Abstract
Background
Rapid-acting antidepressants (RAADs), including dissociative anesthetics, psychedelics, and empathogens, elicit rapid and sustained therapeutic improvements in psychiatric disorders by purportedly modulating neuroplasticity, neurotransmission, and immunity. These outcomes may be mediated by, or result in, an acute and/or sustained entrainment of epigenetic processes, which remodel chromatin structure and alter DNA accessibility to regulate gene expression.
Methods
In this perspective, we present an overview of the known mechanisms, knowledge gaps, and future directions surrounding the epigenetic effects of RAADs, with a focus on the regulation of stress-responsive DNA and brain regions, and on the comparison with conventional antidepressants.
Main body
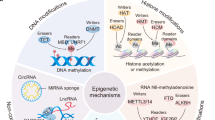
Preliminary correlative evidence indicates that administration of RAADs is accompanied by epigenetic effects which are similar to those elicited by conventional antidepressants. These include changes in DNA methylation, post-translational modifications of histones, and differential regulation of non-coding RNAs in stress-responsive chromatin areas involved in neurotrophism, neurotransmission, and immunomodulation, in stress-responsive brain regions. Whether these epigenetic changes causally contribute to the therapeutic effects of RAADs, are a consequence thereof, or are unrelated, remains unknown. Moreover, the potential cell type-specificity and mechanisms involved are yet to be fully elucidated. Candidate mechanisms include neuronal activity- and serotonin and Tropomyosine Receptor Kinase B (TRKB) signaling-mediated epigenetic changes, and direct interaction with DNA, histones, or chromatin remodeling complexes.
Conclusion
Correlative evidence suggests that epigenetic changes induced by RAADs accompany therapeutic and side effects, although causation, mechanisms, and cell type-specificity remain largely unknown. Addressing these research gaps may lead to the development of novel neuroepigenetics-based precision therapeutics.
Similar content being viewed by others
Introduction
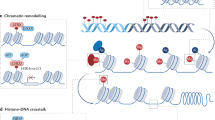
Chromatin is a plastic entity, which adapts to external stimuli such as a changing environment and stress [1, 2]. This feature shapes the endogenous response to a changing environment via long-term modulation of gene expression [3, 4]. Psychosocial stress causes an epigenetic remodeling of stress-responsive DNA and chromatin regions, and associated histone proteins in susceptible brain areas such as the ventral hippocampus [1, 5], amygdala [6], nucleus accumbens (NAc) [7], and the prefrontal cortex (PFC) [8] – dorsal raphe nucleus loop [9,10,11]. Similarly, and in an opposite fashion, a remodeling of chromatin regions involved with synaptic plasticity, neurotransmission, neurogenesis, and neuroinflammation is required in infralimbic and prelimbic neurons to elicit antidepressant effects [1, 9,10,11,12,13]. Hence, drugs which directly or indirectly affect the epigenetic control of chromatin regions linked to neurotransmission, neurogenesis, and neuroinflammation, represent promising epigenetics-based therapeutics.
Rapid-acting antidepressants (referred herein as RAADs) are being investigated as novel therapeutics in psychiatry [14,15,16]. These compounds are generally classified as serotonin (5-HT)2A receptor agonists such as psilocybin, lysergic acid diethylamide (LSD), N, N-Dimethyltryptamine (DMT) and 2,5-Dimethoxy-4-iodoamphetamine (DOI) [14]; N-Methyl-D-Aspartate (NMDA) antagonists such as ketamine [17]; and empathogens such as 3,4-methylenedioxymethamphetamine (MDMA), which act mainly through neurotransmitter transporters [18]. The long-term improvements in psychiatric symptoms and neuropsychological function elicited by RAADs [19,20,21,22,23,24] are accompanied by enduring serotonergic (such as a peripheral increase of serotonin -5-HT- platelets uptake sites in long-term Ayahuasca users) [25], and neuromorphological changes (such as thinner precuneus and posterior cingulate cortex and thicker anterior cingulate cortex in long-term Ayahuasca users) [26], which suggest the entrainment of epigenetic mechanisms.
Preliminary evidence indicates that the therapeutic effects of RAADs, akin to conventional antidepressants, are accompanied by a remodeling of DNA methylation, histone post-translational modifications (PTMs), and non-coding RNAs (ncRNAs) dynamics in stress-responsive brain areas. Interestingly, repeated, binge, or high-dose administration of RAADs elicit side effects mediated by overlapping yet opposite epigenetic mechanisms, such as hippocampal apoptosis, reduced neurotrophic signaling, increased neuroinflammation, and cognitive impairments (see Involvement of Epigenetic Mechanisms in Potential Side Effects Elicited by RAADs). These effects, contrasting with those of clinically relevant doses and resembling stress-induced epigenetic responses, underscore the importance of identifying the optimal dosages and regimens for clinical RAADs applications to maximize desirable epigenetic outcomes and minimize undesirable ones.
Epigenetic Control in Stress-Related Disorders, and the Effects of Conventional and Rapid-Acting Antidepressants
DNA methylation, stress, and rapid-acting antidepressants
DNA methylation consists in the reversible addition of methyl groups to cytosines in CpG dinucleotides by DNA methyltransferases (DNMTs). This modification generally modulates transcription by inhibiting the binding of transcription factors or recruiting proteins involved in gene repression [27]. Stress and psychosocial trauma modulate the methylation status of CpG dinucleotides in DNA sequences involved with the stress response, and synaptic and neuronal plasticity [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. For example, the methylation status of genes encoding the glucocorticoid receptor, arginin-vasopressin, and the brain-derived neurotrophic factor (BDNF) can be modulated by early-life adversities as early as 1 day after birth [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] and specific cell types such as astrocytes carryover the epigenetic marks of stress [35, 44]. Epigenetically-active compounds with DNA demethylase activity [45] have antidepressant effects [46, 47], confirming that the methylation state and the associated psychiatric symptoms can be reversed pharmacologically [48]. Stressors also affect the regulation of DNA-methylating enzymes such as DNMT3a and 3b [49] in stress-susceptible brain areas such as the PFC and hippocampus [35, 44, 50]. Therefore, stress exposure is physically “remembered” in specific cell types and brain areas, causing altered gene expression and ultimately lasting effects on behavior. The most studied example of epigenetic programming by stress, and its normalization by antidepressants, is the BDNF gene. Prenatal and life adversities decrease the promoter IV-mediated BDNF expression via increasing its methylation [36, 43, 49, 51,52,53,54,55,56,57,58], and psychiatric disorders largely share increased BDNF promoter methylation, which can be reversed by conventional antidepressants [36, 54,55,56,57,58,59,60,61].
Like conventional antidepressants, but only requiring one or few administrations, ketamine restores some of the stress-induced aberrant DNA methylation, including on the BDNF gene (Fig. 1 and Table 1). Delving into the molecular mechanisms, ketamine induces a Extracellular Signal-Regulated Kinase 1 (ERK1) / nuclear receptor binding protein 1 (NRBP1)-mediated microglial decrease of methyl-CpG binding protein 2 (MeCP2), which upregulates the cAMP response-binding protein (CREB)-mediated BDNF exon IV transcription [62]. The enhancement of fear extinction elicited by ketamine is also accompanied by demethylation of PFC and hippocampal BDNF exon IV, and increased BDNF transcription [49]. Similarly, fluoxetine dissociates the MeCP2–CREB complex from BDNF promoter IV through the protein kinase A (PKA)-mediated phosphorylation of MeCP2, thus increasing BDNF transcription [55]. Resembling chronic imipramine’s effects in the PFC [50], acute ketamine reversed the nerve injury-induced increase in the hippocampal DNMT1, 3a, and 3b. It also restored the decrease in hippocampal total BDNF and BDNF exon I transcription and protein levels [52]. In rodents, chronic fluoxetine, imipramine, and desipramine blunted the stress-induced increase in hippocampal DNA methylation, and restored the decrease elicited in the PFC [50, 63, 64]. Antidepressant use increased the peripheral methylation level of the 5-HT transporter [65], and the interleukin (IL)-6 gene [66], as well as telomerase activity in individuals with PTSD [67]. While it has been speculated that RAADs such as psilocybin might increase telomerase activity and telomere length, this hypothesis remains to be tested [68].
Abbreviations: 5-HTRs, Serotonin Receptors; SIGMAR1, Sigma-1 Receptor; SERT, Serotonin Transporter; NET, Norepinephrine Transporter; TRKB, Tropomyosin Receptor Kinase B; BDNF, Brain-Derived Neurotrophic Factor; NMDA, N-Methyl-D-Aspartate; PLC, Phospholipase C; IP3, Inositol trisphosphate; DAG, Diacylglycerol; MAPK, Mitogen-Activated Protein Kinase; ERK, Extracellular Signal-Regulated Kinase; PI3K, Phosphoinositide 3-Kinase; Akt, AKT Serine/Threonine Kinase 1; PKC, Protein Kinase C; GSK-3, Glycogen Synthase Kinase 3; NF-kB, Nuclear Factor Kappa-B; CaMKs, Calcium Calmodulin Dependent Protein Kinases; Wnt, Proto-Oncogene Wnt-1; JAK, Janus Kinase; STAT, Signal Transducer and Activator of Transcription; PLCγ, Phospholipase C Gamma 1; SSRI, Selective Serotonin Reuptake Inhibitors; SNRI, Serotonin-Norepinephrine Reuptake Inhibitors; MAOI, Monoamine Oxidase Inhibitors; H3, Histone 3; H4, Histone 4; H3K4, Histone 3 Lysine 4; H3K9, Histone 3 Lysine 9; H3K12, Histone 3 Lysine 12; H3K27, Histone 3 Lysine 27; PKA, Protein Kinase A; Mecp2, Methyl-Cpg Binding Protein 2; CREB, cAMP Response Element-Binding Protein; G9a, Histone Methyltransferase G9a; HDAC, Histone Deacetylase; DNMT, DNA Methyltransferase; lncRNA, Long non-Coding RNA; circRNA, Circular RNA; miRNA, micro RNA; NR3C1, Glucocorticoid Receptor; CRH31, Corticotropin Releasing Hormone; PTSD, Post-Traumatic Stress Disorder; LSD, lysergic acid diethylamide.
Amongst psychedelics, preliminary evidence indicates that LSD and Ayahuasca affect DNA methylation (Fig. 1 and Table 1). Repeated LSD largely increased the methylation level of 635 CpG sites in the PFC of adult male mice receiving a prosocial, anxiolytic, and synaptoplastic repeated regimen of LSD [69,70,71,72]. The genes interested were involved with neurotropic-, neurotrophic-, and neuroplasticity-related signaling. Proteomics profiling and qRT-PCR validation suggested specificity and functional significance of the changes observed in DNA methylation [69]. Given that cytosine methylation can affect the likelihood of mutations [73, 74] future studies are warranted to investigate the potential effects of the increased DNA methylation elicited by LSD on genomic stability and mutation rates. Despite the fact that the use of RAADs has been associated with decreased likelihood of suicide [75, 76], DNA hypermethylation is also observed in the blood of individuals who attempted suicide [77] and in the PFC of suicide completers [78]. Hence, future studies are required to better characterize the relationships between the epigenetic effects elicited by psychedelics and suicidality. Participation in a ceremonial Ayahuasca retreat in the Amazon consisting of multiple administrations increased the saliva methylation level of 5 CpG sites within the Sigma-1 receptor gene promoter (in a greater fashion in individuals with greater childhood trauma), but did not affect the methylation level of one CpG site within the FKBP5 gene, which is involved with trauma and the stress response [79]. These changes were accompanied by improved depression and anxiety scores for up to 6 months [79]. While they are preliminary findings, previous biochemical and neurostructural evidence supports the existence of epigenetic mechanisms accompanying the regular consumption of Ayahuasca: increased platelets 5-HT uptake sites [25], thinner precuneus and posterior cingulate cortex, and thicker anterior cingulate cortex [26] were identified in long term Ayahuasca users. Speculations exist that Ayahuasca might extinguish the fear response via an epigenetic mechanism mediated by the DMT-activated Sigma-1 receptor [80], although this hypothesis remains to be tested. The β-carbolines contained in the Ayahuasca brew might also inhibit histone deacetylase activity, given that engineered derivatives containing β-carbolines motifs are potent HDACs inhibitors [81, 82]. However, no studies have insofar investigated the epigenetic effects elicited by β-carboline, or those elicited by the combination of β-carboline with DMT-containing plants (as in the case of Ayahuasca).
MDMA-assisted psychotherapy led to peripheral epigenetic changes correlated to therapeutic improvement [83]. The increase in saliva methylation level of one CpG site (cg08276280) in the corticotropin-releasing factor receptor 1 gene and one (cg01391283) within the glucocorticoid receptor gene was correlated with symptom reduction in individuals with severe PTSD receiving MDMA-assisted psychotherapy [83]. Further studies should characterize the epigenetic effects of psychotherapy coupled to RAADs, and if those treatments synergistically or additively interact to produce determinate epigenetic outcomes. Putative prophylactic or acute effects of RAADs on attenuating stress-induced DNA methylation changes remain to be elucidated.
Histone post-translational modifications, stress, and RAADs
Histones form protein cores around which DNA wraps. Stress and antidepressants affect histone post-translational modifications (PTMs) such as acetylation, methylation, and phosphorylation in stress-responsive chromatin areas, altering chromatin accessibility and transcription. For example, stress elicits hypoacetylation of hippocampal histone 3 (H3) at BDNF III and IV promoters, and increases the levels of histone deacetylases (HDACs) including HDAC5 and Sirtuin 2, ultimately decreasing BDNF expression [1, 84]. Indeed, specific HDACs such as HDAC2 and HDAC5, which are critical regulators of adult neurogenesis [85] and cognition [86], are altered in depression [87].
Some of the aberrant PTMs dynamics elicited by stress are restored by chronic administration of conventional antidepressants such as fluoxetine, paroxetine, reboxetine, citalopram, imipramine and mirtazapine. These drugs rescue the H3-H4 (i.e., H3 lysine (K)9 and H3K27) hypoacetylation of the BDNF promoters, reverse the stress-induced decrease in BDNF transcription and protein level, promote HDAC5 phosphorylation and nuclear export, and attenuate the stress-induced increase in HDACs in stress-susceptible brain areas [1, 55, 84, 88,89,90,91,92]. Delving into the mechanisms, fluoxetine was shown to disassociate the MeCP2-CREB-BDNF promoter IV complex via Protein Kinase A (PKA)- mediated phosphorylation of MeCP2, leading to CREB-mediated BDNF transcription [55]. Fluoxetine also inhibited the binding of delta-FosB to the Calcium/calmodulin-dependent protein kinase IIa (CaMKIIa) promoter by reducing its acetylation and increasing its H3K9 dimethylation, ultimately decreasing CaMKIIa expression in the NAc [93]. Eight-week escitalopram treatment in individuals with depression decreased H3K27 histone trimethylation, and these changes were negatively correlated with increased BDNF levels [58]. Importantly, the stress-induced hippocampal BDNF promoter-associated H3K9 hypoacetylation and HDAC2 overexpression can also be restored by non-pharmacological strategies such as acupuncture [94].
The epigenetic outcomes elicited by a single administration of ketamine largely overlap those elicited by chronic conventional antidepressants. Indeed, one or few ketamine administrations are sufficient to reverse the stress-induced histone hypoacetylation and downregulated neurotrophic-related transcription (Fig. 1 and Table 1). In a mouse model of Gulf War Illness, ketamine decreased the hippocampal HDAC1 and HDAC5 levels and increased the H3K9 acetylation of BDNF promoter IV, restoring BDNF levels and neuroplasticity [95]. Similarly, in rats exposed to early life adversities, ketamine decreased the depressive-like behaviors and restored NAc HDAC activity [7]. Moreover, high-dose ketamine had a prophylactic effect on attenuating the stress-induced increase in hippocampal H3K9 methylation [96]. Similarly to conventional antidepressants, ketamine increases HDAC5 phosphorylation and nuclear export, resulting in enhanced H3-H4 acetylation. This triggers the myocyte enhancer factor 2-mediated transcription of the plasticity-related genes Eukaryotic Translation Initiation Factor 4E Binding Protein 1 (eIF4EBP1) and CREB, and their target genes, and ultimately the onset of antidepressant-like effects [97].
Psychedelics such as LSD and DOI also have histone-acetylating properties, which might be involved in their therapeutic effects (Fig. 1 and Table 1). Early studies reported that LSD acutely increases histone acetylation in the midbrain and cortex, but not the cerebellum of rabbits [98]. The increased histone acetylation activity elicited by LSD suggests gene activation, and indeed, LSD activates the transcription [99] of immediate early genes and genes involved with synaptic potentiation and neurotropism in the hippocampus, cortex, midbrain [100], and brainstem [99]. Given that enhancing hippocampal and PFC histone acetylation ameliorates fear extinction [101] and the consolidation of cued fear extinction [102] via a permissive effect over transcription [101], the increased histone acetylation elicited by LSD could be involved in its therapeutic action for alcohol addiction, and distress associated with a life-threatening condition [103,104,105,106].
A single administration of the LSD analog DOI also elicited a sustained modulation of the acetylation level of the transcriptional enhancer histone H3K27 in neurons of the mouse PFC [107]. This sustained effect had highly specific spatio-temporal dynamics, was accompanied by neurotrophic-related transcriptional shifts, and was associated to enhanced synaptic plasticity and antidepressant-like activity [107]. Lastly, the recently identified histone PTMs serotonylation and dopaminylation [108,109,110] might potentially be involved in the therapeutic effects of psychedelics, although further investigations are required. Together, the therapeutic effects of RAADs, similarly to conventional antidepressants, are accompanied by PTMs opposite to those elicited by stress, leading to increased accessibility of genes involved with neurotrophic signaling and antidepressant response.
Non-coding RNAs, stress, and RAADs
Non-coding RNAs (ncRNAs) are portions of the genome which are transcribed but not translated, with roles in tissue-specific selective or cooperative regulation of splicing, transcription, and translation [111,112,113,114]. ncRNAs, including stress-responsive ones, are intricately linked to neuroplasticity, psychiatric disorders, and suicide, offering potential as biomarkers and therapeutics for psychiatric disorders [115,116,117,118,119,120,121,122]. Early-life and chronic stressors affect the PFC, amygdalar and hippocampal regulatory RNA network, influencing neurotransmission, neuroplasticity, neurogenesis, and behavior [118, 123,124,125,126,127]. Among these networks, microRNAs (miRNAs), approximately 22 nucleotides in length, stand out for their tissue-specific regulation of a significant proportion of protein-coding genes expression by binding mRNA untranslated regions. Dysregulated miRNA dynamics are observed in depression and suicide, with studies pinpointing alterations in the PFC and hippocampus during depressive states [121, 122, 128, 129]. Conventional antidepressants influence miRNA expression patterns. For instance, miRNAs such as miR-16, miR-124, miR-132, and miR-135 are implicated in antidepressant response, with drugs like fluoxetine and venlafaxine, imipramine, sertraline, and citalopram modulating their expression, as well as the expression of miR-18a, miR-34a, miR-326, miR-1202 and miR-1971 [117, 130,131,132,133,134,135,136,137,138,139,140,141].
RAADs such as ketamine also alter miRNA pathways, with PFC miR-29b-3p, miR-98-5p, and miR-132-5p playing important roles in mediating the upregulation of BDNF leading to ketamine’s antidepressant-like effects (Fig. 1 and Table 1) [142,143,144]. In preclinical studies, ketamine modulated some of the stress-dysregulated hippocampal miRNAs (i.e., miR-598), which are also regulated by other antidepressant strategies such as fluoxetine and electroconvulsive therapy (ECT) [124]. Ketamine also decreased miR-451 levels and increased those of the RNA-binding protein hur-6, a de-repressor miRNA “sponge” for deleterious miRNAs involved in inflammatory responses [124, 145, 146]. ECT and ketamine in rodents exposed to early-life adversities affected 43 common miRNA targets, 7 of which were reversals of stress-induced changes. Interestingly, ketamine rescued some of the stress-induced miRNAs alterations which were not reversed by fluoxetine [124], suggesting that some of the epigenetic mechanisms engaged by conventional and RAADs diverge. A three-day ketamine regimen upregulated 23 miRNAs and downregulated 15 including miR-206 [147] (a negative modulator of BDNF) in vivo and in vitro, while increasing BDNF and decreasing apoptosis [147,148,149]. Ketamine time-dependently modulated the transcription of a cluster of hippocampal (but not PFC) miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p, and 448-3p) which are hosted in the 5-HT2C gene locus, mediated by the inhibition of glycogen synthase kinase 3 [150]. Acutely, ketamine increased the prefrontal level of miR-148a-3p and decreased miR-128-3p, miRNAs involved with the ubiquitin proteasome system [151]. Ketamine also elicited immunomodulatory prophylactic effects in lipopolysaccharide (LPS)-treated mice through miR-149 [152], indicating that some of the immunomodulatory effects of ketamine involved in its antidepressant effects [153] might be mediated by ncRNAs.
Similarly to what observed with conventional antidepressants [140, 154,155,156,157,158,159] the miRNome might predict the therapeutic response elicited by RAADs. For example, the non-responder status to ketamine was predicted by lower pretreatment level of miR-548d-5p and miR-605 in individuals with neuropathic pain [160, 161]. However, in a study in individuals with treatment-resistant depression receiving a ketamine infusion, no significant effects on whole blood miRNA levels were detected 24 hours after [162]. Further studies are required to identify miRNAs that can predict favorable treatment outcome in response to RAADs to increase therapeutic precision.
Circular RNAs (circRNAs), ncRNAs generated by back-splicing, have extensive complementarity to target mRNAs and can encode proteins or increase the expression of the target gene(s) [163]. A regimen of repeated ketamine in rats increased the hippocampal expression of 4, and decreased the expression of 1 circRNA, with predicted downstream effects on genes involved in calcium signaling, G protein signaling, protein phosphorylation, Mammalian Target of Rapamycin (mTOR) signaling, transcription, alternative splicing, and neuroplasticity [164]. Long non-coding RNAs (lncRNAs) are a class of ncRNAs longer than 200 nucleotides with brain area-specific expression patterns involved with neural differentiation and plasticity [165]. lncRNA dynamics are stress- and antidepressant-responsive, are altered in several neuropsychiatric disorders including Major Depressive Disorder (MDD), and might predict the antidepressant response [118, 127, 166]. For example, greater decreases of the lncRNA FEDORA predicted the decrease in depression severity following ketamine in individuals identifying as female who experience MDD [167].
Potential mechanisms of rapid-acting antidepressants-induced epigenetic changes
Neuronal activity-mediated epigenetic effects
One mechanism through which psychedelics might affect the epigenetic regulation of neurotrophic-related gene expression is via neuronal activity-induced modification of chromatin structure and accessibility. The membrane depolarization of cortical neurons results in chromatin remodeling and enhanced BDNF gene accessibility via a) decreased methylation of BDNF promoter III and IV [168, 169], b) H3K4 dimethylation of BDNF promoter IV, and c) HDAC1 and mammalian Switch-independent 3 A promoter dissociation [169]. Additionally, upon neuronal depolarization, CaMKII-mediated phosphorylation and release of the methyl-binding protein MeCP2 from the BDNF promoter III permits BDNF promoter III-dependent transcription, causing dendritic plasticity in neurons [168, 170]. Additionally, the ketamine-induced loss of somatostatin-expressing interneurons dendritic inhibition leads to heightened synaptic calcium transients in the dendritic spines of pyramidal neurons in the PFC. This process might play a role in the epigenetic effects observed following ketamine administration through altered calcium signaling, leading to increased activity-dependent synaptic plasticity [171,172,173]. DOI activates specific subsets of 5-HT2A- and metabotropic glutamate receptor 2 (mGlu2)-expressing glutamatergic neurons and interneurons, as well as astrocytes of deep-layer prefrontal and somatosensory cortices, the claustrum, and the insula [174], and this activation might be involved in the sustained epigenetic and antidepressant-like effects elicited [107]. A single administration of DOI induced prolonged alterations in the acetylation level of neuronal transcriptional enhancers, causing sustained effects over axonogenesis, synapse organization, assembly, and function, and receptor internalization and activity [107]. Despite this preliminary correlative evidence, given that physiological neuronal activation itself can profoundly modulate the epigenetic landscape de novo [175], this calls into questions whether the epigenetic effects elicited by RAADs are causally involved in the therapeutic effects, or neuronal activity-driven changes. Future studies are encouraged to address this research question.
5-HT receptors signaling-mediated epigenetic effects
Psychedelics might activate similar epigenetic mechanisms to those activated by endogenous 5-HT signaling through various 5-HT receptors. 5-HT triggers a transient remodeling of chromatin structure mediated by 5-HT1A and BDNF-TRKB signaling which decreases HDAC5 expression, and increases cortical H3K9 acetylation of the BDNF promoter and BDNF levels, reinstating plasticity in the adult nervous system [176]. 5-HT signaling also induces changes in the methylation status of the promoter of CREB2, a plasticity-related transcription factor involved in long-term facilitation [177, 178]. The properties of psychedelics resemble those of 5-HT: they interact with 5-HT receptors such as the 5-HT1A and 5-HT2A receptors, triggering biased signaling and the transcription of genes involved in neurotransmission, neuroplasticity, and neuroimmunomodulation [14,15,16]. Given that these 5-HT receptors are fundamentally involved in governing cortical circuits mediating cognitive and executive functions [179, 180], the activation of these receptors by RAADs might result in specific epigenomic fingerprints and chromatin architecture changes, ultimately leading to structural and functional synaptic changes. The epigenetic effects elicited in discrete cell types through activation of 5-HT receptors, might alter circuit and network activities, putatively mediating therapeutic improvement at the circuit-, network-, and system-levels.
TRKB signaling-mediated epigenetic effects
Like classical antidepressants [181], a mechanism that might mediate the epigenetic effects of RAADs is the activation of endogenous BDNF signaling through direct binding to and activation of the TRKB receptor [182,183,184,185]. Activation of TRKB signaling results in the activation of other cascades such as the CaMKII, RAS/MAPK and phosphoinositide 3 kinase pathways [14, 17, 186,187,188]. Moreover, TRKB signaling leads to altered chromatin structure of the BDNF gene, and transcriptional upregulation of the BDNF gene and its downstream effector target AKT Serine/Threonine Kinase 1 (Akt)-mTOR. These events ultimately cause an increase in neuroplasticity, synaptic potentiation, and antidepressant effects. The outcome is the reopening of a period of structural and functional plasticity within the CNS that can be harnessed therapeutically [182,183,184, 189, 190]. Supporting a fundamental role of the reopening of critical periods of plasticity, RAADs lead to the degradation of the extracellular matrix in the NAc, creating the prerequisites to enable metaplasticity [190]. Whether epigenetic mechanisms are involved in this process remains unknown.
Interestingly, the epigenetic changes elicited by LSD resemble those observed during critical periods of neurodevelopment and synaptic plasticity, thus seemingly re-creating in the adult nervous system the epigenetic correlates observed during neurodevelopment and learning [69]. Indeed, CCAAT enhancer binding protein beta (CEBP2), a co-activator and target gene of CREB, was a “top-hit” in the PFC in terms of differential DNA methylation following repeated LSD [69]. In accordance, CEBP2 together with several other neuroplasticity-related genes is dose-dependently transcriptionally modulated in the PFC following psilocybin administration [191]. Accordingly, a single administration of psilocybin following chronic stress during adolescence in rats was sufficient to normalize depressive-like and cognitive-like behaviors [192]. Lastly, given that (i) psychedelics interact with TRKB, 5-HT2A, and mGlu2 receptors, that (ii) TRKB interacts with the 5-HT2A [193] and the mGlu2 [194] receptors, and that (iii) the latter two receptors interact with each other [195], these interactions may be functionally relevant from an epigenetic standpoint.
Direct interaction with DNA, histones, or chromatin remodeling complexes
Another intriguing mechanism supported by several lines of evidence is that RAADs may directly interact with DNA, histones, or chromatin remodeling complexes, thus acting as direct epigenetic modulators. Indeed, the β-carbolines contained in the brew Ayahuasca directly interact with DNA in vitro [196], and early studies observed that LSD directly binds DNA or histone proteins [197,198,199]. For psychedelics to interact with DNA or histones in vivo, they would first need to reach the intracellular space and the nucleus. Indeed, membrane permeability is essential for the neuroplastic effects of psychedelics, and ligand-bound 5-HT2A receptors are internalized in vivo and in vitro [200,201,202,203]. Additionally, neurotrophic factors are uptaken and transported to the cell body through retrograde axonal transport. Given that RAADs bind the TRKB receptor [186], they might similarly be internalized by nerve terminals. In support of this hypothesis, DMT is internalized via a three-step process requiring active transport: 1) it is actively transported across the blood-brain barrier, 2) acts as a 5-HT transporter substrate on the neuronal plasma membrane, and 3) it is internalized by neuronal cells, and stored into synaptic vesicles by the neuronal vesicle monoamine transporter 2 for up to 1 week [204,205,206]. Whether the sequestered DMT in synaptic vesicles or the DMT-activated Sigma-1 receptor interact with DNA or chromatin remodeling complexes remains to be assessed [206, 207]. Recently, it was reported that 5-HT can covalently bind to glutamine 5 on the trimethylated H3K4 histone, eliciting a permissive transcriptional influence that modulates the interaction of histone PTMs with chromatin readers during neuronal differentiation [109]. Due to the structural similarity of 5-HTergic psychedelics to 5-HT, it remains to be assessed whether they modulate the relationship between histone PTMs and chromatin readers.
The mechanisms discussed here are not necessarily mutually exclusive and may be part of a series of events responsible for reopening a period of neurodevelopmental-like neuroplasticity, providing a neurobiological substrate that can be harnessed therapeutically. Achieving this seems to require appropriate support and integration before, during, and following psychedelic-assisted psychotherapy. Given that the different mechanisms modulating chromatin structure and DNA accessibility may interact with one another, deciphering the “psychedelic epigenome” represents a new frontier in psychiatry.
Involvement of epigenetic mechanisms in the potential side effects elicited by RAADs
While preliminary evidence suggests that the epigenetic effects induced by RAADs may contribute to therapeutic improvement, uncertainties persist regarding side effects. Studies have documented epigenetic alterations following the abuse of RAADs, which appear antithetical to those elicited by clinically relevant doses, yet similar to those elicited by stress. These changes are reminiscent of neuronal, immune and cardiac dysfunction. Most of the potential epigenetic-mediated side effects associated with RAADs were observed following repeated administration of higher doses of ketamine during gestation, lactation, and the neonatal period (Table 1).
Immune-related side effects
High or repeated doses of ketamine may cause side effects through the epigenetic modulation of immune function, via (i) upregulation of permissive H3K4 trimethylation, (ii) downregulation of repressive H3K27 and H3K36 trimethylation, and (iii) DNA hypomethylation at nuclear factor-κB (NF-κB)-responsive promoters [208, 209], as well as iv) modulation of proinflammatory, neurotoxic, and oxidative signaling through RNA regulatory pathways [152, 210,211,212,213,214,215,216]. Repeated MDMA administration also causes epigenetic marks reminiscent of immune dysfunction such as hypermethylation of the Terminal Nucleotidyltransferase 5B gene, a chaperone involved with cyclooxygenase 2 maturation [217]. Future studies are warranted to further investigate potential epigenetics-mediated immune side effects arising from repeated use and abuse of RAADs.
Cardiac hypertrophy
In a model of ketamine abuse during early gestation, ketamine decreased the acetylation level of cardiac histone H3K9 at the Myosin Light Chain 2 promoter by increasing HDAC3 level and histone deacetylase activity. These effects were accompanied by altered expression of several genes related to cardiomyogenesis, resulting in enlarged heart and ventricular chamber, thinner ventricular wall, degeneration of cardiomyocytes, and reduced systolic function [218]. Repeated MDMA exposure also led to hypermethylation of cardiac DNA promoter regions resulting in cardiac hypertrophy and progressive damage [217]. Given the concern surrounding the potential impact of RAADs on cardiovascular function through cardiac 5-HT receptors [219], future investigations are recommended to explore potential deleterious effects at clinically relevant doses and regimens.
Deleterious gestational and transgenerational effects
While few studies have assessed the transgenerational effects of RAADs, maternal intake during pregnancy and lactation may similarly to conventional antidepressants lead to epigenetic modifications and altered anxiety and depressive-like behavior in the offspring [56, 220,221,222]. For example, ketamine abuse during gestation impacts the epigenetic make-up of the fetus, and can result in impaired neurocognitive function in the offspring [218, 223]. Similarly, sociability, emotional behavior, and increased PFC dopamine levels are observed in the offspring of rats following repeated Ayahuasca administration during pregnancy and lactation [224].
Potential for addiction
While there is general consensus that psychedelics have low potential for addiction, psychological dependence or reinforcing effects, the NMDA antagonist ketamine [225, 226] and the empathogen MDMA [227, 228] have been associated with drug-seeking behaviors accompanied by epigenetic changes, similarly to drugs of abuse [229, 230]. For instance, one study found that ketamine-induced conditioned place preference resulted in differential expression of 34 hippocampal miRNAs [225], while another study showed that the conditioned place preference was accompanied by altered expression of serum exosomal miRNAs associated with processes such as nervous system development, neuron generation and differentiation, and apoptosis [231]. Acute and repeated MDMA exposure altered histone PTMs in the NAc promoter regions of the opioid-related nociceptin/orphaninFQ (N/OFQ)- Nociceptin Receptor (NOP) and dynorphin (DYN)- Kappa-Opioid Receptor (KOP) systems [232]. Specifically, acute MDMA increased the H3K4 methylation at N/OFQ and DYN promoters, while increasing H3K9 acetylation and decreasing H3K9 dymethylation at the DYN promoter. Acute and repeated MDMA also decreased H3K9 acetylation at the N/OFQ promoter [232]. These opioidergic effects could have direct implications for the therapeutic effects of MDMA on trauma [233], and for MDMA’s potential for addiction and neurotoxicity [234]. Considering the known involvement of epigenetics and transcriptional changes in addiction, it is prudent to investigate through cell-type and brain area-specific approaches whether epigenetic mechanisms may play a role in putative addictive, or anti-addictive, properties of RAADs.
Neurotoxicity
Several studies have reported neurotoxic effects of RAADs arising from repeated administration or higher doses. While such effects are not triggered in vivo by clinically relevant doses, this knowledge remains relevant from a harm-reduction perspective. Repeated ketamine increased histone deacetylase and DNMT activity, in an opposite fashion to the homolog changes elicited by clinically-relevant doses [235]. Additionally, repeated high-dose ketamine in young mice led to neurotoxicity, neuronal degeneration and memory impairments during adulthood, at least partially through miR-34c [236], miR-124 [237], miR-137 [238], and miR-199a-5p [239] signaling. In adolescent rats, repeated ketamine high-doses elicited hippocampal apoptosis and cognitive impairments, putatively mediated by hippocampal miR-214 downregulation/ Phosphatase And Tensin Homolog upregulation [240] and miR-34a upregulation/ Fibroblast Growth Factor Receptor 1 downregulation [241]. Repeated high-dose ketamine also elicited schizophrenia-like behavior accompanied by modulation of the miRNome and neurotrophic-related gene expression in the PFC and hippocampus [242, 243]. In vitro studies corroborate the neurotoxic effects of ketamine at higher doses through epigenetic mechanisms involving HDAC6, miR-22, miR-124, miR-497-5p, the lncRNAs SPRY4-IT1, LINC00641, and SNHG16, and the BDNF antisense RNA [214, 215, 237, 244,245,246,247,248,249,250,251]. Together, future research exploring the neurotoxic effects of RAADs abuse through epigenetic changes is encouraged.
Cancer
Several of the epigenetic effects elicited by RAADs involve signaling cascades related to cell growth, proliferation, and cancer, such as mTOR and BDNF [252, 253]. Therefore, epigenetic changes in these genes might induce or affect the progression of cancer. Considering that psychedelic-assisted psychotherapy is a promising treatment to attenuate psychological and existential distress in individuals facing a life-threatening conditions such as cancer, it is important to assess the neoplastic potential of RAADs [254,255,256]. If RAADs affected DNA methylation and other epigenetic mechanisms indiscriminately, for example by increasing or decreasing global DNA methylation aspecifically, this could pose a risk factor by causing genomic instability or repressing the transcription of tumor-suppressor genes [257,258,259]. A recent study reported oxidative DNA damage following the administration of higher-than clinically-relevant doses of psilocybin in the PFC and hippocampus [260]. Early reports suggested that ingesting or being exposed in utero to psychedelics such as LSD might be mutagenic and cause chromosomal damage and potential delayed mutations in the offspring (reviewed in [261]). However, subsequent experimental and epidemiological studies failed to replicate the findings, even in long-term psychedelic users [261, 262]. One investigation found that ketamine elicited ferropoptosis, and may thus elicit anti-neoplastic effects through the lncRNA Pvt1 oncogene /miR-214-3p signaling [263]. Given this evidence, further studies are warranted to investigate the potential of RAADs to induce, accelerate, or perhaps counteract, cancer.
Conclusion
Preliminary correlative evidence indicates that the epigenetic mechanisms engaged by RAADs converge, like conventional antidepressants, on permissive mechanisms over chromatin areas involved in neuroplasticity within stress-vulnerable brain regions. Importantly, these epigenetic changes counteract those induced by psychosocial stress, early-life adversities, and substance abuse. Yet, the abuse of RAADs triggers epigenetic marks resembling those elicited by stress.
Significant knowledge gaps remain, such as the mechanistic relationship between RAADs and epigenetic effects, the timing of onset of these epigenetic changes and their duration, the mechanisms responsible, cell type specificity, the role of metabolites, and the effects of “microdosing” (the ingestion of 1/10th–1/20th of a “large” dose). The polypharmacological nature of RAADs implies that each compound, dose, and regimen may uniquely affect the epigenetic control of gene expression, with potential indications or contraindications for various psychiatric disorders. Understanding how the subjective experience and environment modulate the epigenetic effects accompanying the administration of RAADs is an area ripe for exploration. Determining the potential modulation by the environment on the epigenetic effects of psychedelics could inform ongoing clinical investigations and expand our understanding of epigenetics. Brain area- and cell type-specific methods, facilitated by techniques like cell-sorting approaches and single-cell omics approaches, could address these questions [264, 265]. Identifying predictors [266] and modulators (i.e., set and setting, placebo effect) [267] of epigenetic responses accompanying RAADs administration could lead to improved therapeutic precision and efficacy.
While the epigenetic outcomes may causally contribute to therapeutic benefits, causative investigations lack. Given that epigenetic changes are observed in all cell types under disparate conditions (for example following neuronal activity or neurotoxic stimuli), the observed epigenetic changes could be secondary to behavioral changes induced by psychedelics. The potential epigenetic-mediated side effects, such as immune-related effects, cardiac hypertrophy, gestational and transgenerational effects, and cancer, also require further investigation. Future studies addressing these knowledge gaps could lead the way for developing novel neuroepigenetics-based precision therapeutics.
References
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. https://doi.org/10.1038/nn1659.
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. https://doi.org/10.1038/nn.2270.
Szyf M. The early life social environment and DNA methylation. Epigenetics. 2011;6:971–8. https://doi.org/10.4161/epi.6.8.16793.
Stajic D, Jansen LET. Empirical evidence for epigenetic inheritance driving evolutionary adaptation. Philos Trans R Soc B: Biol Sci. 2021;376:20200121. https://doi.org/10.1098/rstb.2020.0121.
Floriou-Servou A, von Ziegler L, Stalder L, Sturman O, Privitera M, Rassi A, et al. Distinct proteomic, transcriptomic, and epigenetic stress responses in dorsal and ventral hippocampus. Biol Psychiatry. 2018;84:531–41. https://doi.org/10.1016/j.biopsych.2018.02.003.
van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–55. https://doi.org/10.1016/j.biopsych.2009.05.014.
Reus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV, et al. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res. 2013;256:451–6. https://doi.org/10.1016/j.bbr.2013.08.041.
Chen ES, Ernst C, Turecki G. The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression. Int J Neuropsychopharmacol. 2011;14:427–9. https://doi.org/10.1017/S1461145710001422.
Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71.
Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharm Toxicol. 2013;53:59–87. https://doi.org/10.1146/annurev-pharmtox-010611-134540.
Labonte B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69:722–31. https://doi.org/10.1001/archgenpsychiatry.2011.2287.
Lim DA, Huang Y-C, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–33. https://doi.org/10.1038/nature07726.
Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–74. https://doi.org/10.1016/j.neuron.2008.10.012.
Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharm Rev. 2021;73:202–77. https://doi.org/10.1124/pharmrev.120.000056.
Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci. 2020;21:611–24. https://doi.org/10.1038/s41583-020-0367-2.
Nichols DE. Psychedelics. Pharm Rev. 2016;68:264–355. https://doi.org/10.1124/pr.115.011478.
Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology 2023. https://doi.org/10.1038/s41386-023-01629-w
Rudnick, G, Wall, SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–21.
Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abus. 2017;43:55–60. https://doi.org/10.3109/00952990.2016.1170135.
Murphy-Beiner A, Soar K. Ayahuasca’s ‘afterglow’: improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology (Berl) 2020. https://doi.org/10.1007/s00213-019-05445-3
Zeifman RJ, Palhano-Fontes F, Hallak J, Arcoverde E, Maia-Oliveira JP, Araujo DB. The Impact of Ayahuasca on Suicidality: Results From a Randomized Controlled Trial. Front Pharm. 2019;10:1325. https://doi.org/10.3389/fphar.2019.01325.
Kuypers KP, Riba J, de la Fuente Revenga M, Barker S, Theunissen EL, Ramaekers JG. Ayahuasca enhances creative divergent thinking while decreasing conventional convergent thinking. Psychopharmacol (Berl). 2016;233:3395–403. https://doi.org/10.1007/s00213-016-4377-8.
Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E. The epidemiology of 5-methoxy- N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J Psychopharmacol. 2018;32:779–92. https://doi.org/10.1177/0269881118769063.
Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abus Rev. 2014;7:157–64. https://doi.org/10.2174/1874473708666150107121331.
Callaway JC, Airaksinen MM, McKenna DJ, Brito GS, Grob CS. Platelet serotonin uptake sites increased in drinkers of ayahuasca. Psychopharmacol (Berl). 1994;116:385–7. https://doi.org/10.1007/bf02245347.
Bouso JC, Palhano-Fontes F, Rodríguez-Fornells A, Ribeiro S, Sanches R, Crippa JAS, et al. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur Neuropsychopharmacol. 2015;25:483–92. https://doi.org/10.1016/j.euroneuro.2015.01.008.
Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. https://doi.org/10.1038/nrg1655.
Weaver ICG, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early Environmental Regulation of Hippocampal Glucocorticoid Receptor Gene Expression: Characterization of Intracellular Mediators and Potential Genomic Target Sites. Ann N. Y Acad Sci. 2004;1024:182–212. https://doi.org/10.1196/annals.1321.099.
Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci USA. 2015;112:6807–13. https://doi.org/10.1073/pnas.1408355111.
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. https://doi.org/10.1038/nn.2436.
Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, et al. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–5. https://doi.org/10.1038/nature06640.
Labonté B, Suderman M, Maussion G, Lopez JP, Navarro-Sánchez L, Yerko V, et al. Genome-wide methylation changes in the brains of suicide completers. Am J Psychiatry. 2013;170:511–20. https://doi.org/10.1176/appi.ajp.2012.12050627.
Provencal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32:15626–42. https://doi.org/10.1523/JNEUROSCI.1470-12.2012.
McGowan PO, Sasaki A, Huang TCT, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS ONE. 2008;3:e2085. https://doi.org/10.1371/journal.pone.0002085.
Ernst C, Deleva V, Deng X, Sequeira A, Pomarenski A, Klempan T, et al. Alternative Splicing, Methylation State, and Expression Profile of Tropomyosin-Related Kinase B in the Frontal Cortex of Suicide Completers. Arch Gen Psychiatry. 2009;66:22–32. https://doi.org/10.1001/archpsyc.66.1.22.
Kang H-J, Kim J-M, Lee J-Y, Kim S-Y, Bae K-Y, Kim S-W, et al. BDNF promoter methylation and suicidal behavior in depressive patients. J Affect Disord. 2013;151:679–85. https://doi.org/10.1016/j.jad.2013.08.001.
Kang H-J, Kim J-M, Bae K-Y, Kim S-W, Shin I-S, Kim H-R, et al. Longitudinal associations between BDNF promoter methylation and late-life depression. Neurobiol Aging. 2015;36:1764.e1761–1764.e1767. https://doi.org/10.1016/j.neurobiolaging.2014.12.035.
Januar V, Ancelin ML, Ritchie K, Saffery R, Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry. 2015;5:e619. https://doi.org/10.1038/tp.2015.114.
Kang HJ, Kim JM, Kim SY, Kim SW, Shin IS, Kim HR, et al. A longitudinal study of BDNF promoter methylation and depression in breast cancer. Psychiatry Investig. 2015;12:523–31. https://doi.org/10.4306/pi.2015.12.4.523.
Kim J-M, Stewart R, Kang H-J, Kim S-Y, Kim S-W, Shin I-S, et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord. 2013;149:93–99. https://doi.org/10.1016/j.jad.2013.01.008.
Ferrer A, Labad J, Salvat-Pujol N, Barrachina M, Costas J, Urretavizcaya M, et al. BDNF genetic variants and methylation: effects on cognition in major depressive disorder. Transl Psychiatry. 2019;9:265. https://doi.org/10.1038/s41398-019-0601-8.
Ikegame T, Bundo M, Murata Y, Kasai K, Kato T, Iwamoto K. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. J Hum Genet. 2013;58:434–8. https://doi.org/10.1038/jhg.2013.65.
Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. https://doi.org/10.1016/j.biopsych.2008.11.028.
Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–8. https://doi.org/10.1038/mp.2014.21.
Detich N, Bovenzi V, Szyf M. Valproate induces replication-independent active DNA demethylation. J Biol Chem. 2003;278:27586–92. https://doi.org/10.1074/jbc.M303740200.
Lima IVA, Almeida-Santos AF, Ferreira-Vieira TH, Aguiar DC, Ribeiro FM, Campos AC, et al. Antidepressant-like effect of valproic acid-possible involvement of PI3K/Akt/mTOR pathway. Behav Brain Res. 2017;329:166–71. https://doi.org/10.1016/j.bbr.2017.04.015.
Ghabrash MF, Comai S, Tabaka J, Saint-Laurent M, Booij L, Gobbi G. Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J Biol Psychiatry. 2016;17:165–70. https://doi.org/10.3109/15622975.2015.1073856.
Ramchandani S, Bhattacharya SK, Cervoni N, Szyf M. DNA methylation is a reversible biological signal. Proc Natl Acad Sci USA. 1999;96:6107–12. https://doi.org/10.1073/pnas.96.11.6107.
Ju LS, Yang JJ, Lei L, Xia JY, Luo D, Ji MH, et al. The combination of long-term ketamine and extinction training contributes to fear erasure by Bdnf methylation. Front Cell Neurosci. 2017;11:100. https://doi.org/10.3389/fncel.2017.00100.
Sales AJ, Joca SRL. Antidepressant administration modulates stress-induced DNA methylation and DNA methyltransferase expression in rat prefrontal cortex and hippocampus. Behavioural Brain Res. 2018;343:8–15. https://doi.org/10.1016/j.bbr.2018.01.022.
Sakata K, Duke S. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260:265–75.
Liu R, Wu X-M, He X, Wang R-Z, Yin X-Y, Zhou F, et al. Contribution of DNA methyltransferases to spared nerve injury induced depression partially through epigenetically repressing Bdnf in hippocampus: reversal by ketamine. Pharm Biochem Behav. 2021;200:173079. https://doi.org/10.1016/j.pbb.2020.173079.
Xu H, Wang J, Zhang K, Zhao M, Ellenbroek B, Shao F, et al. Effects of adolescent social stress and antidepressant treatment on cognitive inflexibility and Bdnf epigenetic modifications in the mPFC of adult mice. Psychoneuroendocrinology. 2018;88:92–101. https://doi.org/10.1016/j.psyneuen.2017.11.013.
Wang P, Zhang C, Lv Q, Bao C, Sun H, Ma G, et al. Association of DNA methylation in BDNF with escitalopram treatment response in depressed Chinese Han patients. Eur J Clin Pharm. 2018;74:1011–20. https://doi.org/10.1007/s00228-018-2463-z.
Jin H-J, Pei L, Li Y-N, Zheng H, Yang S, Wan Y, et al. Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci Rep. 2017;7:14926. https://doi.org/10.1038/s41598-017-13929-5.
Boulle F, Pawluski JL, Homberg JR, Machiels B, Kroeze Y, Kumar N, et al. Prenatal stress and early-life exposure to fluoxetine have enduring effects on anxiety and hippocampal BDNF gene expression in adult male offspring. Dev Psychobiol. 2016;58:427–38. https://doi.org/10.1002/dev.21385.
Allen AP, Naughton M, Dowling J, Walsh A, Ismail F, Shorten G, et al. Serum BDNF as a peripheral biomarker of treatment-resistant depression and the rapid antidepressant response: a comparison of ketamine and ECT. J Affect Disord. 2015;186:306–11. https://doi.org/10.1016/j.jad.2015.06.033.
Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT, et al. Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry. 2013;18:398–9. https://doi.org/10.1038/mp.2012.38.
D’Addario C, Dell’Osso B, Palazzo MC, Benatti B, Lietti L, Cattaneo E, et al. Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacology. 2012;37:1647–55. https://doi.org/10.1038/npp.2012.10.
D’Addario C, Dell’Osso B, Galimberti D, Palazzo MC, Benatti B, Di Francesco A, et al. Epigenetic modulation of BDNF gene in patients with major depressive disorder. Biol Psychiatry. 2013;73:e6–e7. https://doi.org/10.1016/j.biopsych.2012.07.009.
Carlberg L, Scheibelreiter J, Hassler MR, Schloegelhofer M, Schmoeger M, Ludwig B, et al. Brain-derived neurotrophic factor (BDNF)—Epigenetic regulation in unipolar and bipolar affective disorder. J Affect Disord. 2014;168:399–406. https://doi.org/10.1016/j.jad.2014.07.022.
Yao W, Cao Q, Luo S, He L, Yang C, Chen J, et al. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry. 2022;27:1618–29. https://doi.org/10.1038/s41380-021-01377-7.
Joca SRL, Sales AJ. Effects of DNA methylation inhibitors and conventional antidepressants on mice behaviour and brain DNA methylation levels. Acta Neuropsychiatrica. 2016;28:11–22. https://doi.org/10.1017/neu.2015.40.
Toffoli LV, Rodrigues GM, Oliveira JF, Silva AS, Moreira EG, Pelosi GG, et al. Maternal exposure to fluoxetine during gestation and lactation affects the DNA methylation programming of rat’s offspring: modulation by folic acid supplementation. Behav Brain Res. 2014;265:142–7. https://doi.org/10.1016/j.bbr.2014.02.031.
Booij L, Szyf M, Carballedo A, Frey EM, Morris D, Dymov S, et al. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: a study in depressed patients and healthy controls. PLoS ONE. 2015;10:e0119061. https://doi.org/10.1371/journal.pone.0119061.
Ryan J, Pilkington L, Neuhaus K, Ritchie K, Ancelin M-L, Saffery R. Investigating the epigenetic profile of the inflammatory gene IL-6 in late-life depression. BMC Psychiatry. 2017;17:354. https://doi.org/10.1186/s12888-017-1515-8.
Verhoeven JE, Yang R, Wolkowitz OM, Bersani FS, Lindqvist D, Mellon SH, et al. Epigenetic age in male combat-exposed War Veterans: Associations with Posttraumatic Stress Disorder Status. Mol Neuropsychiatry. 2018;4:90–9. https://doi.org/10.1159/000491431.
Germann CB. The Psilocybin-Telomere Hypothesis: An empirically falsifiable prediction concerning the beneficial neuropsychopharmacological effects of psilocybin on genetic aging. Med Hypotheses. 2020;134:109406. https://doi.org/10.1016/j.mehy.2019.109406.
Inserra A, Campanale A, Cheishvili D, Dymov S, Wong A, Marcal N, et al. Modulation of DNA methylation and protein expression in the prefrontal cortex by repeated administration of D-lysergic acid diethylamide (LSD): Impact on neurotropic, neurotrophic, and neuroplasticity signaling. Prog Neuro-Psychopharmacol Biol Psychiatry. 2022;119:110594. https://doi.org/10.1016/j.pnpbp.2022.110594.
De Gregorio D, Popic J, Enns JP, Inserra A, Skalecka A, Markopoulos A, et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc Natl Acad Sci USA. 2021;118:e2020705118. https://doi.org/10.1073/pnas.2020705118.
De Gregorio D, Inserra A, Enns JP, Markopoulos A, Pileggi M, El Rahimy Y, et al. Repeated lysergic acid diethylamide (LSD) reverses stress-induced anxiety-like behavior, cortical synaptogenesis deficits and serotonergic neurotransmission decline. Neuropsychopharmacology. 2022;47:1188–98. https://doi.org/10.1038/s41386-022-01301-9.
Inserra A, Giorgini G, Lacroix S, Bertazzo A, Choo J, Markopolous A, et al. Effects of repeated lysergic acid diethylamide (LSD) on the mouse brain endocannabinoidome and gut microbiome. Br J Pharm. 2023;180:721–39. https://doi.org/10.1111/bph.15977.
Hanson HE, Liebl AL. The mutagenic consequences of DNA methylation within and across generations. Epigenomes 2022;6.
Supek F, Lehner B, Hajkova P, Warnecke T. Hydroxymethylated cytosines are associated with elevated C to G transversion rates. PLoS Genet. 2014;10:e1004585. https://doi.org/10.1371/journal.pgen.1004585.
Zeifman RJ, Yu D, Singhal N, Wang G, Nayak SM, Weissman CR. Decreases in suicidality following psychedelic therapy: a meta-analysis of individual patient data across clinical trials. J Clin Psychiatry. 2022;83. https://doi.org/10.4088/JCP.21r14057
Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol. 2015;29:280–8. https://doi.org/10.1177/0269881114565653.
Murphy TM, Mullins N, Ryan M, Foster T, Kelly C, McClelland R, et al. Genetic variation in DNMT3B and increased global DNA methylation is associated with suicide attempts in psychiatric patients. Genes Brain Behav. 2013;12:125–32. https://doi.org/10.1111/j.1601-183X.2012.00865.x.
Haghighi F, Xin Y, Chanrion B, O’Donnell AH, Ge Y, Dwork AJ, et al. Increased DNA methylation in the suicide brain. Dialogues Clin Neurosci. 2014;16:430–8. https://doi.org/10.31887/DCNS.2014.16.3/jmann.
Ruffell SGD, Netzband N, Tsang W, Davies M, Butler M, Rucker JJH et al. Ceremonial Ayahuasca in Amazonian retreats—mental health and epigenetic outcomes from a six-month naturalistic study. Front Psychiatry 2021;12. https://doi.org/10.3389/fpsyt.2021.687615
Inserra A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front Pharm. 2018;9:330. https://doi.org/10.3389/fphar.2018.00330.
Ling Y, Feng J, Luo L, Guo J, Peng Y, Wang T, et al. Design and Synthesis of C3-Substituted β-Carboline-Based Histone Deacetylase Inhibitors with Potent Antitumor Activities. ChemMedChem. 2017;12:646–51. https://doi.org/10.1002/cmdc.201700133.
Leonhardt M, Sellmer A, Krämer OH, Dove S, Elz S, Kraus B, et al. Design and biological evaluation of tetrahydro-β-carboline derivatives as highly potent histone deacetylase 6 (HDAC6) inhibitors. Eur J Medicinal Chem. 2018;152:329–57. https://doi.org/10.1016/j.ejmech.2018.04.046.
Lewis, CR, Tafur, J, Spencer, S, Green, JM, Harrison, C, Kelmendi, B, et al. Pilot study suggests DNA methylation of the glucocorticoid receptor gene (NR3C1) is associated with MDMA-assisted therapy treatment response for severe PTSD. Frontiers in Psychiatry 2023;14 https://doi.org/10.3389/fpsyt.2023.959590
Erburu M, Muñoz-Cobo I, Domínguez-Andrés J, Beltran E, Suzuki T, Mai A, et al. Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol. 2015;25:2036–48. https://doi.org/10.1016/j.euroneuro.2015.08.016.
Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, et al. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol. 2010;6:93–107. https://doi.org/10.1017/S1740925X10000049.
Gräff J, Rei D, Guan J-S, Wang W-Y, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–6. https://doi.org/10.1038/nature10849.
Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–60. https://doi.org/10.1523/JNEUROSCI.1758-09.2009.
Qiao M, Jiang QS, Liu YJ, Hu XY, Wang LJ, Zhou QX, et al. Antidepressant mechanisms of venlafaxine involving increasing histone acetylation and modulating tyrosine hydroxylase and tryptophan hydroxylase expression in hippocampus of depressive rats. Neuroreport. 2019;30:255–61. https://doi.org/10.1097/wnr.0000000000001191.
Nghia NA, Hirasawa T, Kasai H, Obata C, Moriishi K, Mochizuki K, et al. Long-term imipramine treatment increases N-methyl-d-aspartate receptor activity and expression via epigenetic mechanisms. Eur J Pharm. 2015;752:69–77. https://doi.org/10.1016/j.ejphar.2015.02.010.
El-Saiy KA, Sayed RH, El-Sahar AE, Kandil EA. Modulation of histone deacetylase, the ubiquitin proteasome system, and autophagy underlies the neuroprotective effects of venlafaxine in a rotenone-induced Parkinson’s disease model in rats. Chem-Biol Interact. 2022;354:109841. https://doi.org/10.1016/j.cbi.2022.109841.
Ookubo M, Kanai H, Aoki H, Yamada N. Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: Brain region specific changes. J Psychiatr Res. 2013;47:1204–14. https://doi.org/10.1016/j.jpsychires.2013.05.028.
Li W, Ali T, Zheng C, Liu Z, He K, Shah FA, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021;18:38. https://doi.org/10.1186/s12974-021-02091-5.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, et al. Fluoxetine Epigenetically Alters the CaMKIIα Promoter in Nucleus Accumbens to Regulate ΔFosB Binding and Antidepressant Effects. Neuropsychopharmacology. 2014;39:1178–86. https://doi.org/10.1038/npp.2013.319.
Jiang H, Zhang X, Lu J, Meng H, Sun Y, Yang X, et al. Antidepressant-Like Effects of Acupuncture-Insights From DNA Methylation and Histone Modifications of Brain-Derived Neurotrophic Factor. Front Psychiatry. 2018;9:102. https://doi.org/10.3389/fpsyt.2018.00102.
Ana R-D, Dalia YAS, Fay MJ, Elisa H, Joseph LM, Laxmikant SD. Ketamine Produces Antidepressant Effects by Inhibiting Histone Deacetylases and Upregulating Hippocampal Brain-Derived Neurotrophic Factor Levels in a Diisopropyl Fluorophosphate–Based Rat Model of Gulf War Illness. J Pharm Exp Therapeutics. 2024;388:647. https://doi.org/10.1124/jpet.123.001824.
Levina AS, Shiryaeva NV, Vaido AI, Dyuzhikova NA. Effect of NMDA receptor activity on histone H3 methylation and its asymmetry in the hippocampal pyramidal neurons of rats with different excitability thresholds under normal and stress conditions. J Evol Biochem Physiol. 2013;49:615–23. https://doi.org/10.1134/S0022093013060091.
Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS, et al. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci USA. 2015;112:15755–60. https://doi.org/10.1073/pnas.1513913112.
Brown I, Liew C. Lysergic acid diethylamide: effect on histone acetylation in rabbit brain. Science. 1975;188:1122–3. https://doi.org/10.1126/science.1215990.
Brown IR. RNA synthesis in isolated brian nuclei after administration of d-lysergic acid diethylamide (LSD) in vivo. Proc Natl Acad Sci USA. 1975;72:837–9. https://doi.org/10.1073/pnas.72.3.837.
Nichols CD, Sanders-Bush E. A Single Dose of Lysergic Acid Diethylamide Influences Gene Expression Patterns within the Mammalian Brain. Neuropsychopharmacology. 2002;26:634–42. https://doi.org/10.1016/S0893-133X(01)00405-5.
Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry. 2012;72:25–33. https://doi.org/10.1016/j.biopsych.2011.12.012.
Bowers ME, Xia B, Carreiro S, Ressler KJ. The Class I HDAC inhibitor RGFP963 enhances consolidation of cued fear extinction. Learn Mem. 2015;22:225–31. https://doi.org/10.1101/lm.036699.114.
Eisner BG, Cohen S. Psychotherapy with lysergic acid diethylamide. J Nerv Ment Dis. 1958;127:528–39. https://doi.org/10.1097/00005053-195812000-00006.
Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202:513–20. https://doi.org/10.1097/nmd.0000000000000113.
Spencer AM. Permissive Group Therapy with Lysergic Acid Diethylamide. Br J Psychiatry. 1963;109:37–45. https://doi.org/10.1192/bjp.109.458.37.
Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–99. https://doi.org/10.1177/0269881114565144.
de la Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021;37:109836. https://doi.org/10.1016/j.celrep.2021.109836.
Chan JC, Maze I. Nothing Is Yet Set in (Hi)stone: Novel Post-Translational Modifications Regulating Chromatin Function. Trends Biochemical Sci. 2020;45:829–44. https://doi.org/10.1016/j.tibs.2020.05.009.
Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV, et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–9. https://doi.org/10.1038/s41586-019-1024-7.
Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, et al. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 2020;368:197–201. https://doi.org/10.1126/science.aaw8806.
Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, et al. New class of microRNA targets containing simultaneous 5’-UTR and 3’-UTR interaction sites. Genome Res. 2009;19:1175–83. https://doi.org/10.1101/gr.089367.108.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. https://doi.org/10.1038/nrg3074.
Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–405. https://doi.org/10.1038/npp.2008.131.
Daskalakis NP, Provost AC, Hunter RG, Guffanti G. Noncoding RNAs: Stress, Glucocorticoids, and Posttraumatic Stress Disorder. Biol Psychiatry. 2018;83:849–65. https://doi.org/10.1016/j.biopsych.2018.01.009.
Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, et al. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602–11. https://doi.org/10.1016/j.euroneuro.2012.06.013.
Enatescu VR, Papava I, Enatescu I, Antonescu M, Anghel A, Seclaman E, et al. Circulating Plasma Micro RNAs in Patients with Major Depressive Disorder Treated with Antidepressants: A Pilot Study. Psychiatry Investig. 2016;13:549–57. https://doi.org/10.4306/pi.2016.13.5.549.
Ahmadimanesh M, Etemad L, Morshedi Rad D, Ghahremani MH, Mohammadpour AH, Jafarzadeh Esfehani R, et al. Effect of citalopram and sertraline on the expression of miRNA- 124, 132, and 16 and their protein targets in patients with depression. Iran J Basic Med Sci. 2023;26:820–9. https://doi.org/10.22038/ijbms.2023.66496.14595.
Barry G. Integrating the roles of long and small non-coding RNA in brain function and disease. Mol Psychiatry. 2014;19:410–6. https://doi.org/10.1038/mp.2013.196.
Zhou Y, Lutz PE, Wang YC, Ragoussis J, Turecki G. Global long non-coding RNA expression in the rostral anterior cingulate cortex of depressed suicides. Transl Psychiatry. 2018;8:224. https://doi.org/10.1038/s41398-018-0267-7.
Maffioletti E, Cattaneo A, Rosso G, Maina G, Maj C, Gennarelli M, et al. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J Affect Disord. 2016;200:250–8. https://doi.org/10.1016/j.jad.2016.04.021.
Roy B, Wang Q, Palkovits M, Faludi G, Dwivedi Y. Altered miRNA expression network in locus coeruleus of depressed suicide subjects. Sci Rep. 2017;7:4387. https://doi.org/10.1038/s41598-017-04300-9.
Rasheed, M, Asghar, R, Firdoos, S, Ahmad, N, Nazir, A, Ullah, KM, et al. A Systematic Review of Circulatory microRNAs in Major Depressive Disorder: Potential Biomarkers for Disease Prognosis. Int JMol Sci. 2022;23:1294 https://doi.org/10.3390/ijms23031294.
Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40:47–55. https://doi.org/10.1007/s12031-009-9252-1.
O’Connor RM, Grenham S, Dinan TG, Cryan JF. microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int J Neuropsychopharmacol. 2013;16:1885–92. https://doi.org/10.1017/s1461145713000448.
Wang SS, Mu RH, Li CF, Dong SQ, Geng D, Liu Q, et al. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:417–25. https://doi.org/10.1016/j.pnpbp.2017.07.024.
Torres-Berrío A, Lopez JP, Bagot RC, Nouel D, Dal Bo G, Cuesta S, et al. DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218. Biol Psychiatry. 2017;81:306–15. https://doi.org/10.1016/j.biopsych.2016.08.017.
Zuo L, Tan Y, Wang Z, Wang KS, Zhang X, Chen X, et al. Long noncoding RNAs in psychiatric disorders. Psychiatr Genet. 2016;26:109–16. https://doi.org/10.1097/ypg.0000000000000129.
Belzeaux R, Lin R, Turecki G. Potential Use of MicroRNA for Monitoring Therapeutic Response to Antidepressants. CNS Drugs. 2017;31:253–62. https://doi.org/10.1007/s40263-017-0418-z.
Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA Expression Is Down-Regulated and Reorganized in Prefrontal Cortex of Depressed Suicide Subjects. PLOS ONE. 2012;7:e33201. https://doi.org/10.1371/journal.pone.0033201.
Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–60.
He S, Liu X, Jiang K, Peng D, Hong W, Fang Y, et al. Alterations of microRNA-124 expression in peripheral blood mononuclear cells in pre- and post-treatment patients with major depressive disorder. J Psychiatr Res. 2016;78:65–71. https://doi.org/10.1016/j.jpsychires.2016.03.015.
Guan W, Wu XY, Jin X, Sheng XM, Fan Y. miR-204-5p Plays a Critical Role in the Pathogenesis of Depression and Anti-depression Action of Venlafaxine in the Hippocampus of Mice. Curr Med Chem. 2023. https://doi.org/10.2174/0929867330666230623163315
Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–41. https://doi.org/10.1126/science.1193692.
Roy B, Wang Q, Dwivedi Y. Long noncoding RNA-associated transcriptomic changes in resiliency or susceptibility to depression and response to antidepressant treatment. Int J Neuropsychopharmacol. 2018;21:461–72. https://doi.org/10.1093/ijnp/pyy010.
Zhang CL, Li YJ, Lu S, Zhang T, Xiao R, Luo HR. Fluoxetine ameliorates depressive symptoms by regulating lncRNA expression in the mouse hippocampus. Zool Res. 2021;42:28–42. https://doi.org/10.24272/j.issn.2095-8137.2020.294.
Schmidt U, Herrmann L, Hagl K, Novak B, Huber C, Holsboer F, et al. Therapeutic action of fluoxetine is associated with a reduction in prefrontal cortical miR-1971 expression levels in a mouse model of posttraumatic stress disorder. Front Psychiatry. 2013;4. https://doi.org/10.3389/fpsyt.2013.00066
Pan B, Liu Y. Effects of duloxetine on microRNA expression profile in frontal lobe and hippocampus in a mouse model of depression. Int J Clin Exp Pathol. 2015;8:15454–61.
Lo Iacono L, Ielpo D, Parisi C, Napoli G, Accoto A, Di Segni M, et al. MicroRNA-34a regulates 5-HT2C expression in dorsal raphe and contributes to the anti-depressant-like effect of fluoxetine. Neuropharmacology. 2021;190:108559. https://doi.org/10.1016/j.neuropharm.2021.108559.
Zhang Y, Wang Y, Wang L, Bai M, Zhang X, Zhu X. Dopamine receptor D2 and associated microRNAs are involved in stress susceptibility and resistance to escitalopram treatment. Int J Neuropsychopharmacol. 2015;18. https://doi.org/10.1093/ijnp/pyv025
Lopez JP, Pereira F, Richard-Devantoy S, Berlim M, Chachamovich E, Fiori LM, et al. Co-variation of peripheral levels of miR-1202 and brain activity and connectivity during antidepressant treatment. Neuropsychopharmacology. 2017;42:2043–51. https://doi.org/10.1038/npp.2017.9.
Launay JM, Mouillet-Richard S, Baudry A, Pietri M, Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl Psychiatry. 2011;1:e56. https://doi.org/10.1038/tp.2011.54.
Wan Y-Q, Feng J-G, Li M, Wang M-Z, Liu L, Liu X, et al. Prefrontal cortex miR-29b-3p plays a key role in the antidepressant-like effect of ketamine in rats. Exp Mol Med. 2018;50:1–14. https://doi.org/10.1038/s12276-018-0164-4.
Huang C, Wang Y, Wu Z, Xu J, Zhou L, Wang D, et al. miR-98-5p plays a critical role in depression and antidepressant effect of ketamine. Transl Psychiatry. 2021;11:454. https://doi.org/10.1038/s41398-021-01588-0.
Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, et al. A key role of miR-132-5p in the prefrontal cortex for persistent prophylactic actions of (R)-ketamine in mice. Transl Psychiatry. 2022;12:417. https://doi.org/10.1038/s41398-022-02192-6.
De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–10. https://doi.org/10.1111/cns.12104.
Goswami A, Mukherjee K, Mazumder A, Ganguly S, Mukherjee I, Chakrabarti S, et al. MicroRNA exporter HuR clears the internalized pathogens by promoting pro-inflammatory response in infected macrophages. EMBO Mol Med. 2020;12:e11011. https://doi.org/10.15252/emmm.201911011.
Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, et al. MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med. 2014;16:594–605. https://doi.org/10.1007/s12017-014-8312-z.
Lepack, AE, Fuchikami, M, Dwyer, JM, Banasr, M, Duman, RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18. https://doi.org/10.1093/ijnp/pyu033
Nibuya M, Morinobu S, Duman R. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–47. https://doi.org/10.1523/jneurosci.15-11-07539.1995.
Grieco SF, Velmeshev D, Magistri M, Eldar-Finkelman H, Faghihi MA, Jope RS, et al. Ketamine up-regulates a cluster of intronic miRNAs within the serotonin receptor 2C gene by inhibiting glycogen synthase kinase-3. World J Biol Psychiatry. 2017;18:445–56. https://doi.org/10.1080/15622975.2016.1224927.
Choi Y, Kim B, Ham S, Chung S, Maeng S, Kim H-S, et al. Subanesthetic ketamine rapidly alters medial prefrontal miRNAs involved in ubiquitin-mediated proteolysis. PLoS ONE. 2021;16:e0256390. https://doi.org/10.1371/journal.pone.0256390.
Ma L, Wang L, Chang L, Shan J, Qu Y, Wang X, et al. A role of microRNA-149 in the prefrontal cortex for prophylactic actions of (R)-ketamine in inflammation model. Neuropharmacology. 2022;219:109250. https://doi.org/10.1016/j.neuropharm.2022.109250.
Zhang N, Yao L, Wang P, Liu Z. Immunoregulation and antidepressant effect of ketamine. 12, 218-36 (2021). https://doi.org/10.1515/tnsci-2020-0167
Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med. 2014;20:764–8. https://doi.org/10.1038/nm.3582.
Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun. 2017;8:15497. https://doi.org/10.1038/ncomms15497.
Kuang, W-H, Dong Z-Q, Tian L-T, Li J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res. 2018;51.
Hung Y-Y, Chou C-K, Yang Y-C, Fu H-C, Loh E-W, Kang H-Y. Exosomal let-7e, miR-21-5p, miR-145, miR-146a and miR-155 in Predicting Antidepressants Response in Patients with Major Depressive Disorder. Biomedicines 9 (2021).
Lin C-C, Tsai M-C, Lee C-T, Sun M-H, Huang T-L. Antidepressant treatment increased serum miR-183 and miR-212 levels in patients with major depressive disorder. Psychiatry Res. 2018;270:232–7. https://doi.org/10.1016/j.psychres.2018.09.025.
Kato M, Ogata H, Tahara H, Shimamoto A, Takekita Y, Koshikawa Y, et al. Multiple Pre-Treatment miRNAs Levels in Untreated Major Depressive Disorder Patients Predict Early Response to Antidepressants and Interact with Key Pathways. Int J Mol Sci. 2022;23:3873.
Douglas SR, Shenoda BB, Qureshi RA, Sacan A, Alexander GM, Perreault M, et al. Analgesic Response to Intravenous Ketamine Is Linked to a Circulating microRNA Signature in Female Patients With Complex Regional Pain Syndrome. J Pain. 2015;16:814–24. https://doi.org/10.1016/j.jpain.2015.05.008.
Pande R, Parikh A, Shenoda B, Ramanathan S, Alexander GM, Schwartzman RJ, et al. Hsa-miR-605 regulates the proinflammatory chemokine CXCL5 in complex regional pain syndrome. Biomedicine Pharmacother. 2021;140:111788. https://doi.org/10.1016/j.biopha.2021.111788.
Gururajan A, Naughton ME, Scott KA, O’Connor RM, Moloney G, Clarke G, et al. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl Psychiatry. 2016;6:e862. https://doi.org/10.1038/tp.2016.131.
Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52. https://doi.org/10.1038/nature12986.
Mao J, Li T, Fan D, Zhou H, Feng J, Liu L, et al. Abnormal expression of rno_circRNA_014900 and rno_circRNA_005442 induced by ketamine in the rat hippocampus. BMC Psychiatry. 2020;20:1. https://doi.org/10.1186/s12888-019-2374-2.
Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron. 2015;88:861–77.
Seki T, Yamagata H, Uchida S, Chen C, Kobayashi A, Kobayashi M, et al. Altered expression of long noncoding RNAs in patients with major depressive disorder. J Psychiatr Res. 2019;117:92–9. https://doi.org/10.1016/j.jpsychires.2019.07.004.
Issler O, van der Zee YY, Ramakrishnan A, Xia S, Zinsmaier AK, Tan C, et al. The long noncoding RNA FEDORA is a cell type– and sex-specific regulator of depression. Sci Adv. 8, eabn9494. https://doi.org/10.1126/sciadv.abn9494
Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–9. https://doi.org/10.1126/science.1086446.
Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent Bdnf gene regulation. Science. 2003;302:890–3. https://doi.org/10.1126/science.1090842.
Zhou Z, Hong EJ, Cohen S, Zhao W-N, Ho H-YH, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–69.
Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun. 2020;11:72. https://doi.org/10.1038/s41467-019-13809-8.
Hernández-Oliveras A, Zarain-Herzberg A. The role of Ca2+-signaling in the regulation of epigenetic mechanisms. Cell Calcium. 2024;117:102836. https://doi.org/10.1016/j.ceca.2023.102836.
Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–4. https://doi.org/10.1126/science.2845577.
Martin DA, Nichols CD. Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine. 2016;11:262–77. https://doi.org/10.1016/j.ebiom.2016.08.049.
Guo JU, Ma DK, Mo H, Ball MP, Jang M-H, Bonaguidi MA, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci. 2011;14:1345–51. https://doi.org/10.1038/nn.2900.
Vetencourt JFM, Tiraboschi E, Spolidoro M, Castrén E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. https://doi.org/10.1111/j.1460-9568.2010.07488.x.
Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, et al. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707.
Chen S, Cai D, Pearce K, Sun PY, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife. 2014;3:e03896.
Ögren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekström JC, Svenningsson P, et al. The role of 5-HT1A receptors in learning and memory. Behav Brain Res. 2008;195:54–77. https://doi.org/10.1016/j.bbr.2008.02.023.
Zhang, G, Stackman, RW. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015;6. https://doi.org/10.3389/fphar.2015.00225
Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–62. https://doi.org/10.1038/sj.npp.1301345.
Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55.
Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, et al. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–10. https://doi.org/10.1523/jneurosci.3878-11.2011.
Mikics É, Guirado R, Umemori J, Tóth M, Biró L, Miskolczi C, et al. Social learning requires plasticity enhanced by fluoxetine through prefrontal Bdnf-TrkB signaling to limit aggression induced by post-weaning social isolation. Neuropsychopharmacology. 2018;43:235–45. https://doi.org/10.1038/npp.2017.142.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–1313.e1219. https://doi.org/10.1016/j.cell.2021.01.034.
Moliner, R, Girych M, Brunello CA, Kovaleva V, Biojone C, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023;26:1032–41. https://doi.org/10.1038/s41593-023-01316-5.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82. https://doi.org/10.1016/j.celrep.2018.05.022.
Lin P-Y, Ma ZZ, Mahgoub M, Kavalali ET, Monteggia LM. A synaptic locus for TrkB signaling underlying ketamine rapid antidepressant action. Cell Rep. 2021;36:109513. https://doi.org/10.1016/j.celrep.2021.109513.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. https://doi.org/10.1038/nature10130.
Nardou R, Sawyer E, Song YJ, Wilkinson M, Padovan-Hernandez Y, de Deus JL, et al. Psychedelics reopen the social reward learning critical period. Nature. 2023;618:790–8. https://doi.org/10.1038/s41586-023-06204-3.
Jefsen OH, Elfving B, Wegener G, Müller HK. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J Psychopharmacol. 2021;35:483–93. https://doi.org/10.1177/0269881120959614.
Hibicke M, Kramer HM, Nichols CD. A single administration of psilocybin persistently rescues cognitive deficits caused by adolescent chronic restraint stress without long-term changes in synaptic protein gene expression in a rat experimental system with translational relevance to depression. Psychedelic Med. 2023;1:54–67. https://doi.org/10.1089/psymed.2022.0012.
Ilchibaeva, T, Tsybko, A, Zeug, A, Müller, FE, Guseva, D, Bischoff, S, et al. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells. 2022;11.
Philibert CE, Disdier C, Lafon P-A, Bouyssou A, Oosterlaken M, Galant S, et al. TrkB receptor interacts with mGlu2 receptor and mediates antipsychotic-like effects of mGlu2 receptor activation in the mouse. Sci Adv.;10 eadg1679. https://doi.org/10.1126/sciadv.adg1679
Moreno JL, Miranda-Azpiazu P, García-Bea A, Younkin J, Cui M, Kozlenkov A, et al. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal. 2016;9:ra5. https://doi.org/10.1126/scisignal.aab0467.
Nafisi S, Bonsaii M, Maali P, Khalilzadeh MA, Manouchehri F. Beta-carboline alkaloids bind DNA. J Photochem Photobio B. 2010;100:84–91. https://doi.org/10.1016/j.jphotobiol.2010.05.005.
Wagner TE. In vitro Interaction of LSD with Purified Calf Thymus DNA. Nature. 1969;222:1170–2. https://doi.org/10.1038/2221170b0.
Smythies JR, Antun F. Binding of tryptamine and allied compounds to nucleic acids. Nature. 1969;223:1061–3. https://doi.org/10.1038/2231061a0.
Yielding KL, Sterglanz H. Lysergic acid diethylamide (LSD) binding to deoxyribonucleic acid (DNA). Proc Soc Exp Biol Med. 1968;128:1096–8. https://doi.org/10.3181/00379727-128-33303.
Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. 2023;379:700–6. https://doi.org/10.1126/science.adf0435.
Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, et al. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. https://doi.org/10.1016/s0306-4522(98)00653-8.
Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–77. https://doi.org/10.1074/jbc.M006968200.
Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharm. 1996;50:306–13.
Frecska E, Szabo A, Winkelman MJ, Luna LE, McKenna DJ. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J Neural Transm (Vienna). 2013;120:1295–303. https://doi.org/10.1007/s00702-013-1024-y.
Carbonaro TM, Gatch MB. Neuropharmacology of N,N-dimethyltryptamine. Brain Res Bull. 2016;126:74–88. https://doi.org/10.1016/j.brainresbull.2016.04.016.
Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–7. https://doi.org/10.1126/science.1166127.
Tsai SY, Chuang JY, Tsai MS, Wang XF, Xi ZX, Hung JJ, et al. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc Natl Acad Sci USA. 2015;112:E6562–70. https://doi.org/10.1073/pnas.1518894112.
Chuang SM, Lu JH, Lin KL, Long CY, Lee YC, Hsiao HP, et al. Epigenetic regulation of COX2 expression by DNA hypomethylation via NFkappaB activation in ketamineinduced ulcerative cystitis. Int J Mol Med. 2019;44:797–812. https://doi.org/10.3892/ijmm.2019.4252.
Jhang JF, Hsu YH, Jiang YH, Kuo HC. Elevated serum IgE may be associated with development of ketamine cystitis. J Urol. 2014;192:1249–56. https://doi.org/10.1016/j.juro.2014.05.084.
Fan X, Bian W, Liu M, Li J, Wang Y. MiRNA-429 alleviates ketamine-induced neurotoxicity through targeting BAG5. Environ Toxicol. 2021;36:620–7. https://doi.org/10.1002/tox.23066.
Li H, Li K, Zhu Q, Tang Z, Wang Z. Transcriptomic analysis of bladder tissue in a rat model of ketamine-induced bladder fibrosis. Neurourol Urodyn. 2022;41:765–76. https://doi.org/10.1002/nau.24892.
Zeng F, Wu Q, Song M, Kang X, Ou Z, Yang Z, et al. Circ-SFMBT2 sponges miR-224-5p to induce ketamine-induced cystitis by up-regulating metadherin (MTDH). Hum Cell. 2023;36:2040–54. https://doi.org/10.1007/s13577-023-00972-w.
Li X, Ma G, Zhang C, Chen M, Huang X, Gu C. miR-34a overexpression protects against hippocampal neuron damage caused by ketamine-induced anesthesia in immature rats through the Notch-1/NF-κB signaling pathway. Am J Transl Res. 2021;13:13452–61.
Chen Q, Yan J, Xie W, Xie W, Li M, Ye Y. LncRNA LINC00641 Sponges miR-497-5p to Ameliorate Neural Injury Induced by Anesthesia via up-regulating BDNF. Front. Mol. Neurosci. 2020;13. https://doi.org/10.3389/fnmol.2020.00095
Zhao X, Shu F, Wang X, Wang F, Wu L, Li L, et al. Inhibition of microRNA-375 ameliorated ketamine-induced neurotoxicity in human embryonic stem cell derived neurons. Eur J Pharmacol. 2019;844:56–64. https://doi.org/10.1016/j.ejphar.2018.11.035
Huang C-H, Chang M-C, Lai Y-C, Lin C-Y, Hsu C-H, Tseng B-Y, et al. Mitochondrial DNA methylation profiling of the human prefrontal cortex and nucleus accumbens: correlations with aging and drug use. Clin Epigenetics. 2022;14:79. https://doi.org/10.1186/s13148-022-01300-z.
Koczor CA, Ludlow I, Hight RS 2nd, Jiao Z, Fields E, Ludaway T, et al. Ecstasy (MDMA) alters cardiac gene expression and DNA methylation: implications for circadian rhythm dysfunction in the heart. Toxicol Sci. 2015;148:183–91. https://doi.org/10.1093/toxsci/kfv170.
Yu Y, Quan J, Zou M, Zhao W, Su Y, Xu Y. Effects of ketamine-induced H3K9 hypoacetylation during pregnancy on cardiogenesis of mouse offspring. Birth Defects Res. 2023;115:770–81. https://doi.org/10.1002/bdr2.2168.
Tagen M, Mantuani D, van Heerden L, Holstein A, Klumpers LE, Knowles R. The risk of chronic psychedelic and MDMA microdosing for valvular heart disease. J Psychopharmacol. 2023;37:876–90. https://doi.org/10.1177/02698811231190865.
Gemmel M, Rayen I, van Donkelaar E, Loftus T, Steinbusch HW, Kokras N, et al. Gestational stress and fluoxetine treatment differentially affect plasticity, methylation and serotonin levels in the PFC and hippocampus of rat dams. Neuroscience. 2016;327:32–43. https://doi.org/10.1016/j.neuroscience.2016.03.068.
Silva AS, Toffoli LV, Estrada VB, Veríssimo LF, Francis-Oliveira J, Moreira EG, et al. Maternal exposure to fluoxetine during gestation and lactation induces long lasting changes in the DNA methylation profile of offspring’s brain and affects the social interaction of rat. Brain Res Bull. 2018;142:409–13. https://doi.org/10.1016/j.brainresbull.2018.09.007.
Boulle F, Pawluski JL, Homberg JR, Machiels B, Kroeze Y, Kumar N, et al. Developmental fluoxetine exposure increases behavioral despair and alters epigenetic regulation of the hippocampal BDNF gene in adult female offspring. Hormones Behav. 2016;80:47–57. https://doi.org/10.1016/j.yhbeh.2016.01.017.
Cheung HM, Yew DTW. Effects of perinatal exposure to ketamine on the developing brain. Front Neurosci. 2019;13:138. https://doi.org/10.3389/fnins.2019.00138.
Oliveira CDRD, Moreira CQ, Spinosa HDS, Yonamine M. Neurobehavioral, reflexological and physical development of Wistar rat offspring exposed to ayahuasca during pregnancy and lactation. Rev Brasileira de Farmacogn. 2011;21:1065–76.
Li C, Tu G, Luo C, Guo Y, Fang M, Zhu C, et al. Effects of rhynchophylline on the hippocampal miRNA expression profile in ketamine-addicted rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:379–89. https://doi.org/10.1016/j.pnpbp.2018.02.009.
De Luca MT, Badiani A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology. 2011;214:549–56. https://doi.org/10.1007/s00213-010-2062-x.
Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology. 2003;169:21–7. https://doi.org/10.1007/s00213-003-1407-0.
Cole JC, Sumnall HR, O’Shea E, Marsden CA. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology. 2003;166:383–90. https://doi.org/10.1007/s00213-002-1374-x.
Bisagno V, Cadet JL. Histone Deacetylases and Immediate early genes: key players in psychostimulant-induced neuronal plasticity. Neurotox Res. 2021;39:2134–40. https://doi.org/10.1007/s12640-021-00420-3.
Wong CCY, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011;106:480–9. https://doi.org/10.1111/j.1360-0443.2010.03321.x.
Li H, Li C, Zhou Y, Luo C, Ou J, Li J, et al. Expression of microRNAs in the serum exosomes of methamphetamine-dependent rats vs. ketamine-dependent rats. Exp Ther Med. 2018;15:3369–75. https://doi.org/10.3892/etm.2018.5814.
Caputi FF, Palmisano M, Carboni L, Candeletti S, Romualdi P. Opioid gene expression changes and post-translational histone modifications at promoter regions in the rat nucleus accumbens after acute and repeated 3,4-methylenedioxy-methamphetamine (MDMA) exposure. Pharm Res. 2016;114:209–18. https://doi.org/10.1016/j.phrs.2016.10.023.
Mithoefer MC, Mithoefer AT, Feduccia AA, Jerome L, Wagner M, Wymer J, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5:486–97. https://doi.org/10.1016/S2215-0366(18)30135-4.
Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42:155–68. https://doi.org/10.1016/s0165-0173(03)00173-5.
Valvassori SS, da Rosa RT, Dal-Pont GC, Varela RB, Mastella GA, Daminelli T, et al. Haloperidol alters neurotrophic factors and epigenetic parameters in an animal model of schizophrenia induced by ketamine. Int J Dev Neurosci. 2023;83:691–702. https://doi.org/10.1002/jdn.10296.
Cao S-E, Tian J, Chen S, Zhang X, Zhang Y. Role of miR-34c in ketamine-induced neurotoxicity in neonatal mice hippocampus. Cell Biol Int. 2015;39:164–8. https://doi.org/10.1002/cbin.10349.
Xu H, Zhang J, Zhou W, Feng Y, Teng S, Song X. The role of miR-124 in modulating hippocampal neurotoxicity induced by ketamine anesthesia. Int J Neurosci. 2015;125:213–20. https://doi.org/10.3109/00207454.2014.919915.
Huang C, Zhang X, Zheng J, Chen C, Chen Y, Yi J. Upregulation of miR-137 protects anesthesia-induced hippocampal neurodegeneration. Int J Clin Exp Pathol. 2014;7:5000–7.
Yan J, Yu Y, Sun Y, Hu R, Jiang H. Ketamine induces neuronal apoptosis and cognitive disorder via miR-199a-5p/HIF-1α in neonatal rats. Mol Cell Toxicol. 2017;13:395–404. https://doi.org/10.1007/s13273-017-0044-3.
Wang J, Zhou M, Wang X, Yang X, Wang M, Zhang C, et al. Impact of ketamine on learning and memory function, neuronal apoptosis and its potential association with miR-214 and PTEN in adolescent rats. PLoS ONE. 2014;9:e99855. https://doi.org/10.1371/journal.pone.0099855.
Jiang XL, Du BX, Chen J, Liu L, Shao WB, Song J. MicroRNA-34a negatively regulates anesthesia-induced hippocampal apoptosis and memory impairment through FGFR1. Int J Clin Exp Pathol. 2014;7:6760–7.
Li X-J, Yu J-H, Wu X, Zhu X-M, Lv P, Du Z, et al. Ketamine enhances dopamine D1 receptor expression by modulating microRNAs in a ketamine-induced schizophrenia-like mouse model. Neurotoxicology Teratol. 2022;91:107079. https://doi.org/10.1016/j.ntt.2022.107079.
Yao Y, Wang X, Gao J. LncRNA KCNQ1OT1 sponges miR-206 to ameliorate neural injury induced by anesthesia via up-regulating BDNF. Drug Des Dev Ther. 2020;14:4789–4800. https://doi.org/10.2147/DDDT.S256319.
Li X, Saiyin H, Chen X, Yu Q, Ma L, Liang W. Ketamine impairs growth cone and synaptogenesis in human GABAergic projection neurons via GSK-3β and HDAC6 signaling. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01864-5.
Julie E, Sarah LT, Avin V, Janette B, Collin C, Jeanine J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci. 2012;32:4400. https://doi.org/10.1523/JNEUROSCI.5634-11.2012.
Pang X, Su Z, Sun Y, Zhang W, Wang H. Long noncoding RNA SNHG16 reduced ketamine-induced neurotoxicity in human embryonic stem cell-derived neurons. J Chem Neuroanat. 2018;94:39–45. https://doi.org/10.1016/j.jchemneu.2018.08.005.
Jiang JD, Zheng XC, Huang FY, Gao F, You MZ, Zheng T. MicroRNA-107 regulates anesthesia-induced neural injury in embryonic stem cell derived neurons. IUBMB Life. 2019;71:20–7. https://doi.org/10.1002/iub.1911.
Li X, Saiyin H, Zhou J-H, Yu Q, Liang W-M. HDAC6 is critical for ketamine-induced impairment of dendritic and spine growth in GABAergic projection neurons. Acta Pharmacol Sin. 2021;42:861–70. https://doi.org/10.1038/s41401-020-00521-3.
Wang X-S, Li L-C, Zhang X, Gao J. Lipoxin A4 methyl ester protects PC12 cells from ketamine-induced neurotoxicity via the miR-22/BAG5 pathway. Hum Exp Toxicol. 2021;40:S519–S529. https://doi.org/10.1177/09603271211051602.
Huang J, Xu Y, Wang F, Wang H, Li L, Deng Y, et al. Long Noncoding RNA SPRY4-IT1 modulates Ketamine-induced neurotoxicity in human embryonic stem cell-derived neurons through EZH2. Dev Neurosci. 2021;43:9–17. https://doi.org/10.1159/000513535.
Zheng X, Lin C, Li Y, Ye J, Zhou J, Guo P. Long noncoding RNA BDNF-AS regulates ketamine-induced neurotoxicity in neural stem cell derived neurons. Biomed Pharmacother. 2016;82:722–8. https://doi.org/10.1016/j.biopha.2016.05.050.
Meng L, Liu B, Ji R, Jiang X, Yan X, Xin Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol Lett. 2019;17:2031–9. https://doi.org/10.3892/ol.2018.9854.
Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10:31. https://doi.org/10.1186/s13578-020-00396-1.
Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30:1181–97.
Holze F, Gasser P, Müller F, Dolder PC, Liechti ME. Lysergic acid diethylamide–assisted therapy in patients with anxiety with and without a life-threatening illness: a randomized, double-blind, placebo-controlled phase II study. Biol Psychiatry. 2023;93:215–23.
Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol. 2020;34:155–66.
Feinberg AP, Gehrke CW, Kuo KC, Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988;48:1159–61.
Howard G, Eiges R, Gaudet F, Jaenisch R, Eden A. Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene. 2008;27:404–8. https://doi.org/10.1038/sj.onc.1210631.
Schuebel KE, Chen W, Cope L, Glöckner SC, Suzuki H, Yi J-M, et al. Comparing the DNA hypermethylome with gene mutations in human colorectal cancer. PLoS Genet. 2007;3:e157. https://doi.org/10.1371/journal.pgen.0030157.
Wojtas A, Bysiek A, Wawrzczak-Bargiela A, Szych Z, Majcher-Maślanka I, Herian M, et al. Effect of psilocybin and ketamine on brain neurotransmitters, glutamate receptors, DNA and rat behavior. Int J Mol Sci. 2022;23.
Barnett BS, Ziegler K, Doblin R, Carlo AD. Is psychedelic use associated with cancer?: Interrogating a half-century-old claim using contemporary population-level data. J Psychopharmacol. 2022;36:1118–28. https://doi.org/10.1177/02698811221117536.
Simonsson O, Hendricks PS, Carhart-Harris R, Kettner H, Osika W. Association between lifetime classic psychedelic use and hypertension in the past year. Hypertension. 2021;77:1510–6. https://doi.org/10.1161/hypertensionaha.120.16715.
He G-N, Bao N-R, Wang S, Xi M, Zhang T-H, Chen F-S. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Dev Ther. 2021;15:3965–78. https://doi.org/10.2147/DDDT.S332847.
Jakovcevski M, Akbarian S, Di Benedetto B. Pharmacological modulation of astrocytes and the role of cell type-specific histone modifications for the treatment of mood disorders. Curr Opin Pharm. 2016;26:61–6. https://doi.org/10.1016/j.coph.2015.10.002.
Filošević Vujnović A, Stanković Matić I, Saftić Martinović L, Dević Pavlić S. Breaking the chains: advances in substance addiction research through single-cell sequencing, epigenetics, and epitranscriptomic. Future Pharm. 2024;4:115–38.
Engelmann J, Zillich L, Frank J, Wagner S, Cetin M, Herzog DP, et al. Epigenetic signatures in antidepressant treatment response: a methylome-wide association study in the EMC trial. Transl Psychiatry. 2022;12:268. https://doi.org/10.1038/s41398-022-02032-7.
Hartogsohn I. Set and setting, psychedelics and the placebo response: an extra-pharmacological perspective on psychopharmacology. J Psychopharmacol. 2016;30:1259–67. https://doi.org/10.1177/0269881116677852.
Andersen PR, Gibbs P, Kubinski H. Effects of neuropharmacological agents on in vitro formation of complexes between nucleic acids and proteins. Neuropharmacology. 1974;13:111–7. https://doi.org/10.1016/0028-3908(74)90028-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Antonio Inserra has received postdoctoral fellowships from the CIHR (Canadian Institutes of Health Research), FRQS (Fonds de Recherche Santé du Québec), and the Canadian Neurodevelopmental Research Training Platform (CanRT). Antonella Campanale has received Graduate Excellence Awards form the Faculty of Medicine of McGill University. Tamim Rezai, Patrizia Romualdi, and Tiziana Rubino have no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Inserra, A., Campanale, A., Rezai, T. et al. Epigenetic mechanisms of rapid-acting antidepressants. Transl Psychiatry 14, 359 (2024). https://doi.org/10.1038/s41398-024-03055-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03055-y
This article is cited by
-
The compulsive eating paradigm: can psychedelics help in treating obesity?
Journal of Eating Disorders (2025)
-
Psilocybin treatment extends cellular lifespan and improves survival of aged mice
npj Aging (2025)
-
Milnacipran and Vanillin Alleviate Fibromyalgia-Associated Depression in Reserpine-Induced Rat Model: Role of Wnt/β-Catenin Signaling
Molecular Neurobiology (2025)
-
The HPA axis and kynurenine pathway: exploring the role of stress and neuroinflammation in treatment-resistant depression
Pharmacological Reports (2025)