Abstract
Study design
An eDelphi survey.
Objective
To develop the Spinal Cord Injury (SCI) basic and extended nutrition datasets for adults with SCI for the International Spinal Cord Society (ISCoS).
Setting
This international eDelphi study, administered in Australia, will be conducted virtually, overseen by a Research Advisory Group.
Methods
An expert panel will be recruited internationally to participate in a three-round eDelphi survey to develop the ISCoS basic and extended nutrition datasets. An a priori criterion will be implemented, defining strong consensus as an interquartile range (IQR) ≤ 1 and consensus as an IQR > 1/≤2. Mean and standard deviation will be calculated to measure convergence and stability depending on the data. Agreement will be determined as ≥ 80% per statement (Likert scale ratings of 4 and 5). A content analysis approach will be utilised to synthesise free-text responses.

Similar content being viewed by others
Introduction
A spinal cord injury (SCI) resulting from trauma or disease disrupts spinal cord pathways, leading to paralysis and adverse alterations in whole-body metabolism. The rapid onset of immobility and dysfunctional physiological health stemming from autonomic dysfunction [1], sublesional myopenia [2], imposed or adopted sedentary behaviour [3], and the overconsumption of energy [4] relative to reduced total energy expenditure [5, 6] contributes to the development of neurogenic obesity [7, 8]. These aberrant injury-related changes heighten cardiometabolic risk [8, 9]. Additionally, the prevalence of malnutrition in this population ranges from 40% to over 60% across studies, largely due to considerable variation in the methods used for assessment and diagnosis of malnutrition [10,11,12].
The wide variability of nutrition-related data stems from a lack of standardisation in study procedures, including the assessment methods, tools, and instruments used to evaluate nutritional outcomes. A standardised nutritional dataset would allow for the evaluation of outcomes, facilitate comparisons across studies and sub-populations, enable the incorporation of such a tool into clinical settings, and support progression in the field of SCI nutrition care. While the International Spinal Cord Society (ISCoS) has developed common datasets for standardised reporting in SCI related to cardiovascular and endocrine and metabolism health [13], these datasets do not capture diet-related and nutritional health status assessments and outcomes.
The objective of the proposed study therefore is to develop both a basic and extended nutrition dataset for adults with SCI through an electronic Delphi (eDelphi) approach. This will involve a diverse panel of experts’ including clinicians and researchers with extensive experience in nutrition for SCI. For this study, the basic dataset is defined as those items considered essential to be collected at a minimum. Whereas the extended dataset will include items which could be beneficial to collect but not critical for minimum standardised care. We hypothesise that an international panel of experts in SCI and nutrition will reach consensus on a set of specific, clinically relevant items to be included in both basic and extended nutrition datasets for adults with SCI. Furthermore, these datasets will encompass key nutrition areas such as anthropometric measurements, dietary intake assessment, and nutrition-related complications specific to SCI. The basic dataset will offer consistent data suited for standard clinical practice, whereas the extended dataset will provide more detailed and specific information, enhancing the depth and applicability for research outputs. The eDelphi approach is well-suited for creating a comprehensive SCI nutrition dataset as expert-derived information tends to be practical, enabling consensus through expert opinion and ensuring usability that reflects real-world applications [14]. The finalised nutrition dataset would be utilised alongside other ISCoS datasets, such as the core [15], endocrine and metabolic [16] datasets or any other relevant datasets as appropriate.
Methods
Study design
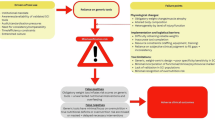
A three-round eDelphi (Fig. 1) [17] guided by Conducting and REporting DElphi Studies (CREDES) guidelines [18], will be employed for this study. This method is selected as it aligns well with the objectives of the study and has been proven to be effective in reaching consensus on emerging and underexplored topics in healthcare research [19].
Study oversight
A Research Advisory Group (RAG) was established by soliciting expressions of interest from the ISCoS Nutrition Special Interest Group. The representative group consists of a dietitian from Sydney, Australia (PI), with over two decades of clinical experience; a dietitian from London, United Kingdom (SW), with more than a decade of expertise; two academics specialising in SCI and nutrition research from Chicago, Illinois (SLL) and Miami, Florida (GJF); and a health psychologist and academic from London, United Kingdom (SPH), with extensive experience in applied health research. Due to the governance and oversight role in the study, RAG members will refrain from participating directly in the eDelphi surveys.
Identification of the expert panel
An international group of experts with expertise in SCI, including academic researchers and clinical professionals from various backgrounds (e.g., dietetics, medicine, nursing, pharmacy, exercise physiology, and other allied health disciplines), will be recruited to participate in the eDelphi expert panel (EP). EP members will actively participate in the eDelphi surveys to rate statements and provide expert justification and feedback to enable RAG to assess responses to achieve consensus. The criteria that individuals must meet to qualify as EP members include (1) a minimum of three years of recent clinical and/or research experience in the field of SCI OR (2) published one or more papers in the field of SCI and/or nutrition in SCI, and (3) able to read and write in the English language.
Recruitment and informed consent
A combination of purposive and snowball sampling will be used to recruit participants for the eDelphi survey [20] as Phase A strategy. A recruitment flyer with links and a quick response (QR) code to an eligibility screener (Supplement 1), participant information, and a consent form will be shared by email via ISCoS interest groups. Additionally, the RAG will distribute the recruitment flyer through their respective professional networks to reach a wider and more diverse pool of potential experts. A gentle email reminder will be sent in a week after initial email circulation. Recipients of the flyer will be encouraged to forward the recruitment flyer to other experts, utilising a snowball strategy while maintaining the confidentiality and privacy of potential participants. If the initial recruitment strategies do not achieve the target sample size of 15 participants within two weeks of the initial email distribution, a secondary recruitment phase (Phase B) will be implemented during the International Nutrition Special Interest Group meeting. All eligible participants must provide informed consent as approved by the ethics committee at the host institution before participating in the survey. Those who consent will be prompted to complete a brief demographics survey (Supplement 2) to gather information on their professional role, age, sex, geographical location, number of publications and years of experience.
Sample size of the expert panel
Although no precise recommendations exist for EP size, a sample size of approximately 10 to 18 diverse members are deemed adequate to build consensus [17, 21]. Considering the dropout rate of 20–30% in eDelphi studies [22], a minimum sample size of 15 is considered sufficient to establish a consensus [21]. Although no upper limit of participants has been established, the recruitment timeframe will be managed to ensure that the minimum number of participants is reached while preventing an excessively large group from posing challenges to manage within available resources. Diversity will be ensured by seeking representation from various disciplines and other demographic factors (e.g., sex, age etc) from across the globe.
eDelphi survey development
Surveys will be managed using the Research Electronic Data Capture (REDCap) platform, a secure online software that enables seamless data management for research [23]. Utilising predefined items initially created by RAG member (SW) refined during the initial ISCoS Nutrition Dataset Subgroup meeting as a preliminary reference, another RAG member (PI) developed the initial survey statements. This was done by modifying the existing items and incorporating additional statements based on practical knowledge and experience in the field. The statements are grouped into sections guided by the Nutrition Care Process terminologies commonly used within dietetic practice [24]. The survey was further refined via numerous iterations and feedback from all members of the RAG before being finalised (Supplement 3). This version will be pilot tested by four experts (dietitians and researchers) independent of the RAG for content validity (measuring what it intends to measure) and readability (Supplement 4). The feedback and modification of the survey will be managed using an iterative process by the RAG, guided by simple analysis of content validity index scores to ascertain content validity and clarity.
Consensus
An interquartile range (IQR) of ≤1 (strong consensus) and >1 but ≤2 (consensus) will be accepted as consensus being reached [25, 26]. Mean and standard deviation (SD) will be calculated if data are normally distributed to measure convergence and stability [27]. Agreement will be established if ≥80% of the responses to a statement fall within the 4 (agree) or 5 (strongly agree) range on the 5-point Likert scale. Conversely, disagreement will be noted if ≥80% of the responses to a statement fall within the 1 (strongly disagree) or 2 (disagree) range on the Likert scale [18]. The RAG will use an iterative process via virtual meetings to deliberate on statements with <80% agreement or disagreement to decide whether to retain, revise, or discard items based on their contextual importance. This iterative process will involve review of qualitative data, critical value of the stability of disagreement and open team discussions using anonymised results [18].The frequency and proportions of responses will be calculated for non-Likert scale questions/statements where experts choose between a basic or extended dataset. Consensus will be established if a choice receives a response rate of ≥75%. Responses <75% will be reviewed by the RAG and revisited in the next eDelphi round. If consensus has been reached on the dataset statement, only the type of dataset question will be presented in subsequent rounds.
eDelphi rounds procedure
The experts who agree to participate in the eDelphi survey will receive an email containing a QR code and weblink for accessing the survey in each round, with two weeks allowed for completion per round [18]. Automated email reminders will be sent to non-responders on the seventh and tenth days of each eDelphi round. To achieve consensus on the recommended dataset, the EP will be asked to rate their level of agreement using a standard 5-point Likert scale (1 = strongly disagree to 5 = strongly agree) and categorise each item into the basic or extended dataset or neither dataset during the three rounds.
-
Round 1: The EP will be asked to rate the initial set of statements regarding developing the nutrition dataset for SCI. Each statement will be accompanied by a textbox for respondents to provide any additional questions or comments or suggest any extra items for inclusion in the dataset. Responses will be exported into Excel (Microsoft 365, Microsoft Corporation, Redmond, WA) by an RAG member (PI) for quantitative data aggregation and compilation of comments. De-identified descriptive data and free text comments from Round 1 will be summarised and shared as feedback for Round 2. Statements meeting pre-defined criteria for consensus and agreement will be excluded from Round 2. Statements not meeting these criteria but deemed critical by the RAG or supported by experts’ feedback will be reviewed iteratively for potential inclusion in Round 2. Based on these considerations, the RAG will then design the survey for the second eDelphi round.
-
Round 2: The Round 2 weblink, QR code, and summarised feedback of aggregated Round 1 results and comments will be emailed individually to the EP. eDelphi panel members will be asked to rate the statements following the same steps as in Round 1. Additionally, new statements or suggestions from Round 1 deemed relevant by the RAG will be incorporated into this round for consideration. A summary report will be prepared highlighting changes and any emerging consensus. New items suggested by the EP and statements that failed to reach an agreement, or consensus will be included for a rating in Round 3.
-
Round 3: The third eDelphi round will proceed to gather feedback and achieve consensus among the EP on the key components of the nutrition dataset for SCI. This round will serve as the final round, and the EP will not have the option to propose new data items. Following a process like that of earlier rounds, the RAG will analyse the revised ratings and comments from Round 3 to finalise a consensus on the components of the nutrition dataset for SCI, encompassing basic and extended data items. The RAG will deliberate on the EP’s collective input, focusing on the dataset’s format, structure, and accessibility to ensure usability by all stakeholders. A summary report will be compiled and emailed to the EP for feedback, along with a unique link for an optional post-participation evaluation survey to assess their eDelphi experiences and improvement suggestions.
Data management and analysis
Quantitative data will be calculated using SPSS (IBM, Armonk, NY) and reported using descriptive statistics ascertaining the normality of data. Free-text comments will be managed using NVivo 14 (Lumivero (2023) Version 14, www.lumivero.com) and analysed qualitatively using a simple content analysis approach [28]. Emerging themes at each round will be used to assess stability, whereby no new themes generated at consecutive rounds will be considered as achieving stability [29].
External validation of the dataset
The final basic and extended nutrition datasets from the eDelphi consensus will undergo an established, standard ISCoS approval process [30]. The ISCoS Nutrition SIG’s dataset working group will undertake the first review before this is passed onto the ISCoS SCI Dataset Committee. Based on their feedback, the SIG dataset working group will further refine the dataset. Then, the dataset will be submitted to the American Spinal Injury Association (ASIA) Board of Directors and ISCoS Scientific and Executive Committee for review and feedback. Based on their input, subsequent updates will be made upon ISCoS Nutrition SIG deliberation. The updated dataset will be circulated to relevant international organisations for input. Simultaneously, the dataset will be available on the ISCoS website for a month to seek wider input. After this, SIG dataset working group will further refine the dataset, which will incorporate collective feedback. The ASIA Board of Directors and ISCoS Scientific and Executive Committee will conduct a final review and approve the dataset. The approved dataset will then undergo review by the National Institutes of Health National Institute of Neurological Disorders and Stroke Common Data Elements (NIH NINDS CDE). The SIG dataset working group and RAG will then develop standardised variable names and a database structure for the basic and extended datasets. Identification of standard measurement approaches and tools followed by implementation and evaluation of the dataset will be considered as the next steps following the establishment of a consensus-based framework for nutrition datasets in SCI.
Ethics and dissemination
Ethics approval (2024/HE000939) was obtained from the University of Sydney Human Ethics Committee, and the protocol was registered with the Open Science Framework (https://osf.io/xdq9a). All relevant institutional guidelines and ethical requirements will be adhered to in the conduct of this study. As part of the ISCoS approval process, the finalised datasets will be published on the ISCoS and NIH NINDS CDE websites and in the official peer-reviewed journal of the ISCoS, Spinal Cord. Furthermore, the nutrition dataset will be disseminated through ISCoS website, academic publications, professional conferences (including the Annual Scientific Meetings of ISCoS and ASIA), and online social media platforms to promote its integration into clinical practice, clinical practice guidelines, educational resources, and research initiatives.
Data availability
All data will be available within the published paper as part of the main article and Supplementary Materials.
References
Henke AM, Billington ZJ, Gater DR Jr. Autonomic dysfunction and management after spinal cord injury: a narrative review. J Pers Med. 2022;12:1110.
Castro MJ, Apple DF, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. J Appl Physiol. 1999;80:373–8.
Pelletier CA, Hicks AL. Importance of exercise in the rehabilitation process after spinal cord injury. Crit Rev Phys Rehabil Med. 2013;25:143–58.
Farkas GJ, Gorgey AS, Dolbow DR, Berg AS, Gater DR. Caloric intake relative to total daily energy expenditure using a spinal cord injury-specific correction factor: an analysis by level of injury. Am J Phys Med Rehabil. 2019;98:947–52.
Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68:1223–7.
Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371–8.
Groah SL, Nash MS, Ward EA, Libin A, Mendez AJ, Burns P, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31:73–80.
Farkas GJ, Gater DR. Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J Spinal Cord Med. 2018;41:378–87.
Gater DR, Farkas GJ, Tiozzo E. Pathophysiology of neurogenic obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2021;27:1–10.
Steensgaard R, Bonne S, Wojke P, Kasch H. SCI-SCREEN: a more targeted nutrition screening model to detect spinal cord-injured patients at risk of malnutrition. Rehabil Nurs J. 2019;44:11–9.
Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. The prevalence of malnutrition in spinal cord injuries patients: a UK multicentre study. Br J Nutr. 2012;108:918–23.
Slettahjell HB, Bastakis M, Biering-Sørensen F, Strøm V, Henriksen C. Defining malnutrition in persons with spinal cord injury – does the global criteria for malnutrition work? Food Nutr Res. 2024; 68. https://doi.org/10.29219/fnr.v68.9989.
Biering-Sørensen F, Charlifue S, Devivo MJ, Grinnon ST, Kleitman N, Lu Y, et al. Using the spinal cord injury common data elements. Top Spinal Cord Inj Rehabil. 2012;18:23–7.
Shang Z. Use of Delphi in health sciences research: a narrative review. Medicine. 2023;102:e32829.
Biering-Sørensen F, Charliefue S, Chen Y, New PW, Noonan V, Post MWM, et al. International spinal cord injury core data set (version 3.0)—including standardization of reporting. Spinal Cord. 2023;61:65–8.
Bauman WA, Biering-Sørensen F, Krassioukov A. The international spinal cord injury endocrine and metabolic function basic data set. Spinal Cord. 2011;49:1068–72.
Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: How to decide its appropriateness. World J Methodol. 2021;11:116–29.
Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on conducting and REporting DElphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31:684–706.
Salminen P, Kow L, Aminian A, Kaplan LM, Nimeri A, Prager G, et al. IFSO consensus on definitions and clinical practice guidelines for obesity management-an international Delphi study. Obes Surg. 2024;34:30–42.
Leighton K, Kardong-Edgren S, Schneidereith T, Foisy-Doll C. Using social media and snowball sampling as an alternative recruitment strategy for research. Clin Simul Nurs. 2021;55:37–42.
Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manag. 2004;42:15–29.
Chalmers J, Armour M. The Delphi technique. In: Liamputtong P, editor. Handbook of research methods in health social sciences. Singapore: Springer Singapore; 2019. p.715–35.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Writing Group of the Nutrition Care Process/Standardized Language Committee. Nutrition care process part II: using the international dietetics and nutrition terminology to document the nutrition care process. J Am Diet Assoc. 2008;108:1291–3.
von der Gracht HA. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc Change. 2012;79:1525–36.
Huijben JA, Wiegers EJA, de Keizer NF, Maas AIR, Menon D, Ercole A, et al. Development of a quality indicator set to measure and improve quality of ICU care for patients with traumatic brain injury. Crit Care. 2019;23:95.
Greatorex J, Dexter T. An accessible analytical approach for investigating what happens between the rounds of a Delphi study. J Adv Nurs. 2000;32:1016–24.
Beiderbeck D, Frevel N, von der Gracht HA, Schmidt SL, Schweitzer VM. Preparing, conducting, and analyzing Delphi surveys: cross-disciplinary practices, new directions, and advancements. MethodsX. 2021;8:101401.
Holey E, Feeley J, Dixon J, Whittaker V. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med Res Methodol. 2007;7:52.
Biering-Sørensen F, Alexander MS, Burns S, Charlifue S, DeVivo M, Dietz V, et al. Recommendations for translation and reliability testing of international spinal cord injury data sets. Spinal Cord. 2011;49:357–60.
Acknowledgements
The authors would like to acknowledge the support of ISCoS Nutrition Special Interest Group. The International SCI Data Sets committee members Prof Fin Biering-Sorensen and Dr Vanessa Noonan in developing this protocol.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
PI: Responsible for designing the eDelphi protocol, first-round survey, recruitment of the RAG, ethics application, content validity analysis and coordination of the study. SW: Facilitated RAG recruitment, provided several pre-determined data elements for the first-round survey, and contributed to the protocol and supported facilitation. GJF: Contributed extensively to the study protocol, survey, choice of survey platform, strengthening of the consensus definition, and reviewing and editing the manuscript. SLL: Valuable contribution to the study protocol, survey, choice of survey platform, consensus, and content validity design, and reviewing and editing of the manuscript. SPH: Provided valuable input to the protocol, survey, content validity, and choice of survey platform.
Corresponding author
Ethics declarations
Competing interests
All authors are active members of the ISCoS Nutrition Special Interest Group, and one author (SW) holds the position of the Chair of this group and are all committed to working on this project as a priority area for the committee. The authors declare no other competing interests. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Ethical approval
Appropriate ethical approval has been obtained for the study (2024/HE000939) and informed consent will be sought from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iyer, P., Farkas, G.J., LaVela, S.L. et al. Protocol for developing the nutrition dataset for the international spinal cord society: an international eDelphi approach. Spinal Cord 63, 432–436 (2025). https://doi.org/10.1038/s41393-025-01102-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-025-01102-z