Abstract
The concept of resilience has changed over time and nowadays it refers to the positive adaptation to life adversities, rather than to the absence of a pathological response normally occurring in susceptible people. Based on our previous data showing that the exposure to the chronic mild stress (CMS) paradigm differently affected bioenergetics in the ventral hippocampus of vulnerable and resilient animals, here we investigated whether resilience is a stable trait or if the energetic strategy set in motion to sustain resilience unveils a vulnerability feature in a more dynamic situation. To this aim, vulnerable and resilient rats after 6 weeks of CMS were subjected to a further acute, unfamiliar restraint stress (ARS) and metabolomic studies were conducted in the ventral hippocampus. We observed that exposure to a single novel challenge negatively affects the fuel utilization of resilient animals. Indeed, while they increase glycolysis to sustain the non-hedonic phenotype when exposed to CMS, they shift to fatty acid β-oxidation after ARS, as vulnerable animals following CMS, suggesting that the energy strategy that guarantees resilience is fragile and can be negatively modified by a different environmental condition. These results suggest that strengthening resilience to foster individuals to bounce back from stressful life events may represent a strategy to decrease vulnerability or prevent the risk of relapsing to a pathological state.
Similar content being viewed by others
Introduction
While for years resilience has referred to the ability of an individual to avoid the negative consequences of adversity [1], more recently it has been defined as an active and adaptive process and not merely the absence of a pathological response that usually occurs in vulnerable persons. Accordingly, it is not a fixed trait, but it may be considered as a dynamic process in which people progress through [2].
Stressful life events precipitate symptoms of depressive disorders [3]. Accordingly, several animal models of depression have been based on stress exposure, such as the chronic mild stress (CMS) model, to capture the neural substrates associated with vulnerability or resilience to develop such pathologies. Interestingly, it has been demonstrated that chronic stress alters the responses to subsequent novel stressors at behavioural and molecular levels by opening a window of plasticity in vulnerable rats [4].
So far most of the studies focused on the systems involved in the vulnerability while recently a growing attention has been paid to the molecular mechanisms underlying resilience.
Recently, we observed that resilience to develop the anhedonic-like behavior following chronic stress exposure in adult male rats was associated with the activation of the Gr-Rack1-Bdnf pathway [5], with the trigger of mitochondrial fission [6], and with the utilization of specific substrates to sustain the energy cost of resilience in the ventral hippocampus [6, 7].
Moreover, considering the concept of the trajectory of resilience [2] and based on the finding that a previous history of chronic stress determines the response to a subsequent challenge [4], we demonstrated that resilient rats exposed to an unfamiliar stressor following a short period of CMS preserved the ability to face the novel challenge in term of neuroplastic mechanisms, capacity that was impaired in rats vulnerable to CMS [8].
Here, our goal was to clarify whether the energetic strategy set in motion to sustain resilience is a stable trait or it veils a feature of vulnerability when challenged by an unexpected event. We focused on the ventral hippocampus (vHip), because all the data mentioned above support a crucial role for this brain region, which is indeed a critical hub for the processing of emotion, in the different response to stress.
To this aim, after 6 weeks of CMS, half of vulnerable and resilient animals were exposed to one hour of acute restraint stress (ARS), a stressor that was not included in the CMS protocol and therefore we can define as unfamiliar and sacrificed one hour later for the metabolomic study. For the first time we demonstrated that, although resilience is an energy-demanding active process, the exposure to a single novel challenge is able to affect the fuel utilization of resilient animals, by switching the utilization of glucose to fatty acids, as previously observed in vulnerable animals [7], suggesting that the energetic strategy that sustained resilience may precipitate, by changing the environmental factor, to a metabolic condition resembling that observed in anhedonic rats. It follows that fostering the mechanisms activated to sustain resilience may play a vital role in avoiding the recurrence of a molecular pathological state at least in the ventral hippocampus.
Material and methods
Animals
Adult male Wistar rats (5 weeks old) (Charles River, Germany) were brought into the animal facility one month before the start of the experiment and were housed in standard laboratory conditions: food and water were freely available on a 12-h light/dark, constant temperature (22 ± 2 °C) and humidity (50 ± 5%). All procedures used in this study have conformed to the rules and principles of the 2010/63 European Communities Council Directive and have been approved by the Local Bioethical Committee at the Maj Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland. All efforts were made to minimize animal suffering and to reduce the number of animals used. The design and procedure of the animal study are conformed to the ARRIVE guidelines. All the procedures were done blindly by experimenters unaware of the experimental group of animals.
Chronic mild stress (CMS) protocol and acute restraint stress (ARS)
Animals were trained to consume 1% sucrose solution, following 14 h food and water deprivation, once a week for 1 h before the starting of the experiment. Throughout the whole experiment, sucrose consumption was similarly monitored at weekly intervals by weighing pre-weighed bottles containing the sucrose solution at the end of the 1-h test.
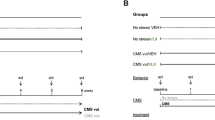
After this period of training, rats (9 weeks old) were randomly divided into two groups. One group of animals was subjected for 6 consecutive weeks to the CMS protocol that consisted of a random combination of periods of stress of the duration of 10–14 h, including food or water deprivation, 45° cage tilt, intermittent illumination every 2 h, soiled cage with 250 ml water in sawdust bedding, paired housing, low intensity stroboscopic illumination-150 flashes/min, and periods of no stress. The other group (No stress) was housed in separate rooms and had no contact with the chronically stressed animals (Fig. 1A).
Panel A: experimental design; panel B: sucrose intake measured through the sucrose consumption test that was performed at weekly intervals during the whole experiment. The cut off to discriminate CMS vul and CMS res was set at mean of sucrose intake at the last week of CMS minus 2sd. The data are the mean ± SEM of 15 independent determinations. ***p < 0.001 vs No stress. (Two-way ANOVA with repeated measures, Tukey multiple comparison test).
Based on the results of the sucrose consumption test obtained at the last week of CMS, we identified animals showing the anhedonic-like behaviour (sucrose intake minor of the mean of the control group at the 6th week of CMS—2 standard deviation (sd)), named CMS vulnerable (CMS vul), and animals showing a similar hedonic phenotype to non-stressed animals (sucrose intake major than the mean of the control group at the 6th week of CMS—2sd), named CMS resilient (CMS res). At the end of the 6th weeks of CMS, each experimental group was randomly divided into two subgroups and was subjected to 1 h of acute restraint stress (ARS), a kind of stressor to which animals were not presented before. Furthermore, ARS was the last stressor to which CMS vul, and CMS res were subjected.
The No stress/ARS (n = 8), CMS vul/ARS (n = 7) and CMS res/ARS (n = 8) were sacrificed one hour after the ARS whereas the naïve counterparts (No stress/Naïve (n = 8), CMS vul/Naive (n = 8) and CMS res/Naïve (n = 7)) were sacrificed 24 h after the last CMS stressor (Fig. 1A) for the collection of ventral hippocampus (vHip) (plates 34–43 according to the atlas of Paxinos and Watson [9]) that was stored at −80 °C.
Metabolomic analysis
Metabolomic data were acquired with a liquid chromatography coupled to tandem mass spectrometry to obtain metabolomic data by using an API-3500 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA) coupled with an ExionLCTM AC System (AB Sciex, Framingham, MA, USA). 10 mg of ventral hippocampus were used for the analysis. Half tissue was smashed in 250 µl of ice-cold methanol/acetonitrile 50:50, while the second half was lysed in 250 µl of ice-cold water/methanol 20:80, respectively. Both solutions contained [U-13C6]-glucose (Merck Life Science S.r.l, Milano, Italy) 1 ng/µl and [U-13C5]-glutamine (Merck Life Science S.r.l, Milano, Italy) 1 ng/µl as internal standards. Lysates were spun at 2000 g for 5 min at 4 °C and supernatants were then passed through a regenerated cellulose filter (4 mm Ø). Samples were then dried under N2 flow at 40 °C and resuspended in 100 µl of methanol for subsequent analysis.
A cyano-phase LUNA column (50 × 4.6 mm, 5 µm; Phenomenex, Torrance, CA, USA) by a 5 min run in negative ion mode with two separated runs was used to quantify energy metabolites. The protocols employed for both detection and quantification are reported in detail in [6]. All metabolites analysed were previously validated by pure standards and the instrument sensitivity was checked with internal standards.
MultiQuant™ software (version 3.0.3, AB Sciex, Framingham, MA, USA) was used for data analysis and peak review of chromatograms. For data processing, raw areas were normalized by the median of all metabolite areas in the same sample. The data were then transformed by generalized log-transformation and Pareto scaled to correct for heteroscedasticity, reduce the skewness of the data, and reduce mask effects [10]. In detail, obtained values were transformed by generalized log (glog) as follows:
where a is a constant with a default value of 1 and x is the sample area for a given metabolites [11]. Then, obtained values underwent Pareto scaling as follows:
where xij is the transformed value in the data matrix (i (metabolites), j (samples)) and si is the standard deviation of transformed metabolite values [12]. Obtained values were considered as relative metabolite levels. Data processing and analysis were performed by MetaboAnalyst 5.0 web tool (https://www.metaboanalyst.ca [13]).
Statistical analysis
The analyses of the behavioural and molecular data were performed with the two way analysis of variance (ANOVA), followed by Tukey multiple comparison test. Each experimental group consists of 7–8 rats. A priori sample size estimation was done with the software G*power 3.1.9.2 (effect size = 0.4, α error probability = 0.05, power = 0.8, degree of freedom = 1), considering the between-subjects factor of stress.
Partial least squares discriminant analysis (PLS-DA) identified differential metabolites between experimental groups and the ranking of metabolites was performed by variable importance in projection (VIP), according to metabolite importance in discriminating groups. A false discovery rate (FDR) < 0.05 was considered in the VIP score analysis to determine the feature selection.
The permutation test was used with a maximum of 1000 permutations, with a separation distance test to assess the significance of class discrimination determined by PLS-DA. Classification and cross-validation were performed with a maximum of 5 components using the leave-one-out method.
Significance for all tests was assumed for p < 0.05. Data are presented as means ± standard error (SEM).
Results
Hedonic-like status in response to the chronic mild stress defined vulnerable and resilient animals
Two-way ANOVA with repeated measures showed a significant effect of stress on sucrose intake (F2-44 = 62.9, p < 0.001). Tukey multiple comparison test revealed that a subgroup of stressed rats, named CMS vulnerable (CMS vul), consumed significantly less sucrose during the test in comparison to non-stressed animals starting from the first week of CMS to the last week of stress protocol (week1: −4.48 g, p < 0.001; week2: −7.37 g, p < 0.001; week3: −6.53 g, p < 0.001; week4: −8.62 g, p < 0.001; week5: −7.82 g, p < 0.001; week6: −7.49 g, p < 0.001 vs No stress/same week), whereas another subgroup, named CMS resilient (CMS res), showed a sucrose intake similar to control animals (Fig. 1B).
Exposure to an acute restraint stressor induced specific changes in the metabolomic profile of vulnerable and resilient animals
To assess the effect of the exposure to an acute stressor on the metabolic asset in vulnerable and resilient rats, we performed a metabolomic analysis in the ventral hippocampus.
As shown in Fig. 2, we observed a different metabolic profile in response to the acute challenge in the subgroups of rats exposed to CMS. Indeed, in the partial least squares discriminant analysis (PLS-DA) plot we observed that No stress/Naive, No stress/ARS, CMS vul/Naive, CMS vul/ARS, CMS res/Naive and CMS res/ARS were grouped into separated clusters (Fig. 2A), with the results being statistically significant (p = 0.026) (Fig. 2B). Moreover, as depicted in Fig. 2C, PLS-DA and ANOVA (FDR < 0.05) identified the most important 25 metabolites.
Further, with the one-way ANOVA and an FDR < 0.05, we identified significant variations of several metabolites (represented as Z-score) reported in the heat map (Fig. 3), which showed the ARS-induced changes of the several metabolites in control, vulnerable and resilient animals.
Considering the major classes of metabolites investigated (Fig. 4—list of metabolites in the Supplementary Table 1), two way ANOVA of the metabolic classes revealed a significant effect of CMS (F2-44 = 3.59, p < 0.05) and of CMS X ARS interaction (F2-44 = 3.15, p < 0.05) on the class of amino acids (AA), with these latter molecules levels being increased specifically in naïve resilient animals (+36%, p < 0.05 vs No stress/Naive) but not in the group subjected to the ARS. Similarly, glycolysis intermediates were significantly modulated by the CMS (F2-44 = 5.96, p < 0.01) with a significant CMS X ARS interaction (F2-44 = 3.28, p < 0.05), supporting an increase in resilient naive rats in comparison to the non stressed counterpart (+116%, p < 0.01 vs No stress/Naive), which is no longer observed after exposure to ARS. Considering the acylcarnitines class, we found a borderline effect of CMS (F2-44 = 3.01, p = 0.06) and a significant CMS X ARS interaction (F2-44 = 9.83, p < 0.001). Indeed, we observed a significant reduction of acylcarnitines specifically in resilient animals (−33%, p < 0.05 vs No stress/Naive) while ARS leads to an increase in the same group (+68%, p < 0.05 vs CMS res/Naive). On the contrary, two-way ANOVA analysis did not reveal any statistically significant change for dNTPs, NTPs and TCA cycle class of metabolites.
Each cluster contains the metabolites listed in Supplementary Table 1. The cluster AA contains the metabolites from Glu to alpha-AAA; the cluster Glycolysis contains the metabolites from glucose to PEP; the cluster Acylcarnitines contains the metabolites from free carnitine to C18:1-Carnitine; the cluster dNTPs contains the metabolites from dAMP to dIMP; the cluster NTPs contains the metabolites from ATP to ITP; the cluster TCA cycle contains the metabolites from AcCoa to CoA. *p < 0.05, **p < 0.01 vs No stress/Naïve; #p < 0.05 vs CMS vul/Naïve; §p < 0.05vs CMS res/Naïve (Two-way ANOVA, Tukey multiple comparison test). Metabolite class relative levels were obtained starting from raw peak areas normalized by the median of all metabolite areas in the same sample. The data were then transformed by generalized log-transformation and Pareto scaled and finally grouped in each cluster as detailed above.
In detail (Fig. 5—Supplementary Table 1), considering the effect of ARS on metabolite levels, Tukey multiple comparison test revealed that aspartate (Asp), methionine (Met), tryptophan (Trp) and free-carnitine were modulated by ARS specifically in the No stress group whereas IMP, cAMP, succinate, glucuronate-6P and NAD+ were differently expressed in vulnerable animal subjected to ARS with respect to their naïve counterpart. Moreover, ARS led to decreased levels of the glycolysis intermediates fructose 1,6-bisphospate (Fructose Bis-P) and dihydroxyacetone-P/glyceraldehyde-3P (DHAP/GAP), and to increased levels of spermidine and of many acylcarnitines (C2-Carnitine, C12-Carnitine, C14-Carnitine, C16-Carnitine, C16:1-Carnitine, C18:1-Carnitine) specifically in resilient rats. Accordingly, resilient animals subjected to the ARS showed increased C2-Carnitine/C0-Carnitine and (C2-Carnitine+ C3-Carnitine/C0-Carnitine) ratio, indirect marker of boosted fatty acid beta-oxidation (Fig. 5), as well as a trend to increase in the (C16:0-Carnitine+ C18:0-Carnitine/C0-Carnitine), indicator of the activity of the Carnitine palmitoyl-transferase (CptI).
Discussion
In this study we found that despite resilient animals did not develop the anhedonic phenotype after 6 weeks of CMS, they were not able to deal with an acute challenge in terms of ventral hippocampus bioenergetics. Indeed, they activated the fatty acid β-oxidation, as vulnerable animals did under basal conditions [7], suggesting that a novel stressful experience can falter the metabolic status of resilient animals, which seem to favor glycolysis, in this brain region, to counteract chronic stress exposure.
These results indicate that resilience is an active process that protects the animal but, apparently, at a high (energy) cost since subsequent exposure to a novel acute stress is sufficient to switch fuel utilization.
At behavioural level, as previously demonstrated [6,7,8, 14, 15], using the sucrose consumption test, we differentiated between animals manifesting the anhedonic-like behavior and animals being resilient thus showing a similar profile to control rats.
Analyzing the whole metabolomic data set, we observed that amino acid levels are increased in unstressed animals after ARS as well as in resilient animals.
Interestingly, it seems that the phenotypic differences observed in the behavioral responses to stress were associated also with sustained anaplerosis at the amino acid level, an enzymatic reaction that replenishes the pools of metabolic intermediates in the TCA cycle, a reaction fundamental to maintaining a balanced neuronal state and conferring metabolic resilience [16]. Accordingly, stress resilience is promoted by branched-chain amino acid supplementation in mice through the activation of the brain derived neurotrophic factor (BDNF) signaling within the hippocampus [17], which we showed increased in rats resilient to 2 weeks of CMS [5].
Moreover, amino acids are used in the brain for the synthesis of neurotransmitters and neuromodulators [18] and, in line, the increase in tryptophan levels in the resilient group was paralleled by a trend toward an increase of serotonin level suggesting a beneficial effect on stress response. Additionally, methionine influences the cellular response to oxidative stress through the production of the antioxidant glutathione [19]. Taken together, these results suggest that these amino acids contribute to coping with stress, as a positive reaction to the acute challenge and as a positive strategy set in motion to sustain resilience.
Shifting to the vulnerable group, we found a more heterogenous scenario since metabolites belonging to different classes were modulated by ARS, such as IMP, cAMP, succinate, glucuronate 6P and NAD+.
When we considered the effect of the acute challenge on animals resilient to chronic stress, we observed that they decreased the levels of the glycolysis in favor to an enhancement of fatty acid β-oxidation as a reaction to the novel stressor. In line with our recent finding demonstrating that resilient animals increased ventral hippocampus glycolysis to produce energy probably to sustain the non-pathological behavioral phenotype [7], while vulnerability is characterized by a shift to fatty acid β-oxidation, the present results suggest that, despite the normal hedonic phenotype, resilient animals show impaired metabolic plasticity when they are requested to face with a new challenge. Actually, even if resilience is known as an active process necessary to maintain and achieve healthy functioning despite adverse events, our metabolic data suggest that the ventral hippocampus seems to shift the substrate utilization to react with the acute stress, similarly to vulnerable animals after a chronic stress exposure [7, 20]. Indeed, it seems that the utilization of lipids to produce energy is set in motion as an emergency mode when the system is no longer able to respond in term of glycolysis. Indeed, although resilience seems to be, from a behavioral point of view, a stable trait maintained by specific molecular mechanisms of adaptation to chronic stress exposure, the presentation of a different event (i.e., exposure to an unfamiliar challenge) may precipitate the “non pathological” phenotype and unmask the frailty, suggesting the possibility that the previous history of chronic stress left some scars of vulnerability.
The choice of a single time-point of sacrifice following acute stress may represent a limitation of our study and we cannot exclude that vulnerable and resilient animals engaged distinct metabolic strategies with different kinetics that may point to a shift, a prolonged response or a lack of termination in substrates utilization. However, such choice is based on our previous studies demonstrating a positive effect on cognitive function associated with several molecular changes, including neuroplastic and antioxidant markers, not only in the ventral subregion of the hippocampus but also in the dorsal counterpart and within the prefrontal cortex [8, 21, 22]. In addition, since we are aware that vulnerability and resilience to stress are dependent on the response domain being studied, we will go beyond the limitation of having considered only the hedonic like behavior by stratifying, in our future studies, vulnerable and resilient animals based on other behavioral readouts. Moreover, although it is well-established that the development of stress-related disorders is influenced by sex, studies on females are lacking; thus, we will plan our future studies including females.
Our findings further emphasize that resilience to chronic mild stress is indeed demanding, at least in terms of energy cost. The system, therefore, implements a series of adaptive mechanisms to preserve the condition of resilience, the main of which is the increase in glycolysis, but the subsequent exposure to further, unexpected stress, evidently exhausts the energy reservoir of the cell shifting the energy balance towards fatty acid catabolism as it occurs in vulnerable animals. All in all, these data confirm previous findings [7] and further point out the involvement of fatty acid β-oxidation not only as a mechanism of vulnerability but also as a process that may underlie relapse. The concept of different fuel utilization as mechanism supporting vulnerability and resilience but also as strategy to react to a novel situation has to be more extensively explored and extended to other brain regions and especially to the blood. The idea is to identify potential biomarkers that are easily measurable in patients with stress-related disorders, to assess the usefulness of the biomarkers’ signature for predictive diagnosis and for improving the monitoring and treatment of MDD.
These results allow us to hypothesize that strengthening resilience to foster individuals to bounce back from stressful life events may represent a strategy to decrease or prevent the risk of stress-related disorders.
Data availability
The data that support the findings of this study are available from the corresponding author FC, upon reasonable request.
References
Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84.
Bhatnagar S. Rethinking stress resilience. Trends Neurosci. 2021;44:936–45.
McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109:17180–5.
Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nisticò R, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA. 2015;112:14960–5.
Brivio P, Buoso E, Masi M, Gallo MT, Gruca P, Lason M, et al. The coupling of RACK1 with the beta isoform of the glucocorticoid receptor promotes resilience to chronic stress exposure. Neurobiol Stress. 2021;15:100372.
Brivio P, Audano M, Gallo MT, Gruca P, Lason M, Litwa E, et al. Metabolomic signature and mitochondrial dynamics outline the difference between vulnerability and resilience to chronic stress. Transl Psychiatry. 2022;12:87.
Brivio P, Audano M, Gallo MT, Miceli E, Gruca P, Lason M, et al. Venlafaxine’s effect on resilience to stress is associated with a shift in the balance between glucose and fatty acid utilization. Neuropsychopharmacology 2023. https://doi.org/10.1038/s41386-023-01633-0.
Brivio P, Gallo MT, Gruca P, Lason M, Litwa E, Fumagalli F, et al. Resilience to chronic mild stress-induced anhedonia preserves the ability of the ventral hippocampus to respond to an acute challenge. Eur Arch Psychiatry Clin Neurosci. 2022. https://doi.org/10.1007/s00406-022-01470-0.
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sixth Edition. Elsevier Academic Press; 2007.
Ghaffari MH, Jahanbekam A, Sadri H, Schuh K, Dusel G, Prehn C, et al. Metabolomics meets machine learning: longitudinal metabolite profiling in serum of normal versus overconditioned cows and pathway analysis. J Dairy Sci. 2019. https://doi.org/10.3168/jds.2019-17114.
Durbin BP, Hardin JS, Hawkins DM, Rocke DM. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics. 2002 https://doi.org/10.1093/bioinformatics/18.suppl_1.S105.
van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 2006. https://doi.org/10.1186/1471-2164-7-142.
Pang Z, Zhou G, Ewald J, Chang L, Hacariz O, Basu N, et al. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc. 2022;17:1735–61.
Brivio P, Sbrini G, Tarantini L, Parravicini C, Gruca P, Lason M, et al. Stress modifies the expression of glucocorticoid-responsive genes by acting at epigenetic levels in the rat prefrontal cortex: modulatory activity of lurasidone. Int J Mol Sci. 2021;22:6197.
Calabrese F, Brivio P, Sbrini G, Gruca P, Lason M, Litwa E, et al. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: the potential role for glucocorticoid receptor signalling. J Psychopharmacol. 2020;34:420–8.
Motori E, Atanassov I, Kochan SM V., Folz-Donahue K, Sakthivelu V, Giavalisco P et al. Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci Adv. 2020; 6. https://doi.org/10.1126/sciadv.aba8271.
Nasrallah P, Haidar EA, Stephan JS, El Hayek L, Karnib N, Khalifeh M, et al. Branched-chain amino acids mediate resilience to chronic social defeat stress by activating BDNF/TRKB signaling. Neurobiol Stress. 2019;11:100170.
Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol (Lausanne). 2019; 10. https://doi.org/10.3389/fendo.2019.00158.
Martínez Y, Li X, Liu G, Bin P, Yan W, Más D, et al. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49:2091–8.
Hamilton PJ, Chen EY, Tolstikov V, Peña CJ, Picone JA, Shah P, et al. Chronic stress and antidepressant treatment alter purine metabolism and beta oxidation within mouse brain and serum. Sci Rep. 2020;10:18134.
Brivio P, Sbrini G, Riva MA, Calabrese F. Acute stress induces cognitive improvement in the novel object recognition task by transiently modulating bdnf in the prefrontal cortex of male rats. Cell Mol Neurobiol. 2020;40:1037–47.
Spero V, Paladini MS, Brivio P, Riva MA, Calabrese F, Molteni R. Altered responsiveness of the antioxidant system in chronically stressed animals: modulation by chronic lurasidone treatment. Psychopharmacology. 2022;239:2547–57.
Funding
The behavioral part of the study was supported by the statutory activity of the Maj Institute of Pharmacology Polish Academy of Sciences (Krakow, Poland). The molecular study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca to F.C. (PRIN2022, Project number 2022P45ZSZ), by the Ministry of University and Research (MUR) Progetto Eccellenza (2023–2027) to the Department of Pharmacological and Biomolecular Sciences “Rodolfo Paoletti”, Università degli Studi di Milano, partially by the Italian Ministry of Health with Ricerca Corrente and 5×1000 funds to N.M and by Piano di Sostegno alla Ricerca from Università degli Studi di Milano. Maria Teresa Gallo was supported by cycle XXXVII of the doctorate in Pharmacological Biomolecular Sciences, Experimental and Clinical, Department of Pharmacological and Biomolecular Sciences “Rodolfo Paoletti”, Università degli Studi di Milano.
Author information
Authors and Affiliations
Contributions
PB: Conceptualization, Formal analysis, Data curation, Investigation, Writing original draft. MTG: Formal analysis. MA: Methodology, Formal analysis. GG: Formal analysis. PG: Methodology. ML: Methodology. EL: Methodology. FF: Review and editing. MP: Methodology, Review and editing. NM: Data curation, Review and editing. FC: Conceptualization, Visualization, Writing original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Brivio, P., Gallo, M.T., Audano, M. et al. Exposure to an acute stress impaired the metabolic plasticity of resilient rats by enhancing fatty acid β-oxidation in the ventral hippocampus. Transl Psychiatry 14, 366 (2024). https://doi.org/10.1038/s41398-024-03080-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03080-x