41:671.
Thyparambil S, Liao W-L, Heaton R, Strasbaugh A, Melkie M, Ghafourian N, et al. Quantitative proteomics of antibody-drug conjugates and chemotherapy targets in prostate cancer. Cancer Res. 2023;83:2160.
Coy S, Lee JS, Chan SJ, Woo T, Jones J, Alexandrescu S, et al. Systematic characterization of antibody-drug conjugate targets in central nervous system tumors. Neuro Oncol. 2024;26:458–72.
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297.
Xu N, Yao Z, Shang G, Ye D, Wang H, Zhang H, et al. Integrated proteogenomic characterization of urothelial carcinoma of the bladder. J Hematol Oncol. 2022;15:76.
Zou X, Lin X, Cheng H, Chen Y, Wang R, Ma M, et al. Characterization of intratumoral tertiary lymphoid structures in pancreatic ductal adenocarcinoma: cellular properties and prognostic significance. J Immunother Cancer. 2023;11:e006698.
Garassino MCSJ, Paz-Ares L, Lisberg A, Johnson M, Pérol M, Carroll D, et al. Normalized membrane ratio of TROP2 by quantitative continuous scoring is predictive of clinical outcomes in TROPION-lung 01. J Thorac Oncol. 2024;19:S2–S3.
Colombo R, Tarantino P, Rich JR, LoRusso PM, de Vries EGE. The journey of antibody-drug conjugates: lessons learned from 40 years of development. Cancer Discov. 2024;14:2089–108.
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2021;27:43–51.
Pereira PMR, Mandleywala K, Ragupathi A, Lewis JS. Acute statin treatment improves antibody accumulation in EGFR- and PSMA-expressing tumors. Clin Cancer Res. 2020;26:6215–29.
Mulati Y, Shen Q, Tian Y, Chen Y, Yao K, Yu W, et al. Characterizing PSMA heterogeneity in prostate cancer and identifying clinically actionable tumor associated antigens in PSMA negative cases. Sci Rep. 2025;15:23902.
Carrasquillo JA, Fine BM, Pandit-Taskar N, Larson SM, Fleming SE, Fox JJ, et al. Imaging patients with metastatic castration-resistant prostate cancer using (89)Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med. 2019;60:1517–23.
Huang W, Li L, Liang Y, Yang Q, Mixdorf JC, Engle JW, et al. ImmunoPET imaging of Nectin4 expression in gastric and bladder cancer using [(64)Cu]Cu-NOTA-Padcev. Mol Pharm. 2025;22:3468–78.
Huang W, Wang T, Chao F, Yang Q, Mixdorf JC, Li L, et al. ImmunoPET imaging of Trop2 expression in bladder cancer using [(64)Cu]Cu-NOTA-Trodelvy. Mol Pharm. 2025;22:2266–75.
Casanova-Salas I, Aguilar D, Cordoba-Terreros S, Agundez L, Brandariz J, Herranz N, et al. Circulating tumor extracellular vesicles to monitor metastatic prostate cancer genomics and transcriptomic evolution. Cancer Cell. 2024;42:1301–12 e7.
Quaglia F, Krishn SR, Daaboul GG, Sarker S, Pippa R, Domingo-Domenech J, et al. Small extracellular vesicles modulated by alphaVbeta3 integrin induce neuroendocrine differentiation in recipient cancer cells. J Extracell Vesicles. 2020;9:1761072.
Wang X, Zhang L, Cheng L, Wang Y, Li M, Yu J, et al. Extracellular vesicle-derived biomarkers in prostate cancer care: opportunities and challenges. Cancer Lett. 2024;601:217184.
Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–63.
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900.
Fabrizio FP, Muscarella LA, Rossi A. B7-H3/CD276 and small-cell lung cancer: what’s new?. Transl Oncol. 2024;39:101801.
Zhang L, Zhang C, Zeng Y, Li Y, Huang S, Wang F, et al. Emergence and characterization of a ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone evolving moderate-level resistance to azithromycin in Shenzhen, China. Infect Drug Resist. 2021;14:4271–6.
Johnson M, Awad M, Koyama T, Gutierrez M, Falchook GS, Piha-Paul SA, et al. Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with refractory SCLC: a subgroup analysis of a phase 1/2 study. J Thorac Oncol. 2023;18:S54–S5.
Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15:2174–80.
Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7-H3 as a therapeutic target in advanced prostate cancer. Eur Urol. 2023;83:224–38.
Gomes IM, Arinto P, Lopes C, Santos CR, Maia CJ. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason score. Urol Oncol. 2014;32:53 e23–9.
Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023;14:2041.
Penny HL, Hainline K, Theoharis N, Wu B, Brandl C, Webhofer C, et al. Characterization and root cause analysis of immunogenicity to pasotuxizumab (AMG 212), a prostate-specific membrane antigen-targeting bispecific T-cell engager therapy. Front Immunol. 2023;14:1261070.
Nauseef JT, Bander NH, Tagawa ST. Emerging prostate-specific membrane antigen-based therapeutics: small molecules, antibodies, and beyond. Eur Urol Focus. 2021;7:254–7.
Hummel HD, Kufer P, Grullich C, Seggewiss-Bernhardt R, Deschler-Baier B, Chatterjee M, et al. Pasotuxizumab, a BiTE((R)) immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy. 2021;13:125–41.
Kelly WK, Danila DC, Lin CC, Lee JL, Matsubara N, Ward PJ, et al. Xaluritamig, a STEAP1 x CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 2024;14:76–89.
Zekri L, Lutz M, Prakash N, Manz T, Klimovich B, Mueller S, et al. An optimized IgG-based B7-H3xCD3 bispecific antibody for treatment of gastrointestinal cancers. Mol Ther. 2023;31:1033–45.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92.
Fallah J, Agrawal S, Gittleman H, Fiero MH, Subramaniam S, John C, et al. FDA approval summary: lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2023;29:1651–7.
Tschernia NP, Norberg SM, Gulley JL. CAR T cells reach clinical milestone in prostate cancer. Nat Med. 2022;28:635–6.
Acknowledgements
This study was supported by: 1. National Natural Science Foundation of China, General Program, Beijing, China (Grant No. 82370773). 2. National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital), Beijing, China (2023IR33). 3. National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital), Beijing, China (2024IR02). 4. Beijing Physician Scientist Training Project, Beijing, China (BJPSTP-2024-20). We would like to thank the Department of Orthopedics and Pathology at Peking University First Hospital for their support and assistance.
Author information
Authors and Affiliations
Contributions
Y.F., Q.Z., and Y.M. conceived and designed the study. Y.M., Y. Chen, Y. Cui, and X.S. contributed to sample acquisition. Y.M., Y.T. and K.Y. performed experiments and collected clinical data. Q.S. and Y.M. interpreted, organized, and analyzed the experimental results. Y.F., Q.Z. and X.S. provided overall supervision. Y.M. and Q.S. drafted the manuscript. Y.F., Q.Z., Z.H., W.Y., X.P. and D.C. provided manuscript guidance and critical revisions. Y.F., Q.Z. and X.S. provided funding support and submission guidance. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experiments involved in this study were approved by the Ethics Committee of Peking University First Hospital with exemption from informed consent (2023-289-001). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mulati, Y., Shen, Q., Chen, Y. et al. Systematic profiling of tumor-associated antigen expression for antibody-drug conjugate in prostate cancer. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-01066-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41391-025-01066-4