Abstract
Many psychiatric disorders, such as anxiety disorders, are characterized by abnormal processing of fear-related information. Parvalbumin (PV) neurons in the prelimbic cortex (PL) are critically involved in fear expression. However, the role of plasticity of the local PV neuron network in the regulation of remote fear memory remains unknown. In this study, we showed that the retrieval of both recent and remote fear memory induced the high-PV plasticity in the PL. Acute chemogenetic inhibition of PV neurons in the PL decreased recent fear memory retrieval and suppressed the fear-induced shift toward high-PV neurons, while these effects were not observed three weeks after chemogenetic inhibition. On the other hand, chronic inhibition of these neurons led to a sustained reduction in fear memory retrieval and persistent suppression of fear-induced high-PV plasticity. Notably, voluntary running mimicked the effects of chronic inhibition of PV neurons and decreased the expression of fear memory, which could be blocked by chemogenetic activation of PV neurons. Together, these findings indicate an essential role for PV network plasticity in the PL in regulating fear memory expression and provide alternative methods for the treatment of certain anxiety disorders.
Similar content being viewed by others
Introduction
Learning to anticipate danger in the environment and adjusting behavior accordingly is essential for survival. Memories of threatening (“fearful”) events can last a lifetime. However, when fear to a threat becomes excessive and persistent, it may lead to psychiatric conditions such as anxiety disorder and post-traumatic stress disorder (PTSD) [1, 2]. Understanding the mechanisms regulating remote fear memory retrieval is critical for understanding the neurobiology of fear expression and for developing treatments for certain anxiety disorders.
Converging evidence indicates that the prelimbic cortex (PL) is necessary for the expression of contextual and tone-cued fear memories beginning hours after conditioned fear training and lasting for weeks thereafter [3,4,5,6]. Reducing PL activity decreases remote memory retrieval [7]; accordingly, in several studies, researchers have been able to alter the activity of the PL and its subcortical downstream targets in memory retrieval tasks [4, 8, 9]. The activity of long-projecting pyramidal neurons is tightly regulated by locally projecting GABAergic neurons [10], among which parvalbumin (PV) neurons are known to inhibit pyramidal neurons through perisomatic and proximal dendritic inhibition [11]. Thus, PV neuronal activity in the PL is also critically involved in fear expression [12, 13]. It has been reported that the activities of PV neurons are negatively correlated with fear behavior and that the inhibition of PV neuronal activity disinhibits prefrontal pyramidal neurons, ultimately leading to fear expression [12]. Moreover, the plasticity of PV neuron networks has been shown to be involved in long-term memory consolidation [14,15,16]. PV neuron networks comprising neurons that express low levels of the PV and the key GABA-synthesizing enzyme GAD67, while exhibiting strong inhibitory connectivity onto these neurons, are referred to as ‘low-PV configuration’ networks [17]. In contrast, networks comprising neurons expressing high levels of PV and GAD67 and exhibiting strong excitatory connectivity onto them, are referred to as ‘high-PV configuration” networks [17]. Notably, low-PV neurons and high-PV neurons present distinct molecular properties, suggesting that they have different functions [18]. Indeed, low-PV configuration networks promote memory acquisition, whereas high-PV configuration networks impede it [14,15,16]. Mice engaged in fear memory retrieval display an increased proportion of high-PV configuration networks in the PL [18]. Although an increasing number of studies have elucidated the role of PV neurons in the regulation of fear expression, the role of local network plasticity in the regulation of fear memory remains largely unknown. Physical exercise is a widely recognized nonpharmacological method for alleviating anxiety disorders [19, 20]. It has been reported that environmental enrichment, a paradigm that provides a range of opportunities for motor, visual, and somatosensory stimulation, can induce the formation of a low-PV configuration network [16]. This raises the possibility that physical exercise could reduce fear memory and alterations in PV neuron network configurations.
In this study, we addressed this issue by showing that the chronic chemogenetic inhibition of PV neurons in the PL induced persistently low plasticity of PV neuron networks and a sustained reduction in fear memory expression. In addition, voluntary running mimicked the effects of chronic inhibition of PV neurons and decreased the expression of fear memory, which could be blocked by chemogenetic activation of PV neurons. These findings suggest that PV neuron network plasticity in the PL plays an important role in the regulation of remote memory retrieval and might shed new light on methods for the treatment of anxiety disorders.
Materials and methods
Animals
The mice had ad libitum access to water and food and were maintained on a 12 hr light/dark cycle. PV-Cre mice were described previously [18]. Behavioral testing was performed during the light cycle between 1:00 P.M. and 5:00 P.M. Only adult male mice were used in the current study.
Virus packaging and stereotaxic injection
AAV vectors were packaged into serotype 2/2 vectors that consisted of the AAV2 ITR genome pseudotyped with AAV2 serotype capsid proteins. AAV-EF1α-DIO-hM3D(Gq)-mCherry (3.5 × 1012 particles/ml), AAV-EF1α-DIO-hM4D(Gi)-mCherry (3.5 × 1012 particles/ml), and AAV-EF1α-DIO-mCherry (4 × 1012 particles/ml) were packaged by Sunbio (Shanghai, China).
All surgeries were performed under aseptic conditions and stereotaxic guidance. The mice were anesthetized with 1% pentobarbital sodium and placed into a stereotaxic instrument (RWD, China). All coordinates are relative to bregma and are expressed in mm. Surgery was performed to transfect the somata of PL cells with viruses (from bregma: +1.75 mm anteroposterior (AP), ±0.3 mm mediolateral (ML), and −2.45 mm dorsoventral (DV)). Viral vectors (0.25 µl) were bilaterally injected into the PL using a 5-µl Hamilton syringe (65460–02, Hamilton, USA) and a microinjector pump (KDS, Stoelting, USA) at a rate of 0.05 µl/min. After completion of the injection, the needle was raised 0.1 mm for an additional 10 min to allow diffusion of the virus into the injection site, after which it was slowly withdrawn. The mice were allowed to recover for two weeks after surgery to allow gene expression.
At the end of the experiments, the mice were perfused for histological verification. Only mice in which viral expression was restricted to the target region were used for subsequent analysis.
Histology
Anesthetized mice were transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS (pH 7.4). The brains were fixed overnight in 4% PFA and then equilibrated in 30% sucrose in PBS. For histological verification, 40 µm-thick coronal slices were cut on a freezing microtome after the brains were dehydrated in sucrose solution. Free-floating sections were washed with PBS and mounted with Vectashield mounting medium. Confocal fluorescence images were acquired on a Nikon A1R confocal microscope. Serial stack images were acquired under constant settings. All imaging steps and analyses were performed under blinded conditions. Only mice in which the virus was injected into the correct site were used.
Immunohistochemistry
The procedures used were described previously [18]. Following perfusion, brain slices were obtained as described above in the “Histology” section. Poorly processed samples (poorly perfused samples, those with residual blood, or those in which the regions of interest were fragmented or inaccessible upon slicing) were not further processed for imaging. These criteria were set before the start of the experiment. The antibodies used were as follows: rabbit anti-PV (Swant Biotechnologies, Cat. No. PV27; 1:5000) and Alexa 488-conjugated secondary antibodies (Molecular Probes, Life Technologies, Cat. No. A-21206; 1:500). The PL was located –1.98 mm from bregma. Images were acquired in parallel under constant settings (laser power, 3%; pinhole, 1.10–1.25 arbitrary units (a.u.); 0.25 µm z-step; GaAsP detector) on an A1R confocal microscope (Nikon) with a 20× objective (Nikon). The fluorescence intensities of individual cells were measured from final output images using ImageJ software. The cell area (number of pixels) and integrated density (whole-cell fluorescence intensity) were measured. Background fluorescence levels were determined for each stained PL section/image. The total fluorescence intensity per cell in a.u. was calculated in an Excel sheet from the measurements obtained for individual cells via the following formula: corrected total cell fluorescence = integrated density - (area of the selected cell × mean background fluorescence intensity). PV neurons from individual mice (4 sections per mouse, 5–6 mice per group) were quantitatively analyzed. A 1000-au scale was adopted to classify PV neurons into four subclasses as follows: low PV expression (low-PV neurons), 0–1000 au; intermediate low PV expression, 1000–2000 au; intermediate-high PV expression, 2000–3000 au; and high PV expression (high-PV neurons), >3000 au.
Fear conditioning
The procedures were modified from previous reports [13, 21]. The mice were trained and tested using the FreezeFrame system (Coulbourn Instruments). For training, the mice were habituated for 2 min on a shock grid (cage setup A: metal fence floor, gray metal wall). Fear conditioning was conducted by pairing four 30-s, 2800-Hz, 70-dB auditory cues (conditioned stimuli (CSs)) with a 1-s, 0.75 mA footshock (unconditioned stimulus (US)). Intertone intervals were randomly set between 60 and 120 s. Mice were returned to their home cages 2 min after presentation of the unpaired stimuli. For contextual fear memory retrieval, the mice were placed in the training cage (cage setup A) for 3 min and placed back into their home cage after the end of the test. Freezing responses were recorded throughout the 3 min testing period. For tone-cued fear memory retrieval, the mice were placed in a different context (cage setup B: white Plexiglas floor, white Plexiglas wall) for an initial 2-min (pre-CS) period, followed by tone presentation (CS) for 3 min. The mice were placed back into their home cage after the end of tone presentation. Freezing responses were recorded in the last three minutes.
For immunostaining, mice were scarified after fear retrieval after 6 h. All are presented in the Results section and in the Figures as an experimental paradigm.
Treatments
Chemogenetic manipulation
The designer drug clozapine-n-oxide (CNO) was purchased from Sigma (#C0832). CNO (3 mg/kg dissolved in saline) was administered intraperitoneally to the animals.
Running
The mice in the running groups were given voluntary access to a single running wheel placed in their home cages.
Statistical analyses
No specific method was used to predetermine the ideal sample size or to randomly assign the subjects to the experimental groups. The sample size for this study was determined on the basis of previous studies in the field and practical considerations. The normality of the data distribution was confirmed by the Shapiro–Wilk normality test. Statistical differences in normally distributed data were determined using an unpaired two-tailed t test or one-way ANOVA followed by post hoc Tukey’s multiple comparisons test. All the data were analyzed via Prism 8.0 software. For all the results, the threshold for significance was set at p = 0.05 (represented by *; p < 0.01 = **, p < 0.001 = ***). All the data are expressed as the means ± SEMs.
Results
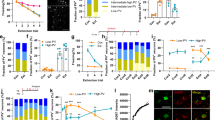
The mice were subjected to fear conditioning, and recent or remote fear memory was tested 1 day or 28 days after fear conditioning (Fig. 1A, B). Consistent with previous reports [9, 22, 23], fear memory persisted for at least four weeks in adult mice (Fig. 1C–F). The configuration of PV neuron network was examined six hours after fear retrieval (Fig. 1A, B). Consistent with our previous results [18], mice that underwent recent fear retrieval presented an increased percentage of high-PV neurons in the PL (Fig. 1G–K). Notably, remote fear retrieval increased the fraction of high-PV neurons without affecting the total number of PV neurons (Fig. 1E–I). The infralimbic cortex (IL), anterior cingulate cortex (ACC), and ventral hippocampus (vHPC) have been shown to be involved in the regulation of fear memory [24, 25]; thus, we examined the configuration of PV neuron networks in these regions. The results revealed that both recent and remote fear memory retrieval had no effect on PV neuron plasticity in the IL (Supplemental Fig. 1A–D) or ACC (Supplemental Fig. 1E–H), whereas recent fear memory retrieval induced a shift toward high-PV neurons in the vHPC (Fig. 1L–O); however, remote fear retrieval had no effect.
A, B Experimental paradigm. The mice were subjected to fear conditioning, and recent or remote fear memory was tested 1 day or 28 days after fear conditioning. The configuration of PV neuron network was examined six hours after fear retrieval. C–F Recent fear memory retrieval was tested one day after fear condoning, including contextual (C) and tone-cued (D) fear memory; remote fear memory retrieval was tested four weeks after fear condoning, including contextual (E) and tone-cued (F) fear memory. G Representative examples of PV immunocytochemistry after fear retrieval. Scale bar = 200 μm. H Summary of PV network configuration in the PL cortex in each group. I Fear retrieval did not affect the fraction of low-PV neurons (F (3,16) = 1.914, p = 0. 1680). J Both recent and remote fear retrieval increased the fraction of high-PV neurons (F (3,16) = 12.88, p = 0.002). K Number of PV neurons in each group (F (3,16) = 1.471, p = 0. 2600). L–N Recent fear retrieval induced a high-PV shift in the vHPC, but remote fear retrieval had no effect. (Low-PV: F, F (3,19) = 1.603, p = 0. 2219; High-PV: G, F (3,19) = 33.13, p < 0. 0001). O, The manipulation did not affect the number of PV neurons (F (3,19) = 0.9614, p = 0.4312). PL, prelimbic cortex; vHPC, ventral hippocampus. I–K, M–O One-way ANOVA with Tukey multiple comparisons, n = 5-6 mice for each group. The data are presented as the mean ± SEM, **p < 0.01, ***p < 0.001.
Next, we explored whether PV network plasticity in the PL is sufficient to regulate fear retrieval. To this end, we used a chemogenetic approach using designer receptors exclusively activated by designer drugs [26] to manipulate the PV neuron network configuration in vivo. hM4Di, an engineered G protein-coupled receptor that suppresses neuronal activity in the presence of its agonist CNO, was expressed in PV neurons via bilateral injection of AAV-DIO-hM4Di-mCherry into the PL of PV-Cre mice (Supplemental Fig. 2A). AAV-DIO-mCherry was injected into another set of animals as a control. Immunostaining revealed that mCherry was specifically expressed in PV neurons (Supplemental Fig. 2B). It has been shown that chemogenetic inhibition of PV neurons specifically induces the formation of a low-PV neuron network configuration in the IL and hippocampus six hours after CNO administration [16, 18]. Similarly, we found that chemogenetic inhibition of PV neurons in the PL was sufficient to induce a low-PV neuron network configuration (Supplemental Fig. 2C–F), as shown by an increased proportion of low-PV neurons (Supplemental Fig. 2E) and a decreased proportion of high-PV neurons (Supplemental Fig. 2F) without affecting the total number of PV neurons (Supplemental Fig. 2G).
We then explored the effects of acute inhibiting PV neurons on fear retrieval. PV-Cre mice were injected with AAV-DIO-hM4Di-mCherry or AAV-DIO-mCherry and subjected to fear training two weeks later. Recent fear retrieval test and remote fear retrieval test were performed one day or four weeks after fear training, respectively. CNO was exclusively administered six hours prior to the recent fear retrieval test (Fig. 2A). Acute inhibition of PV neurons reduced both recent contextual and tone-cued fear memory (Fig. 2B, D), but it did not affect remote fear memory recall (Fig. 2C, E). To rule out the possibility that the effects of chemogenetic manipulation on freezing may be mediated through its impact on locomotion, an open field test was performed on nonconditioned animals (Fig. 2F). The results showed that acute stimulation of PV neurons had no effect on locomotor activity (Fig. 2G).
A Experimental paradigm. CNO was administered 6 h prior to the recent fear retrieval test, which was performed one day after fear conditioning. The remote fear retrieval test was conducted four weeks later. B–E Acute inhibition of PV neurons reduced recent fear expression in both the contextual (B t (14) = 9.815, p < 0.0001) and tone-cued (C t (14) = 14.43, p < 0.0001) fear tests, while it did not affect remote fear memory recall (D t (14) = 0.4410, p = 0.6659; E t (14) = 0.1426, p = 0.8886). F Experimental paradigm. CNO was injected 6 h before open field test. G Acute inhibition of PV neurons had no effect on locomotion (t (13) = 0.4336, p = 0.6717). H, I Experimental paradigm. CNO was exclusively administered six hours prior to the recent fear retrieval test. The mice were sacrificed six hours post-retrieval for PV neuron network configuration analysis at both recent and remote memory retrieval. J–L Administration of CNO six hours before recent fear retrieval suppressed the high-PV shift, while this effect was no longer observed four weeks later. (Low-PV: K F (3,17) = 75.41, p < 0. 0001; High-PV: L F (3,17) = 70.83, p < 0. 0001). M The manipulation had no effect on the number of PV neurons (F (3,17) = 0.4819, p = 0. 6992). B–G unpaired two-tailed Student’s t-test, n = 7-8 mice for each group. J–L One-way ANOVA with Tukey multiple comparisons, n = 5-6 mice for each group. The data are presented as the mean ± SEM, ***p < 0.001.
To examine how acute inhibition of PV neurons modulates fear experience-dependent PV network plasticity, a new cohort of mice were used where CNO was exclusively administered six hours prior to the recent fear retrieval test. The mice were sacrificed six hours post-retrieval for PV neuron network configuration analysis at both recent and remote memory retrieval (Fig. 2H, I). Consistently, we found that recent fear retrieval induced a shift toward high-PV neurons in the PL, as shown by an increased proportion of these neurons (Fig. 2H–K). This plasticity pattern showed temporal specificity, as it was absent following remote retrieval four weeks later (Fig. 2H–K). Control groups presented similar PV neuron network configurations across both recent and remote retrieval (Fig. 2H–K), confirming this plasticity represents an experience-dependent rather than time-dependent phenomenon. The administration of CNO six hours before recent fear memory retrieval suppressed the shift toward high-PV neurons in the PL, with no detectable effect after remote retrieval (Fig. 2H–K). In addition, the manipulation exhibited anatomical specificity, producing no alteration in either the IL or vHPC PV network plasticity (Supplemental Fig. 3).
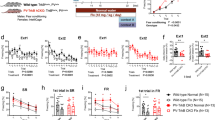
To investigate how chronic inhibition of PV neurons affects fear memory retrieval, we conducted a series of behavioral and histological experiments. Mice received bilateral injections of either AAV-DIO-hM4Di-mCherry or AAV-DIO-mCherry (control) vector, followed by fear conditioning. Following training, the animals received daily CNO administration for one week. Fear memory retrieval was tested one day or three weeks after the final CNO injection (Fig. 3A). The results revealed significant decrease in both contextual and tone-cued fear memory (Fig. 3B, C). Notably, this reduction was still observed three weeks later (Fig. 3D, E). Open field test in non-conditioned animals showed no differences between two groups following chronic inhibition of PV neurons, indicating normal locomotor activity (Fig. 3F, G). PV neuron network configuration analysis showed that chronic inhibition of PV neurons prevented the experience-dependent shift toward high-PV neurons typically observed after fear retrieval, and this effect remained evident even three weeks post-manipulation (Fig. 3J–M). For this analysis, the animals were scarified six hours following their fear retrieval test (Fig. 3H, I).
A Experimental paradigm. Mice were administered CNO daily for one week after fear conditioning, fear retrieval was tested 24 h or three weeks after the last injection. B–E Inhibition of PV neurons one week decreased the contextual (B, t (14) = 10.64, p < 0.0001) and tone-cued (C, t (14) = 10.91, p < 0.0001) fear expression, and the reduction in fear expression was still observed three weeks later (D, t (14) = 19.85, p < 0.0001; E, t (14) = 15.18, p < 0.0001). F Experimental paradigm. CNO was injected daily for one week before open field test. G Chronic inhibition of PV neurons had no effect on locomotion (t (14) = 0.9245, p = 0.3709). H, I Experimental paradigm. Following training, the animals received daily CNO administration for one week. Fear memory retrieval was tested one day or three weeks after the final CNO injection. The animals were scarified six hours following their fear retrieval test for PV staining. J–M Chronic inhibition of PV neurons before fear retrieval suppressed the high-PV shift, and the suppression was still observed three weeks later (Low-PV: K F (3,18) = 34.51, p < 0.0001; High-PV: L F (3,18) = 28.80, p < 0.0001). M The manipulation had no effect on the number of PV neurons (F (3,18) = 0.2013, p = 0.8941). B–G, unpaired two-tailed Student’s t-test, n = 8 mice for each group. K–M One-way ANOVA with Tukey multiple comparisons, n = 5-6 mice for each group. The data are presented as the mean ± SEM, **p < 0.01, ***p < 0.001.
Physical exercise is a widely recognized nonpharmacological method for alleviating anxiety disorders [19, 20]. To test whether physical exercise could modulate both fear memory expression and PV neuron network reorganization, we employed voluntary running, a common form of physical exercise. The mice that underwent fear training were divided into two groups with similar freezing levels (Fig. 4A, B, G). The experimental mice were provided continuous access to running wheels placed in their home cages while controls maintained standard housing conditions. Our behavioral analysis revealed a progressive attenuation of fear memory expression corresponding to exercise duration: one week of running had no effect on fear memory expression (Fig. 4C, H), two weeks of running reduced fear memory (Fig. 4D, I), three weeks of running further diminished it (Fig. 4E, J), and four weeks of running had the strongest effect (Fig. 4F, K). We next examine the effect of running on the PV neuron network configuration.
A Experimental paradigm. After fear conditioning, mice were divided into two groups. One group underwent four weeks of voluntary running, while the control group was housed normally in their cages. Fear recall tests were conducted weekly for both groups. B, G Mice exhibited similar levels of contextual fear memory (B, t (14) = 0.0508, p = 0.9602) and tone-cued fear memory (G, t (14) = 0.2272, p = 0.8235) before undergoing voluntary running. C–F In the control group, context fear memory persisted for at least four weeks; while voluntary running gradually reduced fear expression overtime: two-week running (D, t (14) = 4.034, p = 0.0012), three-week running (E, t (14) = 6.800, p < 0.0001), and four-week running (F, t (14) = 15.34, p < 0.0001) significantly decreased fear expression, but one-week running (C, t (14) = 0.2993, p = 0.7691) had no effect. H–K In the control group, tone-cued fear memory persisted for at least four weeks; while voluntary running gradually reduced fear expression overtime: two-week running (I, t (14) = 5.118, p = 0.0002), three-week running (J, t (14) = 10.11, p < 0.0001), and four-week running (K, t (14) = 14.61, p < 0.0001) significantly decreased fear memory expression, but one week running (H, t (14) = 0.1660, p = 0.8705) had no effect. B-K, unpaired two-tailed Student’s t-test, n = 8 mice for each group. The data are presented as the mean ± SEM, **p < 0.01, ***p < 0.001.
Immunostaining analyses revealed dynamic temporal patterns in the PL following voluntary running (Fig. 5A): we observed the emergence of increased proportion of low-PV neurons after one week of running, accompanied by delayed but significant decrease in the proportion of high-PV neurons after three weeks of running (Fig. 5B–E), while total number of PV neurons was not affected by running (Fig. 5F). To further investigate the lasting effects of voluntary running on fear memory expression, we implemented a delayed-testing paradigm where fear-conditioned mice underwent two weeks of running, followed by a one-month waiting period, after which fear memory recall and the PV neuron network configuration were tested (Supplemental Fig. 4A). The data revealed that this exercise regimen induced persistent modifications: when tested one month after running cessation, the mice exhibited decreased fear memory expression (Supplemental Fig. 4B, C), accompanied by reduced experience-dependent reorganization within high-PV neuronal subpopulations (Supplemental Fig. 4D–F), without alterations to the total number of PV neurons (Supplemental Fig. 4G).
A Experimental paradigm. Mice were sacrificed for PV staining following voluntary running. B Representative examples of PV immunocytochemistry under control conditions and after running. Scale bar = 100 μm. C Summary of PV network configuration in the PL cortex in each group. D Running gradually increased the fraction of low-PV neurons over time (F (5,30) = 41.41, p < 0.0001), with a significant increase observed after just one week of running. E Running gradually decreased the fraction of high-PV neurons over time (F (5,30) = 13.40, p < 0.0001), with a significant reduction observed after three weeks of running. F Number of PV neurons in each group. D, E one-way ANOVA with Tukey’s multiple comparisons test, n = 6 mice for each group. The data are presented as the mean ± SEM, **p < 0.01, ***p < 0.001.
To determine whether running-induced shifts toward low-PV neuron network configuration in the PL directly mediate the reduction in fear memory retrieval, we locally interfered with this shift following running. hM3Dq, an engineered G protein-coupled receptor that enhances neuronal activity in the presence of its agonist CNO, was expressed in PV neurons via bilateral injection of AAV-DIO-hM3Dq-mCherry vectors into the PL of PV-Cre mice. AAV-DIO-mCherry vectors were injected into another set of animals as a control. Immunostaining revealed that mCherry was specifically expressed in PV neurons (Fig. 6A, B). Chemogenetic stimulation of PV neurons induced a shift toward a high-PV neuron network configuration, as shown by a decrease in the proportion of low-PV neurons and an increase in the proportion of high-PV neurons (Fig. 6C–F). When applied chronically during four-week running protocols, this stimulation fully blocked exercised-induced PV network plasticity, preventing both the rise in low-PV neuron proportions and the decline high-PV populations (Fig. 6C–F), without altering total number of PV neurons (Fig. 6G). Behaviorally, two weeks of running reduced contextual and tone-cued fear memory expression (Fig. 6H–J), and this suppression was further enhanced after four weeks of running (Fig. 6K‒M); both effects could be blocked by the chemogenetic activation of PV networks (Fig. 6H–M). In addition, following three weeks of running and aa additional one-week waiting period, the mice maintained reduced fear memory expression (Supplemental Fig. 4H–J). Notably, chemogenetic activation of PL PV neurons six hours prior to fear recall successfully restore fear memory expression (Supplemental Fig. 4H–J).
A Representative image showing hM3Dq-mCherry co-stained with PV. Scale bar = 100 μm. B The calculation data from A. C Up, Experimental paradigm. The study involved four groups of mice: two control groups housed in homecages and two experimental groups subjected to a 4-week running. Within the control groups, one group received the control virus mCherry, while the other received the hM3Dq virus for chemogenetic activation. Similarly, in the experimental groups, one group received the control virus mCherry, and the other received the hM3Dq virus for chemogenetic activation. Throughout the 4-week period, all mice were administered CNO daily. Down, representative examples of PV immunocytochemistry in each group. Scale bar = 100 μm. D Summary of PV network configuration in the PL cortex in each group. E Chemogenetic activation of PV neurons induced a decreased in low-PV fraction in naïve mice; running induced an increased in low-PV fraction, which could be blocked be chemogenetic activation of PV neurons (F (3, 20) = 73.52, p < 0.0001). F Chemogenetic activation of PV neurons induced an increased in high-PV fraction in naïve mice; running induced a decreased in high-PV fraction, which could be blocked be chemogenetic activation of PV neurons (F (3,20) = 25.46, p < 0.0001). G Number of PV neurons in each group (F (3,20) = 3.031, p = 0.0533). H Experimental paradigm. Control mice injected with mCherry vectors were housed in homecages after fear condoning, two experimental groups were subjected to a 2-week running. Within the experimental groups, one group received the control virus mCherry, while the other received the hM3Dq virus for chemogenetic activation. Throughout the 2-week period, all mice were administered CNO daily. I, J Two-week running reduced contextual fear memory (I, F (2,21) = 43.68, p < 0.0001) and tone-cued fear memory (J, F (2,21) = 33.54, p < 0.0001), which could be blocked by chronic chemogenetic stimulation of PV neurons. K Experimental paradigm. Similar to H, except the running period lasted for 4 weeks. L, M Four-week two-week running further reduced contextual fear memory (L, F (2,21) = 190.3, p < 0.0001) and tone-cued fear memory (M, F (2,21) = 134.0, p < 0.0001), which could be blocked by chronic chemogenetic stimulation of PV neurons. A, n = 3 mice for each group. E, F one-way ANOVA with Tukey’s multiple comparisons test, n = 6 mice for each group. I, J, L, M one-way ANOVA with Tukey’s multiple comparisons test, n = 8 mice for each group. The data are presented as the mean ± SEM, **p < 0.01, ***p < 0.001.
Discussion
Our major findings are as follows. Both recent and remote fear retrieval induced high-PV plasticity in the PL. Acute chemogenetic inhibition through single administration of CNO before recent fear retrieval selectively decreased recent fear memory retrieval and suppressed high-PV plasticity induction, without affecting remote fear memories tested three weeks later. On the other hand, chronic week-long chemogenetic inhibition resulted in rapid and sustained (observed 3 weeks after manipulation) alterations in fear memory retrieval and experienced-dependent PV neuron plasticity. In addition, voluntary running mimicked the effects of chronic inhibition of PV neurons and decreased the expression of fear memory, which could be blocked by chemogenetic activation of PV neurons. Together, these findings indicate an essential role for PV neuron network plasticity in the PL cortex in regulating fear memory expression and provide alternative methods for the treatment of anxiety disorders.
Memories of fearful events can last a lifetime. Traditionally, it is believed that memories are initially stored within the hippocampal–entorhinal cortex network (recent memory) and are gradually consolidated within the neocortex for permanent storage (remote memory) [27,28,29]. However, emerging evidence suggests that the hippocampus and cortex encode memories simultaneously, with the cortex gradually playing a more dominant role in storage through mechanisms such as synaptic plasticity and neural circuit interactions [8]. These perspectives underscore the critical role of the prefrontal cortex (PFC) in recent and remote memory storage. Specifically, the PL, a subregion of the PFC, has been shown to play a pivotal role in fear memory retrieval over time [3, 30, 31]. Although numerous studies have highlighted the involvement of PV neurons in the PL in the regulation of fear expression [12, 13, 18], the role of local network plasticity in the regulation of fear memory remains poorly understood. Our findings demonstrated that recent fear retrieval induced high-PV plasticity in the PL and vHPC but not in the IL or ACC, two other PFC subregions. These results align with previous reports showing that mice subjected to fear conditioning and retrieval present an increased proportion of high-PV neurons in the vHPC and PL, respectively [14, 16, 18]. Notably, we observed that remote fear retrieval induced high-PV plasticity in the PL but not in the vHPC. These findings further support the notion that the PL participates in the storage of both recent and remote memory, whereas the vHPC is involved primarily in recent memory storage. Moreover, our results highlight the critical role of PV neurons plasticity in the PL in regulating fear memory expression across different time scales. In contrast to previous studies showing that chronic inhibition of the ACC prevents fear retrieval [32], our work reveals that neither recent nor remote fear retrieval altered PV neuron plasticity in the ACC. These findings suggest that PV network plasticity in the ACC may not be directly involved in fear retrieval. Additionally, while learning in the Morris water maze test has been shown to depend on PV neuron plasticity in the hippocampus [14, 16], whether PV neuron plasticity in the PL plays a similar role remains an open question that warrants further investigation.
Various studies have addressed the involvement of PV neurons in fear expression [33,34,35]. Specifically, the expression of fear was found to be causally linked to the phasic inhibition of PV neurons in the PFC [12]. Real-time optogenetic inhibition of PFC PV neurons increase freezing, whereas the stimulation of these neurons decreases fear expression [12]. In our study, we found that acute chemogenetic inhibition of PV neurons in the PL (6 h after CNO injection) decreased fear expression. This discrepancy may be due to the different manipulation methods employed in these studies, which might have had different cellular effects. Courtin examined the real-time responses of mice to optogenetic manipulation, whereas we assessed the responses of mice 6 h after chemogenetic inhibition. In addition, optogenetic activation of PV neurons induced a shift toward a high-PV neuron network configuration 6 h after light stimulation [16]. Future studies should explore the change in fear expression 6 h after light stimulation.
Altering the frequency and duration of stimulation may result in strikingly different effects on behavior [36]. For example, chemogenetic inhibition of somatostatin neurons by acute administration of CNO results in anxiety-like behaviors, whereas the administration of CNO twice per day for 3 weeks produces an anxiolytic effect [37]. In addition, sustained photostimulation (1 min on/1 min off for 1 h) of pyramidal neurons in the infralimbic cortex produces anxiolytic effects [38], whereas acute stimulation results in the opposite (anxiogenic) effects [39]. In our study, we found that acute inhibition of PV neurons in the PL by a single administration of CNO decreased recent fear memory retrieval but had no effect on remote fear memory retrieval, whereas chronic inhibition of these neurons by daily administration of CNO for 1 week decreased both recent and remote memory retrieval. The sustained effects of chronic chemogenetic inhibition indicate that long-term neuroplasticity of the PV neuron network may reorganize PFC circuits underlying long-term memory formation [8, 40], which needs further exploration.
A previous study revealed that the inhibition of PV neurons in the hippocampus 3 h after fear conditioning prevents fear retrieval 4 weeks later [14]. Our study reveals that chronic inhibition of PV neurons in the PL 24 h after fear memory formation can achieve the same effect, suggesting that the therapeutic window can be extended. Notably, our study reveals that running can decrease fear memory retrieval between 2 and 4 weeks, providing an alternative method for the treatment of certain anxiety disorders.
There are also several limitations in our present study. Multiple studies have demonstrated sex differences in the effects of PV neurons. Indeed, male and female mice exhibit sex differences in the electrophysiological properties of prefrontal PV neurons [41], and PFC PV interneurons undergo sex-dependent changes in response to stress [42, 43]. In addition, there are sex differences in the prevalence and pathology of anxiety disorders and PTSD and the response of these diseases to treatment [44, 45]. For example, anxiety disorders and PTSD are more common in women than in men [46], whereas women respond better to selective serotonin reuptake inhibitors than men [47, 48]. Our study focused solely on male mice, and future studies should extend our research to include female mice. Second, our study demonstrated that the plasticity of local PV neuron networks plays an important role in the regulation of remote fear memory retrieval, but a mechanism-based explanation for how the dominance of low-PV neurons leads to decreased fear expression needs further exploration. Changes in synaptic plasticity or oscillatory activity [14, 16, 18, 32] may represent two important focuses.
Conclusions
In summary, this study reveals a novel local network mechanism involved in regulating fear memory expression. Fear retrieval induced a shift toward a high-PV neuron network configuration in the PL. Acute chemogenetic inhibition of PV neurons in the PL rapidly decreased fear memory retrieval and suppressed the fear-induced shift toward a high-PV neuron network configuration but had no sustained effect, whereas chronic inhibition of these neurons had rapid and sustained effects on fear memory retrieval and PV neuron network plasticity. In addition, voluntary running mimicked the effects of chronic inhibition of PV neurons and promoted the reduced of fear memory, which could be blocked by chemogenetic activation of PV neurons. Together, these findings indicate an essential role for PV neuron network plasticity in the PL in regulating fear memory expression and provide alternative methods for the treatment of anxiety disorders.
Data availability
Data supporting the findings of this study can be made available from the corresponding authors upon request.
References
Craske MG, Stein MB Anxiety. Lancet 2016 Dec 17;388:3048–59.
Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–65.
DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci. 2019;22:460–9.
Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–3.
Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–4.
Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38.
Sierra-Mercado D Jr., Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–8.
Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–78.
Zhang SR, Wu DY, Luo R, Wu JL, Chen H, Li ZM, et al. A prelimbic cortex-thalamus circuit bidirectionally regulates innate and stress-induced anxiety-like behavior. J Neurosci. 2024;44:e2103232024.
Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807.
Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42.
Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96.
Chen YH, Lan YJ, Zhang SR, Li WP, Luo ZY, Lin S, et al. ErbB4 signaling in the prelimbic cortex regulates fear expression. Transl Psychiatry. 2017;7:e1168.
Karunakaran S, Chowdhury A, Donato F, Quairiaux C, Michel CM, Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat Neurosci. 2016;19:454–64.
Donato F, Chowdhury A, Lahr M, Caroni P. Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron. 2015;85:770–86.
Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–6.
Caroni P. Regulation of Parvalbumin Basket cell plasticity in rule learning. Biochem Biophys Res Commun. 2015;460:100–3.
Chen YH, Hu NY, Wu DY, Bi LL, Luo ZY, Huang L, et al. PV network plasticity mediated by neuregulin1-ErbB4 signallingcontrols fear extinction. Mol Psychiatry. 2022;27:896-906.
Yan L, Wang Y, Hu H, Yang D, Wang W, Luo Z, et al. Physical exercise mediates cortical synaptic protein lactylation to improve stress resilience. Cell Metab. 2024;36:2104–2117.e4.
Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, et al. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry. 2018;5:739–46.
Zhang SR, Wu JL, Chen H, Luo R, Chen WJ, Tang LJ, et al. ErbB4 knockdown in serotonergic neurons in the dorsal raphe induces anxiety-like behaviors. Neuropsychopharmacology. 2020;45:1698–706.
Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602.
Gao A, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA, et al. Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. J Neurosci. 2018;38:3190–8.
Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31.
Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T, et al. Neuronal circuits for fear expression and recovery: recent advances and potential therapeutic strategies. Biol Psychiatry. 2015;78:298–306.
Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–6.
Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7.
Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, et al. Schemas and memory consolidation. Science. 2007;316:76–82.
McClelland JL. Incorporating rapid neocortical learning of new schema-consistent information into complementary learning systems theory. J Exp Psychol Gen. 2013;142:1190–210.
Klavir O, Prigge M, Sarel A, Paz R, Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017;20:836–44.
Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30.
Xia F, Richards BA, Tran MM, Josselyn SA, Takehara-Nishiuchi K, Frankland PW. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. Elife. 2017;6:e27868.
Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32:2988–97.
Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, et al. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron. 2014;84:835–46.
Jetsonen E, Didio G, Winkel F, Llach Pou M, Boj C, Kuczynski-Noyau L, et al. Activation of TrkB in Parvalbumin interneurons is required for the promotion of reversal learning in spatial and fear memory by antidepressants. Neuropsychopharmacology. 2023;48:1021–30.
Chen Y, Hu N, Yang J, Gao T. Prefrontal cortical circuits in anxiety and fear: an overview. Front Med. 2022;16:518–39.
Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39:2252–62.
Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci USA. 2015;112:8106–11.
Chen Y-H, Wu J-L, Hu N-Y, Zhuang J-P, Li W-P, Zhang S-R, et al. Distinct projections from the infralimbic cortex exert opposing effects in modulating anxiety and fear. J Clin Invest. 2021;131:e145692.
Do Monte FH, Quirk GJ, Li B, Penzo MA. Retrieving fear memories, as time goes by. Mol Psychiatry. 2016;21:1027–36.
Fabian CB, Jordan ND, Cole RH, Carley LG, Thompson SM, Seney ML, et al. Parvalbumin interneuron mGlu(5) receptors govern sex differences in prefrontal cortex physiology and binge drinking. Neuropsychopharmacology. 2024;49:1861–71.
Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, et al. Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Rep. 2018;25:2299–2307.e2294.
Page CE, Shepard R, Heslin K, Coutellier L. Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci Rep. 2019;9:19772.
Bangasser DA, Cuarenta A. Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci. 2021;22:674–84.
Christiansen DM, Berke ET. Gender- and Sex-Based Contributors to Sex Differences in PTSD. Curr Psychiatry Rep. 2020;22:19.
Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–47.
Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh-Soerensen P. Danish University Antidepressant G. Are gender differences important for the clinical effects of antidepressants? Am J Psychiatry. 2003;160:1643–50.
Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–50.
Acknowledgements
This work was supported by grants from the STI2030-Major Projects (2022ZD0214300 to CYH), the National Natural Science Foundation of China (32271014 to CYH, 32200947 to HNY), Guangdong-Hong Kong Joint Laboratory for Psychiatric Disorders (2023B1212120004), Shenzhen Science and Technology Program (JCYJ20240813115130040 to HNY), the China Postdoctoral Science Foundation Grant (2024M751308 to HNY), the Open Research Fund of the Zhejiang Key Laboratory of Precision Psychiatry (2025A3 to CYH) and Development Subsidy Fund of Pingshan Hospital of Southern Medical University.
Author information
Authors and Affiliations
Contributions
CYH conceived the idea for this study and designed the study. HNY performed all the experiments with the help of HX, LH, HL and DYY. HNY and CYH generated the figures. CYH wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article exclusively involves the use of animals in the research, and no human participants are included or involved in any aspect of the study. All experimental procedures with animals were approved by the Southern Medical University Animal Ethics Committee (Approval number: 2021Animal No. 072) and were performed according to the Chinese Council on Animal Care Guidelines. Efforts were made to minimize animal suffering and reduce the number of animals used.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, NY., Heng, X., Li, H. et al. Exercise-induced PV network plasticity in the prelimbic cortex regulates the expression of fear memory in male mice. Transl Psychiatry 15, 243 (2025). https://doi.org/10.1038/s41398-025-03472-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03472-7