Abstract
NR2A and NR2B are the major GluR2 subunits of N-methyl-D-aspartate (NMDA) receptor. NR2B-containing NMDA receptor was found to be exclusively expressed in post-synapses in layer III pyramidal cells of the prefrontal cortex (PFC). Many studies have suggested the importance of PFC NR2B-containing NMDA receptor for working memory, especially for the persistent delay cell firing. However, direct evidence for the necessity of PFC NR2B-containing NMDA receptor on working memory is still absent, especially in non-human primates. Here, we directly evaluated the necessity of PFC synaptic NR2B for working memory in both rats and monkeys. We first examined the synaptosome expression ratio of NR2B/2A in the PFC, hippocampus and visual cortex, and confirmed a relatively higher expression ratio of NR2B/2A in the PFC than in the hippocampus and visual cortex in both species. We then investigated the effects of intra-PFC blockade of NR2B on the performance of spatial working memory and pattern discrimination, and found that the spatial working memory, but not pattern discrimination, was robustly impaired in a delay length-dependent way in both species. The present study provided behavioral and neuropharmacological evidence for the critical role of PFC NR2B-containing receptors in working memory performance in non-human primates.
Similar content being viewed by others
Introduction
The neocortices of human and non-human primates are hierarchically organized [1]. The hierarchy is not only based on anatomical structures and connections in macroscale, but also on spatially heterogeneous gene expression [2,3,4]. Studies correlating neuroimaging data with the spatial expression profiles of genes in the Allen Human Brain Atlas (AHBA) dataset have suggested that there is a close link between the cortical transcriptome and macroscale specialization of functions. Many genes show transcriptional gradients along with the hierarchical axis [5, 6].
N-methyl-D-aspartate receptor (NMDAR) is one of the major excitatory ionotropic receptors that receive glutamate input in the central nervous system (CNS). An NMDA receptor usually consists of four subunits, two NR1 and two NR2 subunits [7]. There are 4 types of NR2 subunits (A-D) that are heterogeneously distributed in different regions of the brain. In the adult mammalian brain, NR2A- and NR2B-containing receptors are the major types of functional NMDARs. The transcription of NR2B coding gene GRIN2B exhibited a strong positive gradient along with the hierarchical axis of the brain, according to the AHBA dataset [6]. It has been reported that NR2B is more distributed extra-synaptically at the posterior brain regions such as the visual cortex and hippocampus [8,9,10], suggesting that NR2B could less contribute to synaptic transmission than NR2A in these regions. At the anterior brain regions, such as the prefrontal cortex which is on the top of the hierarchy, NR2B subunits are exclusively located in the post-synaptic densities of layer III neurons in adult primates [11]. Since the response kinetics of each NR2 subunit upon activation are quite different, the synaptic NR2A/2B ratio largely determines the receptor properties [12, 13]. For example, the offset decay time constant of NR1-NR2A channel is 3–4 times faster than that of NR1-NR2B channel upon glutamate binding [12].
Working memory is a specific memory type that reflects the ability to maintain currently-obtained information online and use it to instruct behavioral output [14]. It is well known that the prefrontal cortex (PFC) is the critical cortical region to execute working memory function [15]. In primates, the dorsolateral prefrontal cortex (dlPFC) is essential for performing spatial working memory tasks. During a visually-guided delayed response task, there are PFC neurons maintaining spatially-tuned, persistent activity across the delay period. The persistent firing is considered a neuronal representation of working memory [16, 17]. Computational models predict that this persistent firing activity requires stimulation of NMDARs rather than AMPARs, and the slow kinetics of NR2B-containing NMDARs are particularly well suited to generate recurrent pyramidal neuron excitation in the absence of sensory inputs. Indeed, Wang et al. demonstrated that the persistent delay firing in the dlPFC pyramidal cells could be abolished upon local iontophoresis of the NR2B antagonist [11].
While this very local and tiny amount of drug infusion with iontophoresis only affects cells in a small volume surrounding the electrode tip and would not disturb the whole neuronal network and the behavioral performance, larger-dose drug application was necessary to address the importance of NR2B-containing NMDARs for working memory. To clearly verify the functional significance of NR2B subunit in the dlPFC in working memory, here we directly compared the synaptic NR2B/2A expression in the PFC and other brain regions in both rodent and non-human primate and investigated the effects of intra-PFC infusion of NR2B antagonist on the behavioral performance of both rodent- and primate-versions of spatial working memory task.
Material and methods
Subjects
Sprague-Dawley rats (male, 200–250 g, 8–10 weeks old) for T-Maze task were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China) and those (male, 300–350 g, 12 weeks old) for western blot from Jiangxi Province Key Laboratory of Laboratory Animal, Nanchang University (Jiangxi, China). Rats were housed in plastic cages (2–3 rats per cage) at temperature-controlled (24 ± 1 °C) vivarium room on a 12/12 light/dark cycle. Food and water were available ad libitum before the behavioral training.
Two rhesus monkeys (Macaca mulatta, female, 4.0 kg (#1) and 6.0 kg (#2)) were purchased from the Kunming Primate Laboratory Animal Center, Chinese Academy of Sciences (Yunnan, China), with license numbers of SCXK (Dian) 2008–0001 and SYXK (Dian) 2008–0001) respectively. The two monkeys were neighbor-housed individually in an established animal room with a 12 h/12 h light/dark cycle (light from 7:00 am to 7:00 pm) and a controlled-temperature (21 ± 1 °C), and were given free access to water. The monkeys were fed with a diet of monkey chow (Kunming Gaoyun Biological Technology Co. Ltd., Yunnan, China; License number: SCXK (Dian) 2009–0003) immediately after behavioral testing, with the amount of chow designed to keep the monkeys at 90% of the body weights when food was freely available. Fresh skinless peanuts and raisins were used as food rewards during behavioral testing thus minimizing the need for dietary regulation. The monkeys were assigned to a single experimenter who trained them extensively before the behavioral testing and was blind to treatment conditions during the behavioral testing. The experimental protocols were approved by the Laboratory Animal Supervision Committee of Yunnan, China.
The brain tissues of monkey for western blot were obtained from one rhesus monkey (Macaca mulatta, 7 years old, male) that was provided by Guangdong Blue-island Biological Technology Co. Ltd (Guangdong, China). The monkey was sacrificed for other experimental purposes without affecting the CNS.
Isolation of synaptosomes and western blotting
Animals were cardiac perfused with saline under anesthetization with pentobarbital sodium (40 mg/kg, i.p.). Brain tissues were obtained from the PFC (mPFC for rat and principal sulcus for monkey), hippocampus (HIP) and visual cortex (VC) of both species, and were then used immediately or frozen under −80 °C. Brain tissue per sample (50 mg) was homogenized in a weight: volume ratio of 80:1000 using cold lysis buffer (1:50 PI and 1:100 PMSF). Homogenate was then centrifuged at 1000×g for 10 min at 4 °C to separate nuclei (P1). The supernatant (S1) was then centrifuged at 10000×g for 15 min to separate the crude synaptosomes (P2) and supernatant S2. P2 was then lysed with TEVP buffer and spun at 25000×g for 20 min to get purified synaptosomes (LP1) [18].
Western blotting was performed with standard protocol. Proteins were separated by electrophoresis on SDS-PAGE with 200 V voltage for 90 min. Proteins were then translocated to membrane with 300 mA current for 2 hr, and then incubated with blocking media (TBST containing 5% nonfat milk) for 2 hr at room temperature. The membranes were then incubated with primary NR2B (1:1000, #AB1557P, Merckmillipore), NR2A (1:1000, #AB1555P, Merckmillipore), PSD95 (1:1500) antibodies overnight at 4 °C. After washing, HRP labeled secondary antibody (1:1500) was used to incubate for 2 hr at 4 °C. ECL chemiluminescence substrate (Pierce) was used to detect HRP activity and developed onto x-ray films (Kodak).
Behavioral tasks
Delayed alteration task for rats
The delayed alteration T-maze (DAT) task was used to assess the working memory of rats, as described previously [19]. In brief, rats were subject to a restrict diet to maintain at approximately 85% of normal body weight. After habituating to the T-maze until they voluntarily ate the reward food at the end of arms, the training was initiated. The animal was placed at the end of the start arm. The trial was initiated by removing the sliding door to allow the animal to explore the maze. In trial 0, both arms were baited and the animal could get reward by visiting either of them. The animal was then forced back to the start position, without being allowed to enter into the opposite arm. After a certain interval (5 s delay), a formal trial started, and the animal was allowed to explore the maze again. The animal was rewarded by entering into the arm opposite to the visited arm in the previous trial. Error choice was followed by error-correction trials, where the same arm was baited until the animal made a correction choice. Each session contained 10 formal trials. After the animals reached the criteria with 80% correct rate of performance in two consecutive sessions, they were ready for drug infusion testing.
Delayed response task for monkeys
The Wisconsin General Test Apparatus (WGTA) was used to assess the working memory of monkeys. The WGTA was custom made and was placed in a quiet room. The monkeys were tested at the same time of day immediately prior to feeding. Firstly, the monkeys were trained on a two-choice spatial delayed response task, as previously described [20, 21]. The testing board contained two food wells (left and right) spaced 15 cm apart. The monkey and the testing board could be separated by an up-down removable opaque screen. Each trial began with the experimenter placing a food reward into one of the two food wells under the observation of the monkey (“cue”). The food wells were then covered with identical plastic plaques and the opaque screen was lowered down for a while (“delay”). At the end of this delay, the screen was raised up and the monkey was allowed to choose one of the wells (“response”). An inter-trial interval of 20 s was interposed between trials. Rewards were pseudo-randomly distributed between left and right wells over 30 trials in a daily testing session. The delay period was held constantly at ‘0’ s at the initial training session, and increased gradually with a stepwise procedure over a total of 34 sessions. To observe the effects of drug infusion, the delay period was arranged at 0 s, 20 s and 40 s among 30 trials in a daily testing session. The “0” s delay included the performance of lowering-down and immediate raising-up of the opaque screen.
Pattern discrimination task for monkeys
Once they had grasped the performance of the delayed response task, the monkeys received training on a pattern discrimination task in the same WGTA. In the pattern discrimination task, the two food wells were covered with two identical opaque plaques, on the surfaces of which were painted with an equilateral triangle and a crisscross pattern respectively. Each trial began with the experimenter’s putting the food reward into one of the two food wells and covering them with opaque plaques, when the monkeys’ vision was blocked by the lowered screen. The screen was then raised, and the monkeys were allowed to choose between the two patterns. The monkeys could get reward only when they chose the triangle pattern. An inter-trial interval of 20 s was interposed. Each daily session included 10 trials. The two patterns were quasi-randomly distributed between left and right. Once performance was stable at 90% correct for 2 consecutive days, drug treatment was initiated.
Cannula implantation and drug infusion
Cannula implantation
The rats and monkeys were implanted with cannulae before drug infusion and behavioral testing. The rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). Stainless steel guide cannulae (23 G) were bilaterally implanted into the mPFC (Bregma AP: 3.5 mm, ML: ±0.75 mm, DV: 1.5 mm from skull surface). The cannulae were then affixed in place with dental cement secured with sterile stainless-steel screws. Sterile stylets (24 gauge) were inserted into the guide cannulae, at a point equal to the intracranial tip of the cannula, to prevent occlusion. After one-week recovery from surgery, the rats were re-trained on the T-maze delayed alteration task to ensure the performance level of 80%.
The monkeys were pre-anesthetized with hydrochloric ketamine (5 mg/kg, intramuscularly) and pre-treated with sulfate atropine (0.2 mg/kg, intramuscularly). They were then deeply anesthetized with sodium pentobarbital (35 mg/kg, intraperitoneally). Guide cannulae were implanted in the dorsolateral prefrontal skull using aseptic methods as following. An incision was made along the midline of the scalp, and a total of 23 (monkey #1) and 16 (monkey #2) stainless-steel guide cannulae (20 gauge, up to 5 cannulae in a row and 4 rows in total) were implanted into the dorsolateral PFC, using the stereotaxic coordinates of the monkey brain atlas (Paxinos, et al., 2008). The coordinates ranged as: AP, +25 mm ~ +33 mm from the interaural line; ML, ±9 mm ~ ±14 mm from the midline. The cannulae were carefully lowered down to touching the dura without penetration, and then affixed to the skull using dental cement secured with sterile stainless-steel screws. Sterile stylets (24 gauge) were inserted into each guide cannula, at a point equal to the intracranial tip of the cannula, to prevent occlusion. All the cannulae were enclosed in a plastic chamber (fixed to the skull) to avoid being grasped by the monkeys and infected by pathogenic bacteria. Meticulous care was taken to minimize pain and post-operative infection. Surgical incisions were smeared with analgetic antiphlogistine, and the monkeys were treated with penicillin for seven consecutive days of post-surgery. The monkeys were given at least 7 days to recover from surgery before behavioral testing.
Drug infusion
The animals were habituated to the infusion procedure by mock treatment. For the rats, they were gentle restricted with hand, the stylets were removed and replaced with infusion needles (24 gauge). The needle tips were inserted beyond the guide cannulae for 2.7 mm, i.e. 4.2 mm below the skull surface. PBS or the NR2B antagonist Ro25-6981 (1 μl; 0.5 mg/ml) was infused at a speed of 0.1 μl/min. After infusion, the needles were kept in place for additional 3 min. Then the needles were removed, and the stylets were inserted back, and the rats were returned to home cages for 15 min before the behavioral testing started.
For the monkeys, they were seated in a restraint chair. The stylets were removed and replaced with sterile infusion needles (24 gauge with sharp tip for dura penetration). The tip open point of the infusion needles extended 1.0–1.5 mm below the tip of the guide cannulae. The monkeys received bilateral intracranial infusions of 3 μl drug solution which included 7.5 μg muscimol (Sigma Chemical, USA) or 15 μg Ro25-6981 (Tocris, UK) at each cannula. Saline (3 μl) was infused as vehicle control. Infusion was driven by a microsyringe pump (Bioanalytical System Inc., West Lafayette, IN, USA) at a speed of 1.0 μl/min. The infusion needles remained in place for 1.0 min after completion of the infusion, and thereafter the stylets were immediately reinserted. Behavioral testing was performed 30 min after the infusion.
Histology
The positions of infusion cannulae were examined after all behavioral pharmacological experiments were completed. The animals were anesthetized with overdoses of sodium pentobarbital. A stainless-steel electrode (30 gauge) was inserted into the same positions for the infusions to deposit ferric ion in the infusion sites with an anodal current (6 V, 10 s). Cadavers were treated by transcardial infusion of 0.9% (w/v) saline followed by formaldehyde (4%, w/v) solution (containing 1% (w/v) potassium ferrocyanide for the Prussian blue reaction with deposited ferric ion. The cannula traces on the brain surface were photo recorded for position re-construction. The brains were removed and stored in 4% (w/v) formaldehyde solution for several days, and were then sectioned for histological verification of cannulae positions (centers of the sites stained by Prussian blue reaction).
Single unit recordings in dlPFC
Oculomotor delayed response (ODR) task
Single unit recordings were performed on two adult male rhesus monkeys (Macaca mulatta), cared for under the guidelines of the National Institutes of Health and the Yale IACUC. The monkeys were trained in the visuospatial ODR task that was described previously (Wang et al., 2013). The monkeys started a trial by fixating at the central spot and maintaining fixation for 0.5 s (fixation period), whereupon a cue was illuminated for a period of 0.5 s (cue period) at one of eight peripheral targets located at an eccentricity of 13° with respect to the fixation spot. After the cue was extinguished, a 2.5-second delay period followed. The subject was required to maintain central fixation throughout both the cue and delay periods. At the end of the delay, the fixation spot was extinguished, which instructed the monkey to make a memory guided saccade to the location where the cue had been shown prior to the delay period. A trial was considered successful if the saccadic response was completed within 0.5 s of the offset of the fixation spot and was within 2° around the correct cue location. The monkeys were rewarded with fruit juice immediately after every successful response.
Pharmacology, physiology and data acquisition
Iontophoresis was used to apply the NR2B antagonist TCN237 (Tocris) near PFC neurons. Iontophoretic electrodes were constructed with a 20-μm-pitch carbon fiber (ELSI, San Diego, CA) inserted in the central barrel of a seven-barrel non-filamented capillary glass (Friedrich and Dimmock, Millville, NJ). A Neurophore BH2 iontophoretic system (Medical Systems Corp., Greenvale, NY) was used to control the delivery of the drugs. The drug (0.01 M in sterile water, pH 3–4) was ejected at currents varying from 15–25 nA, which was expected to produce roughly 0.1 μM of drug concentration near the recorded neuron [22]. Extracellular voltage was amplified using a custom low-noise preamplifier (SKYLAB) and band-pass filtered (180Hz–6Khz, 20 dB gain, 4-pole Butterworth; Kron-Hite, Avon, MA). Signals were digitized (15 kHz, micro 1401, Cambridge Electronics Design, Cambridge, UK) and acquired using the Spike2 software (CED, Cambridge, UK). Neural activity was analyzed using waveform sorting by a template-matching algorithm, which made it possible to isolate more than one unit at the same recording site. Post-stimulus time histograms (PSTHs) and rastergrams were constructed online to determine the relationship of unit activity with the task events. Single unit activity was measured in spikes per second. If the rastergrams displayed task-related activity, the units were recorded further, and pharmacological testing was performed.
Unit activity data were first collected from the cell under a control condition, in which at least eight trials at each of the eight cue locations were obtained. Upon establishing the stability of the cells’ activity, this control condition was followed by iontophoretic application of drug(s). Dose-dependent effects of the drug were tested in two or more consecutive conditions. Drugs were continuously applied at a relevant current throughout a given condition. Each condition had ~8 (6–12) trials at each location for statistical analysis of effects. The cue epoch was used for Cue cells, the delay epoch for Delay cells, and the response epoch for Response cells.
Data analysis
The band intensities of Western blot for NR2B and NR2A were first normalized with each PSD95 band. For each batch of experiment, the NR2B/2A ratio for the hippocampus (HIP) was set as 1, and the ratios for the prefrontal cortex (PFC) and the visual cortex (VC) were then normalized with the HIP value.
The behavioral data of the delayed-response task performance in drug-treatment sessions were first separated as effective versus non-effective sessions with 85% criteria. A session with more than 25 hits in a 30-trial session was labeled as non-effective session. The effective-session data were then statistically analyzed using a two-way ANOVA with repeated measures. The delay length and drug treatment were used as within-subject factors. If a significant effect was noted, data were refined with the post hoc Tukey’s test by paired t-test (Tdep) or one-way ANOVA.
Results
Synaptic expression of NR2B is enriched more in the PFC than in other brain areas in both rats and monkeys
To evaluate the subunit components of GluR2 across the PFC, hippocampus (HIP) and visual cortex (VC), immunoblot was used to detect the protein of NR2B and NR2A from the brain tissues of adult rats (Fig. 1A) and monkeys. To restrict the protein source from synapses, synaptosomes were isolated from each brain region. As shown in Fig. 1B and C, both NR2B and NR2A protein were detected in all synaptosome samples from the mPFC, HIP and VC of rats. PSD95 was used as inner synaptosome amount control for each batch of samples, and then the NR2B/2A ratio for the HIP was normalized as “1”. For all three Immunoblot runs, the NR2B/2A ratio for the mPFC was all higher than for the HIP (1.771 ± 0.234 fold), while that for the VC was all lower than for the HIP (0.736 ± 0.032 fold) (Fig. 1D). The same experiments were repeated with brain samples of the monkeys (Fig. 1E). Similarly, both NR2B and NR2A were detected in synaptosome samples from the dlPFC (principal sulcus area), HIP and VC (Fig. 1F and G). Consistent with the result in rats, the NR2B/2A ratio was reliably higher in the dlPFC than in the HIP (1.226 ± 0.081 fold), and that in the VC was lower than in the HIP (0.511 ± 0.149 fold) (Fig. 1H). Therefore, our data were consistent with the hypothesis that NR2B is distributed across the brain with an ascending gradient from posterior to anterior, while NR2A is with an opposite gradient.
(A) Anatomy location of the rat medial prefrontal cortex (mPFC). Samples were obtained mainly from prelimbic area (PrL) that covered from anterior bregma 5.0 to 2.5 mm, including partial anterior cingulate (Acg) area. (B) Western blot (WB) showing NR2B expression in purified synaptosomes from the mPFC, hippocampus (HIP) and visual cortex (VC) samples of rats. PSD95 was used as synaptosome amount control, and actin as total protein control. (C) NR2A was paralleled run using the same batch of rat samples for NR2B. (D) Relative NR2B/2A expression ratio analysis showing a higher 2B/2A ratio in the mPFC than in the HIP and VC. Tissue samples for each brain area were pooled from two male rats with age of 3 months. WB was repeated 3 times for all samples. (E) Diagram of the monkey brain showing the dorsolateral prefrontal cortex (dlPFC), HIP and VC. Area marked with the red circle represents the principle sulcus (PS). (F) WB showing synaptic NR2B expression in the samples from the PS, HIP and VC of monkeys. (G) NR2A was paralleled run using the same batch of monkey samples for NR2B. (H) Relative NR2B/2A expression ratio analysis showing a higher 2B/2A ratio in the PFC than in the HIP and VC. Samples were from one rhesus monkey (macaca mulatta) with age of 6 years. Data are presented as mean ± SEM.
Online blockage of NR2B disrupts working memory-related delay cell direction preferences in monkey dlPFC
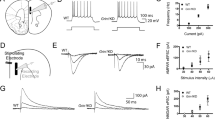
Similar as the previous study by Wang et al. [11], we here again examined the effect of NR2B blockade on the persistent delay firing of dlPFC neurons in monkeys performing an oculo-motor delayed response (ODR) task. Acute single-unit recordings from the dlPFC were established when the monkeys were performing the task. Delay cells exhibited elevated persistent firing during the delay epoch (delay cells) at one or more than one direction. The direction with highest delay firing was defined as the cell’s preferred direction, and its opposite direction was defined as the nonpreferred direction. Two-way ANOVA was used to examine the spatial tuned delay-related activity regarding: (1) different periods of the task (delay vs. fixation) and (2) different cue locations (preferred direction vs. nonpreferred direction). Delay cells that maintained persistent firing through the delay period were real-time identified. After that, the NR2B antagonist TCN237 was iontophoretically applied onto the cells with constant current of 15–25 nA. A total of 14 delay cells were successfully recorded and received the drug administration. As shown in Fig. 2A–C, iontophoresis of TCN237 decreased the cell firing rate overall for both preferred (p < 0.0001, paired t-test) and nonpreferred (p < 0.01, paired t-test) at a dose-dependent manner. However, the decreases in the preferred direction were more dramatic. Thus, the spatial tuning index, i.e. the ratio of firing rate in preferred vs. nonpreferred directions, was significantly decreased (p < 0.001, paired t-test) (Fig. 2D). This result again indicates that NR2B-containing NMDARs are involved in maintaining the working memory-related delay persistent firing of dlPFC neurons.
(A) A single neuron example of the dose-dependent effects of TCN237 on delay-related firing. Raster and histograms for the neuron’s preferred and non-preferred directions are shown. TCN237 produced a dose-dependent decrease in delay-related firing selectively for the neuron’s preferred direction. Activity was partially recovered after drug application was terminated. (B) Population spike density functions for the average of 14 delay cells showing firing for their preferred vs. nonpreferred directions under control condition (blue) and following iontophoresis of TCN237 (red). Results represent mean ± SEM. (C) Histograms showing the mean firing rate (± SEM) of the 14 delay cells during the delay epochs of the task for their preferred vs. nonpreferred directions under control condition (blue) and following iontophoresis of TCN237 (red). Statistical analysis shows that TCN237 significantly reduced the delay firing, especially for neurons’ preferred direction (p < 0.001). (D) Spatial tuning index (d’) is calculated by comparing each neuron’s delay firing for its preferred vs. nonpreferred directions. Greater d’ value indicates greater directional selectivity, i.t., greater spatial tuning. Iontophoresis of TCN237 significantly reduced the spatial tuning of the 14 delay cells. **p < 0.01, ***p < 0.001, compared with control condition; paired t-test.
Intra-PFC infusion of NR2B antagonist impairs spatial working memory performance in both rats and monkeys
To examine the necessity of prefrontal NR2B-containing NMDARs for executing working memory tasks, both rodent and primate versions of spatial working memory tasks were employed.
Rats were trained to perform a delayed alteration T-maze (DAT) task, where the animal had to remember the location where it got food reward in the previous trial and maintain the information during the delay period to instruct the choice to the opposite direction in the next trial (Fig. 3A and B). After the animal reached a reliable performance (less than 2 errors in a 10-trial session), the NR2B antagonist Ro25-6981 (0.5 μg in 1 μl PBS per side) was infused to the medial prefrontal cortex (PrL area) of both hemispheres (Fig. 3C). The DAT testing started at 15 min post-infusion. The rats performed the task at the previous day with 2.00 ± 0.47 win-shift errors and only one rat made 1 lose-shift error. Upon the infusion of Ro25-6981, the win-shift errors increased up to 5.75 ± 0.43 and the lose-shift errors increased to 3.75 ± 0.43, yielding a total of 9.50 ± 0.50 errors (a chance level of performance). The task performance was recovered to the pre-infusion level three days later. However, the control rats treated with PBS were not affected, either on the testing session or in the recovery session (Fig. 3D). Thus, the inhibition of NR2B-containig NMDARs in the mPFC disrupted the spatial working memory capacity of the rats.
(A) Flow of the delayed alteration T-maze task. The initial trial (trial “0”) was initiated with a free choice to left or right arm by the animal. In the following trials, the animal must choose the arm opposite to the one visited in the previous trial to get reward. An incorrect choice was followed by error-correction trial(s), in which the baited arm kept unchanged. The interval between trials, i.e., the delay period, was 5 s. (B) Diagram showing the definition of error types. (C) Reconstruction of the infusion sites for the NR2B antagonist Ro25-6981 in the mPFC (left) and representative coronal section with neutral red straining showing the trace of infusion needle (right). The infusion sites were between Bregma 3.20 to 4.00 mm. (D) T-maze task performance of the rats under control and drug infusion conditions. The task performance at 15 min after infusion of Ro25-6981 (left, n = 8 rats), but not PBS (right, n = 7 rats), was significantly impaired, with both Win-Shift failure and Lose-Shift failure increases. The task performance recovered at post-infusion day 3 in the Ro25-6981 group. ***p < 0.001, paired t-test.
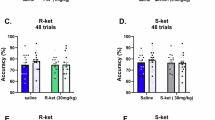
Monkeys were trained to perform a delayed response task using the Wisconsin General Test Apparatus, where the animals had to remember the location of a baited well and make choice between the left and right wells (Fig. 4A). For each session, one pair of symmetrical cannulas were selected for drug infusion. Saline was used as vehicle control. The GABAergic receptor agonist muscimol, which is widely used to inactivate a particular brain site for functional identification, was employed to identify the importance of the infusion sites (Fig. 4B and C) for working memory. As shown in Fig. 4D, the task performance remained intact in the saline sessions in both monkeys. However, infusion of Ro25-6981 (15 μg in 3 μl saline for each cannula) significantly impaired the task performance. Two-way ANOVA with Turkey’s repeated measure showed that there were significantly delay-dependent decreases in the correct rate of performance in both monkeys (p < 0.001 for treatment, p < 0.001 for treatment × delay interaction in both monkeys). Infusion with muscimol (7.5 μg in 3 μl saline for each cannulae) produced similar deficit in the task performance (p < 0.001 for treatment, p < 0.001 for treatment × delay interaction in both monkeys), indicating that the infusion sites were essential for working memory. Paired t-test analysis showed that the performance upon Ro25-6981 or muscimol infusion was intact at the “0”-second delay but was significantly impaired at the 20-second and 40-second delay, in both monkeys (Fig. 4D).
(A) Design of the spatial delayed response task for assessing working memory and pattern discrimination task for assessing long-term memory. For the working memory task, one of two food wells was baited under the gaze of monkey, and for the long-term memory task, food well marked with triangle sign was baited. Pattern discrimination task session always followed delayed response task session immediately. No re-correction trial was used. (B) representative coronal section with Nissl staining showing the principal sulcus and a trace of injection at the lateral bank from monkey #2. (C) Reconstruction of each drug infusion sites for both monkeys. Canulae were implanted with row assembles. For each experimental session, only one symmetrical canulae from each side was used for drug infusion. Effective site was defined as with less than 27 of 30 correct trials, and non-effective site was with 27 or more of 30 correct trials. All infusion sites for monkey #1 were effective and used for statistical analysis (n = 21 for Ro25-6981; n = 16 for muscimol). One canulae row was discarded in monkey #2 since muscimol infusion produced no effect, and the remaining sites were effective and used for statistical analysis (n = 11 for Ro25-6981; n = 9 for muscimol). All sites were repeated with saline infusion. (D) Correct rates of task performance for each monkey under intra-dlPFC infusion of saline, Ro25-6981 and muscimol. The correct rates of performance for the working memory task at the delays of 20 s and 40 s but not 0 s were dramatically reduced upon infusion of Ro25-6981 or muscimol, while those for the pattern discrimination task remained unchanged under all the conditions. Two-way ANOVA showed significant drug effects (p < 0.001) and drug × delay interaction (p < 0.001) for both monkeys, and no difference was detected between Ro25 and muscimol infusions. ***p < 0.001, post-hoc analysis with paired t-test. Data were presented as mean ± SEM.
A pattern discrimination for long-term memory was tested following each delayed-response task sessions. Ro25-6981 or muscimol produced no deficit in the performance of pattern discrimination (Fig. 4D; All T < 1.58, p > 0.14). This is within expectation, as the dlPFC is not essential for the long-term memory for pattern or pattern discrimination. This result further supported that the impaired performance in the delayed response task was not the outcome of impaired capability-in-general for task execution or motivation, but impaired working memory capacity per se.
Discussion
The present study investigated the synaptic expression of NMDA receptor subunit NR2B in the visual cortex, hippocampus and prefrontal cortex. Our data confirmed the hypothesis that NR2B is enriched more in the post-synapses of the prefrontal cortex than in the hippocampus and visual cortex, strengthening the molecular basis of the neocortical hierarchical principle. The striking behavioral impairment of NR2B inhibition in working memory in both rodents and primates emphasized the functional significance of NR2B-containing NMDARs on the cognitive execution of the PFC.
The significance of NR2B-containing in PFC for working memory has been long suggested by studies with NR2B over-expressed transgenic mice [23] and electrophysiological data from both rodents and primates [11, 19]. A previous study in monkeys with systemic administration of drugs also supported the importance of NR2B for working memory [24]. However, the present study provided direct evidence showing that prefrontal cortical NR2B-containing NMDRs are crucial for working memory performance, both in rats and monkeys.
NR2B is required for working memory in the hippocampus and PFC in rodents. The hippocampus could be constantly required for spatial information encoding and interaction with the PFC. Inhibition of NR2B subunit in the hippocampal CA1 impaired delayed alternation working memory performance with longer delay [19]. Genetic depletion of NR2B specifically in the hippocampus did not affect spatial learning in water maze, but impaired working memory for recently-visited places [25]. In the present study, intra-mPFC blockade of NR2B disrupted the task performance of the rats (to a chance level of performance), suggesting a more critical role of NR2B in the mPFC for spatial working memory. A previous study showed that trace fear memory, which involved working memory components, was impaired upon intra-mPFC blockade of NR2B [26]. Similarly, intra-dlPFC blockade of NR2B severely impaired monkey’s performance on the delayed response task, in a delay-dependent way. Taken together, the present study indicates that NR2B-containing NMDARs in the PFC are essential for working memory across mammalian species.
The present study confirmed a relatively higher level of synaptic NR2B in the PFC than in the hippocampus and visual cortex. It is known that NR2B expression occurs prenatally and is maintained at a relatively high level in adult CNS [12, 27,28,29]. There is a down-regulation of NR2B from young to adult and a location shift from post-synapse to extra-synapse during CNS maturation in many brain areas such as the visual cortex and hippocampus, and the synaptic NR2B is partially replaced by NR2A [30,31,32,33,34]. An optimal synaptic NR2B/NR2A ratio is a good balance between plasticity and stability for information processing and storage. The slower kinetics of NR2B was predicted to be suitable for working memory function with computation models [35, 36]. The ability of NR2B to generate recurrent local network firing may be more critical than long-term plasticity in the PFC for cognitive functions such as working memory.
It should be noted that the mPFC in rodents is not equivalent to the dlPFC in primates. While the agranular parts of the PFC emerged in early mammals and are inherited by both rodents and primates [37], the granular part (layer 4) of the PFC is specific to primates but absent in rodents. The evolution of layer 4 granular cells in the PFC could enhance and refine the information processing of local networks within the PFC and enable more powerful capacity and robustness of working memory in primates.
In the electrophysiological part, the present study focused on the effect of NR2B blockade on the persistent spiking during the delay. Micro-iontophoresis could only eject very small amounts of drug molecules that influence nearby neurons, and thus could not affect behavioral performance. The NR2B antagonist was dissolved at 0.01 M concentration in sterile water with pH 3–4 in this study. The range of the iontophoretic currents were 15–25 nA. Although it was difficult to measure the ‘dose’ of the antagonist, the drug ejected at currents of 15–25 nA was expected to produce roughly 0.1 μM of concentration near a recorded neuron [22].
For decades, classic model has proposed persistent spiking for maintenance or representation of working memory [16, 38], which is further supported by computational evidence that the slow kinetics of NR2B-containing NMDARs are involved in generating recurrent and persistent excitation of pyramidal cells [39]. In recent years, a hybrid attractor-dynamic and synaptic model has been proposed for working memory [40]. According to this model, sparse and bursty spiking rather than persistent spiking contribute to working memory maintenance, and synaptic weight changes between sparse bursts of spiking strengthen working memory maintenance, leaving “impressions” in the neocortex [41]. Despite an integrative theory for neuronal representation of working memory remains to be established, it is possible that NR2B-containing NMDARs are involved in both mechanisms: generating recurrent excitation and mediating synaptic weight changes between pyramidal cells in the dlPFC.
Data availability
Data is available upon request to authors.
References
Reid AT, Krumnack A, Wanke E, Kötter R. Optimization of cortical hierarchies with continuous scales and ranges. Neuroimage. 2009;47:611–17.
Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008;28:2539–50.
Hansen JY, Markello RD, Vogel JW, Seidlitz J, Bzdok D, Misic B. Mapping gene transcription and neurocognition across human neocortex. Nat Hum Behav. 2021;5:1240–50.
Siu C, Balsor J, Merlin S, Federer F, Angelucci A. A direct interareal feedback-to-feedforward circuit in primate visual cortex. Nat Commun. 2021;12:4911.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–99.
Burt JB, Demirtas M, Eckner WJ, Navejar NM, Ji JL, Martin WJ, et al. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci. 2018;21:1251.
Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–92.
Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24.
Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–66.
Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–8.
Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–49.
Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40.
Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35.
Baddeley A. The fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13468–72.
Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–97.
Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–6.
Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85.
Tieze SM, Chandra SS, Vidyadhara DJ. Subcellular fractionation for the isolation of synaptic components from the murine brain. Jove-J Vis Exp. 2022;187: https://doi.org/10.3791/64574.
Zhang XH, Liu SS, Yi F, Zhuo M, Li BM. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain. 2013;6:13.
Cai JX, Ma YY, Xu L, Hu XT. Reserpine impairs spatial working memory performance in monkeys: reversal by the α2-adrenergic agonist clonidine. Brain Res. 1993;614:191–96.
Ou LY, Tang XC, Cai JX. Effect of huperzine A on working memory in reserpine- or yohimbine-treated monkeys. Eur J Pharmacol. 2001;433:151–56.
Gerhardt GA, Palmer MR. Characterization of the techniques of pressure ejection and microiontophoresis using in vivo electrochemistry. J Neurosci Methods. 1987;22:147–59.
Cui Y, Jin J, Zhang X, Xu H, Yang L, Du D, et al. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One. 2011;6:e20312.
Weed MR, Bookbinder M, Polino J, Keavy D, Cardinal RN, Simmermacher-Mayer J, et al. Negative allosteric modulators selective for the NR2B subtype of The NMDA Receptor impair cognition in multiple domains. Neuropsychopharmacol. 2016;41:568–77.
von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–60.
Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–4.
Sun L, Shipley MT, Lidow MS. Expression of NR1, NR2A-D, and NR3 subunits of the NMDA receptor in the cerebral cortex and olfactory bulb of adult rat. Synapse. 2000;35:212–21.
Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, et al. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90.
Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci USA. 2008;105:20953–8.
Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76:189–211.
Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999;96:12876–80.
Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–78.
Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–34.
Sans N, Petralia RS, Wang YX, Blahos J 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–71.
Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–63.
Pereira J, Wang XJ. A Tradeoff between accuracy and flexibility in a working memory circuit endowed with slow feedback mechanisms. Cereb Cortex. 2015;25:3586–601.
Preuss TM, Wise SP. Evolution of prefrontal cortex. Neuropsychopharmacol. 2022;47:3–19.
Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–80.
Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–603.
Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405.
Miller EK, Lundqvist M, Bastos AM. Working Memory 2.0. Neuron. 2018;100:463–75.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (STI 2030-Major Project 2021ZD0201705 to B.L.), the National Natural Science Foundation of China (32060199 and 32360197 to J.P.), Jiangxi Provincial Natural Science Foundation (20224ACB206016 to J.P.), China Postdoctoral Science Foundation [20080440581 to G.W.] and US National Institute on Aging (RF1 AG083090 to M.W.).
Author information
Authors and Affiliations
Contributions
B.L. conceived of the study. J.P., G.W., M.W., Y.H. and L.Q. performed the experiments. J.C. and A.F.T.A. monitored the experiments. J.P., G.W. and W.M. analyzed the data. J.P., G.W. and B.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental procedures were performed in accordance with the Use and Care of Laboratory Animals Guidelines issued by the Chinese government and the US National Institute of Health Guidelines for Use and Care of Laboratory Animals. The experimental procedures with rats were approved by the Ethical Committee of Animal Experiments at Fudan University (20080930R) and Nanchang University (2019-0301M). The electrophysiological experimental protocols with monkeys were approved by the Yale University IACUC (2021-07638) and the behavioral experimental protocols with monkeys by the Laboratory Animal Supervision Committee of Yunnan, China (2008-1010M). This study did not involve human participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, J., Wang, G., Han, Y. et al. Prefrontal cortical NR2B-containing NMDA receptors are essential for spatial working memory performance. Transl Psychiatry 15, 357 (2025). https://doi.org/10.1038/s41398-025-03595-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03595-x