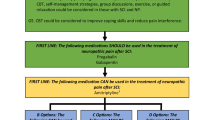
Abstract
Study design
Multicenter, randomized, double-blind, placebo controlled, clinical trial.
Objective
The objective of this paper is to evaluate the effectiveness of cannabinoids and an anti-inflammatory diet, alone and in combination, for the management of neuropathic pain (NP) after spinal cord injury (SCI).
Setting
Two Canadian SCI rehabilitation centers.
Methods
A sample of 144 individuals with SCI will receive either an anti-inflammatory diet, cannabinoids or a placebo for 6 weeks. Following this, a combined effect of these treatments will be evaluated for a further 6 weeks. The primary outcome measure will be the change in NP as assessed by the numeric rating scale (NRS). Secondary outcomes will include changes in inflammation, mood, sleep, spasticity, cost-effectiveness, and function.
Conclusion
This study will assess the efficacy of an anti-inflammatory diet and cannabinoids (individually and in combination) for the treatment of NP following SCI. Results may reveal a cost-effective, side-effect free intervention strategy which could be utilized for the long-term management of NP following SCI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on request.
References
Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. https://doi.org/10.1038/sj.sc.3101116.
Henwood P, Ellis JA. Chronic neuropathic pain in spinal cord injury: the patient’s perspective. Pain Res Manag. 2004;9:39–45. https://doi.org/10.1155/2004/863062.
Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: clinical evidence. Curr Opin Investig Drugs. 2008;9:65–75.
Cardenas DD, Jensen MP. Treatments for Chronic Pain in Persons With Spinal Cord Injury: A Survey Study 2005:109–17.
Corroon JM, Mischley LK, Sexton M. Cannabis as a substitute for prescription drugs—a cross-sectional study. J Pain Res. 2017;10:989–98. https://doi.org/10.2147/JPR.S134330.
Bruce D, Brady JP, Foster E, Shattell M. Preferences for medical marijuana over prescription medications among persons living with chronic conditions: alternative, complementary, and tapering uses. J Alter Complement Med. 2018;24:146–53. https://doi.org/10.1089/acm.2017.0184.
Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2:160–6. https://doi.org/10.1089/can.2017.0012.
Eisenstein M. Medical marijuana: showdown at the cannabis corral. Nature. 2015;525:S15–7. https://doi.org/10.1038/525S15a.
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–9. https://doi.org/10.1212/01.wnl.0000176753.45410.8b.
Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L, Hall W, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep. 2018;18:8. https://doi.org/10.1007/s11910-018-0814-x.
Fine PG, Rosenfeld MJ. Cannabinoids for neuropathic pain. Curr Pain Headache Rep. 2014;18:451. https://doi.org/10.1007/s11916-014-0451-2.
Guy SD, Mehta S, Casalino A, Côté I, Kras-Dupuis A, Moulin DE, et al. The CanPain SCI clinical practice guidelines for rehabilitation management of neuropathic pain after spinal cord: recommendations for treatment. Spinal Cord. 2016;54:S14–23. https://doi.org/10.1038/sc.2016.90.
Allison DJ, Thomas A, Beaudry K, Ditor DS. Targeting inflammation as a treatment modality for neuropathic pain in spinal cord injury: a randomized clinical trial. J Neuroinflammation. 2016;13:152. https://doi.org/10.1186/s12974-016-0625-4.
Vučkovic S, Srebro D, Vujovic KS, Vučetic Č, Prostran M. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9. https://doi.org/10.3389/fphar.2018.01259.
Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–6. https://doi.org/10.1016/S1471-4914(02)02383-3.
Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003;3:310–6. https://doi.org/10.1111/j.1530-7085.2003.03034.x.
Krause SJ, Backonja M-M. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14.
Widerström-Noga E, Biering-Sørensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set (version 2.0). Spinal Cord. 2014;52:282–6. https://doi.org/10.1038/sc.2014.4.
Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10:S73. https://doi.org/10.1016/j.jpain.2009.01.258.
McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Mosby, St. Louis. 1989.
McCaffery M, Beebe A. Pain: clinical manual for nursing practicePain: clinical manual for nursing practice. Nurs Stand. 1994;9:55. https://doi.org/10.7748/ns.9.11.55.s69.
Miller WC, Anton HA, Townson AF. Measurement properties of the CESD scale among individuals with spinal cord injury. Spinal Cord. 2008;46:287–92. https://doi.org/10.1038/sj.sc.3102127.
Morfeld M, Petersen C, Krüger-Bödeker A, von Mackensen S, Bullinger M. The assessment of mood at workplace—psychometric analyses of the revised Profile of Mood States (POMS) questionnaire. Psychosoc Med. 2007;4:Doc06.
Shahid A, Wilkinson K, Marcu S, Shapiro CM. Leeds Sleep Evaluation Questionnaire (LSEQ). In: STOP, THAT one hundred other sleep scales. New York: Springer; 2011. pp. 211–3. https://doi.org/10.1007/978-1-4419-9893-4_48.
Adams MM, Ginis KAM, Hicks AL. The spinal cord injury spasticity evaluation tool: development and evaluation. Arch Phys Med Rehabil. 2007;88:1185–92. https://doi.org/10.1016/j.apmr.2007.06.012.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Golicki D, Niewada M, Karlińska A, Buczek J, Kobayashi A, Janssen MF, et al. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual Life Res. 2015;24:1555–63. https://doi.org/10.1007/s11136-014-0873-7.
Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Roijen Van LH. The iMTA Productivity Cost Questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Heal. 2015;18:753–8. https://doi.org/10.1016/j.jval.2015.05.009.
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–800. https://doi.org/10.1212/01.wnl.0000244422.45278.ff.
Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999–1012. https://doi.org/10.1002/j.1532-2149.2013.00445.x.
Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. https://doi.org/10.1016/S0140-6736(99)01307-0.
Sommer C. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7. https://doi.org/10.1016/s0304-3940(03)01387-9.
Pitchford S, Levine JD. Prostaglandins sensitize nociceptors in cell culture. Neurosci Lett. 1991;132:105–8. https://doi.org/10.1016/0304-3940(91)90444-X.
Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–93. https://doi.org/10.1016/j.apmr.2007.08.004.
Hayes KC, Hull TCL, Delaney GA, Potter PJ, Sequeira KAJ, Campbell K, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–61. https://doi.org/10.1089/08977150260139129.
Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74. https://doi.org/10.3390/nu2030355.
Ware MA. Medical cannabis research: issues and priorities. Neuropsychopharmacology. 2018;43:214–5. https://doi.org/10.1038/npp.2017.222.
Meng H, Johnston B, Englesakis M, Moulin DE, Bhatia A. Selective cannabinoids for chronic neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2017;125:1638–52. https://doi.org/10.1213/ANE.0000000000002110.
Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. https://doi.org/10.1016/j.jneuroim.2005.04.023.
Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharm Toxicol. 2006;46:101–22. https://doi.org/10.1146/annurev.pharmtox.46.120604.141254.
Karschner EL, Darwin WD, McMahon RP, Liu F, Wright S, Goodwin RS, et al. Subjective and physiological effects after controlled sativex and oral THC administration. Clin Pharm Ther. 2011;89:400–7. https://doi.org/10.1038/clpt.2010.318.
Funding
This study is funded by a grant from the Ontario Neurotrauma Foundation.
Author information
Authors and Affiliations
Contributions
DSD and EL were substantially involved in the conception and design of the study. ARA, DJA, DSD, and EL are participating in the coordination of the study and the acquisition of data. DJA drafted the paper with the assistance of DSD, EL, ARA, and BCFC. DSD, EL, BCFC, and AR critically revised the text. The present publication has been approved by all involved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol, informed consent form, recruitment materials, and all participant materials will be submitted for ethical approval through the University of Western REB and the Brock University REB prior to participant recruitment. The study will be conducted in full conformity with the ICH E6, Health Canada Food and Drugs Act, and Good Clinical Practice.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Allison, D.J., Agudelo, A.R., Chan, B.C.F. et al. Cannabinoids and an anti-inflammatory diet for the treatment of neuropathic pain after spinal cord injury (The CATNP Study): study protocol for a randomized controlled trial. Spinal Cord 59, 112–122 (2021). https://doi.org/10.1038/s41393-020-0508-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-020-0508-5