Abstract
Mutations in the RBFOX1 gene are associated with psychiatric disorders but how RBFOX1 influences psychiatric disorder vulnerability remains unclear. Recent studies showed that RBFOX proteins mediate the alternative splicing of PAC1, a critical HPA axis activator. Further, RBFOX1 dysfunction is linked to dysregulation of BDNF/TRKB, a pathway promoting neuroplasticity, neuronal survival and stress resilience. Hence, RBFOX1 dysfunction may increase psychiatric disorder vulnerability via HPA axis dysregulation, leading to disrupted development and allostatic overload. To test this hypothesis, we generated a zebrafish rbfox1 loss of function (LoF) line and examined behavioural and molecular effects during development. We found that rbfox1 LoF mutants exhibited hyperactivity, impulsivity and heightened arousal, alongside alterations in proliferation – traits associated with neurodevelopmental and stress-related disorders. In adults, loss of rbfox1 function led to decreased fertility and survival, consistent with allostatic overload. At the molecular level, at larval stages rbfox1 mutants showed increased cortisol levels and disrupted expression of key stress-related genes (bdnf, trkb2, pac1a-hop, crhb, nr3c2). Pharmacological intervention targeting TRKB restored crhb and nr3c2 gene expression and hyperactive and hyperarousal behaviours. In adults, dysregulation of crhb, nr3c2 and bdnf/trkb2 genes was only seen following acute stress exposure. Our findings reveal a fundamental role for RBFOX1 in integrating stress responses through its regulation of BDNF/TRKB and neuroendocrine signalling.

Similar content being viewed by others
Introduction
Psychiatric disorders are influenced by multiple genes, with a substantial portion of their heritability linked to common genetic variations [1]. Identifying gene variants associated with multiple psychiatric disorders is crucial for understanding their shared genetic underpinnings and enhancing therapeutic strategies.
RNA binding fox-1 homologue 1 (RBFOX1), also known as FOX1 or ataxin 2-binding protein 1 (A2BP1), is a splicing factor highly conserved among vertebrates and expressed in the heart, brain and skeletal muscle, where it contributes to normal development and function [2, 3]. Alterations in RBFOX1 expression or function have been associated with susceptibility to psychiatric disorders, particularly those associated with changes in stress-related behaviour [4,5,6]. In a cross-disorder genome-wide association study (GWAS), RBFOX1 emerged as the second most pleiotropic locus, showing association with seven out of the eight disorders studied: schizophrenia, bipolar disorder, depression, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), obsessive-compulsive disorder and Tourette syndrome [1]. In mice and zebrafish, Rbfox1/rbfox1 knockout caused hyperactivity, increased anxiety-like behaviour and altered social behaviour [2, 6]. However, the mechanisms by which RBFOX1 genetic variants contribute to psychiatric disease are poorly understood.
In both mice and zebrafish, it has been suggested that Rbfox1 regulates the Pituitary Adenylate Cyclase-Activating Polypeptide Type 1 Receptor (Pac1) alternative splicing, an important mediator of corticotropin releasing hormone (CRH) synthesis in the hypothalamus [4, 7]. CRH is the first hormone to be secreted in response to stress by the hypothalamic-pituitary-adrenal (HPA) axis (hypothalamic-pituitary-interrenal (HPI) axis in fish) [8]. In mammals there are several PAC1 isoforms with the predominant isoforms in the brain being PAC1-hop (long isoform) and PAC1-short [9]. In mice and zebrafish, following acute stress exposure, both Pac1-hop/pac1-hop and Pac1-short/pac1-short expression increased, while at the late recovery phase, only Pac1-hop was still up-regulated [4].
Another regulator of the stress response and a resilience factor against chronic stress-induced psychopathology is the brain derived neurotrophic factor (BDNF)/Tropomyosin receptor kinase B (TRKB) pathway [10,11,12]. BDNF is a neurotrophin possessing a pivotal role in the modulation of neurotransmission and synaptic plasticity and dysregulation of the BDNF/TRKB pathway has been associated with several neuropsychiatric diseases, including anxiety/stress disorders [13, 14]. In human neural stem cells, RBFOX1 knockdown increased BDNF expression levels [15], while another study in mice identified TrkB as a target of RBFOX1 within the hippocampus [16]. Further, PAC1-short activation can elevate BDNF levels and potentiate TRKB activity, enhancing BDNF/TRKB neuroprotective and plasticity-promoting effects, especially in the context of stress response and neuropsychiatric health [17].
Given that both PAC1 and BDNF/TRKB influence how the brain adapts to and manages stress, RBFOX1 variants may increase susceptibility to psychiatric disorders through dysregulation of the stress response, leading to adaptive plasticity and disrupted development in the short-term and allostatic overload in the long-term. Allostasis refers to the collective processes by which the body actively maintains stability (or homeostasis) in response to environmental stressors [12]. When allostasis primary mediators (e.g., HPA axis hormones, catecholamines, cytokines) are overactivated or fail to return to normal, it leads to an allostatic state [18, 19]. The cumulative results of an allostatic state are referred to as allostatic load and, when maladaptive, allostatic overload [18]. Excess glucocorticoid exposure during early life and early life stress have been shown to cause prolonged activation of the allostatic systems, ultimately leading to allostatic overload [19,20,21,22], while neurotrophic factors such as BDNF play a key role in regulating adaptive plasticity and mechanisms counteracting damage caused by allostatic overload [12]. Here, to explore the possibility that RBFOX1 loss of function (LoF) leads to increased vulnerability to psychiatric disease through HPA axis hyperactivation and allostasis-induced adaptation during development, we generated a zebrafish line carrying a predicted rbfox1 LoF mutation using CRISPR-Cas9 gene editing and assessed behavioural and molecular changes at different developmental stages. We hypothesised that RBFOX1 regulates HPA axis activity through an effect on BDNF/TRKB signalling leading to disrupted brain development.
Results
Generation of a loss of function rbfox1 line
CRISPR-Cas9 genome editing generated a 19 base pair deletion (NM_001005596.1, nucleotides 120-138, TCCCATCGGCCCAGTTCGC) that introduced a premature termination codon (PTC) at position 58 in the rbfox1 amino acid sequence. This line is recorded on ZFIN as qm4, ZDB-ALT-240222-6. Details regarding the generation of the line, nucleotide and amino acid sequences can be found in our previous study [2]. Wild type animals are denoted throughout as rbfox1+/+, heterozygous mutants as rbfox1+/19del and homozygous mutants as rbfox119del/19del.
To evaluate whether the PTC elicited mRNA non-sense mediated decay (NMD) and consequent reduction of rbfox1 mRNA in mutant fish, we examined rbfox1 expression by quantitative Real-Time polymerase chain reaction (qPCR) and by in situ hybridisation (ISH). As RBFOX1 itself is alternatively spliced to generate nuclear and cytoplasmic isoforms [7], we designed primers targeting all rbfox1 zebrafish isoforms available on NCBI (see Supplementary Table 1). qPCR showed that rbfox1 transcript levels were significantly lower in mutant larvae compared to rbfox1+/+ siblings (prbfox1+/+ vs rbfox1+/19del < 0.05, prbfox1+/+ vs rbfox119del/19del < 0.01), and ISH showed that rbfox1 was not detectable in rbfox119del/19del fish, at either larval or adult stages (Supplementary Figure 1A–C).
These results confirm degradation of defective rbfox1 mRNA in mutant fish.
rbfox1 mutant fish show hyperactivity, impulsivity and hyperarousal behaviour
As mutations in RBFOX1 locus have been linked to several psychiatric diseases in humans, we assessed zebrafish larvae and adult fish for phenotypic traits associated with such disorders and examined rbfox1 mRNA distribution in rbfox1+/+ larvae and adults (Tübingen strain).
In humans, RBFOX1 copy number variants (CNVs) and LoF mutations are risk factors for ADHD [6]. Two major ADHD traits are hyperactivity and increased impulsivity. We therefore assessed hyperactive and impulsive behaviour of rbfox1+/+, rbfox1+/19del and rbfox119del/19del 5 days post fertilisation (dpf) larvae and adults. When we measured larval locomotion, we observed a gene dosage effect on distance travelled, whereby rbfox1 mutant larvae travelled greater distances than wild type siblings (p rbfox1+/+ vs rbfox1+/19del < 0.05, p rbfox1+/+ vs rbfox119del/19del < 0.0001, p rbfox1+/19del VS rbfox119del/19del < 0.05) (Supplementary Figure 2A), and a significant increase in the swimming speed of rbfox119del/19del larvae (p rbfox1+/+ vs rbfox119del/19del < 0.0001, p rbfox1+/19del VS rbfox119del/19del < 0.05) (Supplementary Figure 2B). This is in line with hyperactivity observed in adult rbfox119del/19del, in our previous study and in other rbfox1 model [2, 23]. We also measured larval burst swimming, a parameter previously used as a measure to predict impulsive behaviour in zebrafish larvae [24]. We found a significant increase in the number of peaks (acceleration events when the fish travelled > 5 mm in <12 s) in rbfox119del/19del larvae (p rbfox1+/+ vs rbfox119del/19del < 0.0001, p rbfox1+/19del VS rbfox119del/19del < 0.0001) (Fig. 1A). Impulsive behaviour was then assessed in adult (7 months old) fish using the 5-choice serial reaction time task (5-CSRTT) [25]. The 5-CSRTT assay measures sustained attention and impulsive action by requiring an animal to detect a brief visual cue (white light stimulus) presented randomly across one of five apertures and “nosepoke” that aperture to signal recognition (Supplementary Figure 3). If correct, a “food” signal light comes on (at the opposite end of the five apertures) and the animal collects food reward (Supplementary Figure 3). Crucially, a pause occurs prior to the onset of the stimulus light (called a pre-stimulus interval [PSI]), during which a “premature” response can be interpreted as “impulsivity” - impulsive action [25]. The assay consists of five stages (Supplementary Table 2) each run for at least a week until fish are promoted to the next stage. We found that 79% rbfox1+/+, 72% rbfox1+/19del and 62% rbfox119del/19del learned the task within 9 weeks. We found no significant differences in the correct responses (stages 2–5, Fig. 1B–E). In stage 5, we found a significant difference in the number of premature responses (increased impulsivity) such that rbfox119del/19del fish were more impulsive compared to rbfox1+/+ siblings (p < 0.05) (Fig. 1F).
A number of peaks/swimming burst (acceleration events when the fish travelled > 5 mm in < 12 s) in 5 days post fertilisation (dpf) zebrafish larvae; N = rbfox1+/+ 24; rbfox1+/19del 24; rbfox119del/19del 24. B–F 5-choice serial reaction time task in adult (7 months old) zebrafish (rbfox1+/+, rbfox1+/19del, rbfox119del/19del): B number of initiated trials during stage 2 (weeks 2–4); all rbfox1+/+ (19), all rbfox1+/19del (18) and all rbfox119del/19del (40) moved to the next stage; C-E average correct responses for stages 3–5 (weeks 3–9); C at the end of stage 3 (weeks 3–7), all rbfox1+/+ (19), 17 rbfox1+/19del and 39 rbfox119del/19del moved to the next stage; D at the end of stage 4 (weeks 4–8), all rbfox1+/+ (19), all rbfox1+/19del (17) and 33 rbfox119del/19del moved to the next stage; E at the end of stage 5 (weeks 5–9), 15 rbfox1+/+, 13 rbfox1+/19del and all rbfox119del/19del (33) showed ≥ 50% correct responses; F premature responses during stage 5 (weeks 5-9). Each dot, square or triangle in D-H) represents a single adult zebrafish rbfox1+/+, rbfox1+/19del and rbfox119del/19del respectively; N = rbfox1+/+ 19; rbfox1+/19del 18; rbfox119del/19del 40. G, H Forced light-dark transition assay in 5dpf zebrafish larvae (rbfox1+/+, rbfox1+/19del, rbfox119del/19del): G distance travelled during the 10 min assay; H 1 s time bin resolution plots of the dark-light transition; N = rbfox1+/+ 43; rbfox1+/19del 34; rbfox119del/19del 37. All larvae employed in behavioural experiments were progeny of rbfox1+/19del in-cross and were genotyped after experiments and prior to data analysis. In all graphs: bars represent standard error of the mean (SEM); * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
RBFOX1 CNVs have also been identified in individuals with schizophrenia [6, 15]. Deficits in habituation to acoustic startle reflex are seen in both schizophrenic patients and animal models of schizophrenia [26]. The acoustic startle assay has been widely employed to measure habituation in animals, including zebrafish [27]. The magnitude of the startle response and the extent of habituation can also serve as indicators of hyperarousal linked to heightened stress responses [28]. We therefore tested 5dpf zebrafish larvae (rbfox1+/+, rbfox1+/19del and rbfox119del/19del) in the habituation to acoustic startle response assay. Consistent with our hyperactivity assay, during the baseline (first 10 min), the genotype had a significant main effect on distance travelled [Effect of genotype: χ2(2) = 8.3397, p < 0.05] whereby rbfox119del/19del larvae travelled greater distances than rbfox1+/+ siblings (p < 0.05). During the startle stimuli, we observed a main effect of genotype [Effect of genotype: χ2(2) = 33.536, p < 0.0001] and stimulus number [Effect of stimulus number: χ2(9) = 487.968, p < 0.0001] on distance travelled, and a significant two-way interaction between genotype and stimulus number [Effect of genotype by stimuli: χ2(18) = 31.511, p < 0.05], whereby rbfox119del/19del larvae travelled greater distances than rbfox1+/+ (p < 0.001) and rbfox1+/19del (p < 0.01) (Supplementary Figure 2C). When assessed for the rate of habituation, wild type larvae showed a habituation response to repeated acoustic startle consistent with previous reports [29]: 100% of rbfox1+/+animals responded to the first acoustic stimulus, but only 22% responded to the last. When we examined the rate of habituation over time, in line with previous findings [30], we observed a significant genotype effect [Effect of genotype: χ2(2) = 7.2676, p < 0.05] and a significant two-way interaction between genotype and stimulus number [Effect of genotype by stimulus number: χ2(18) = 132.8476, p < 0.001] whereby rbfox119del/19del showed reduced rate of habituation and a greater proportion of responders compared to rbfox1+/+ siblings (p rbfox1+/+ vs rbfox119del/19del < 0.05) (Supplementary Figure 2D).
SNPs in RBFOX1 locus have also been associated with anxiety [6, 31]. Hence, we assessed anxiety-like behaviour in 5dpf zebrafish larvae using the forced light-dark transition (FLDT) assay and in adult zebrafish using the novel tank diving (NTD) assay. In the FLDT assay, zebrafish are exposed to sudden transitions in illumination with effects on locomotion and amplitude of response on transition from dark to light being used as a measure of anxiety-like behaviour: the increased locomotion/startle response upon the light-dark transition is a measure of anxiety-like behaviour [29, 32, 33]. Consistent with our acoustic startle assay, we found that during the baseline (first 5 min) (Fig. 1G) genotype had a significant main effect on distance travelled [Effect of genotype: χ2(2) = 14.4878, p < 0.001] whereby rbfox119del larvae travelled greater distances than rbfox1+/+ siblings (p rbfox1+/+ vs rbfox1+/19del < 0.05, p rbfox1+/+ vs rbfox119del/19del < 0.01). During the 1 min light flash (min 5–6 of the assay) we found a significant main effect of genotype on distance travelled [Effect of genotype: χ2(2) = 8.8518, p < 0.05] with rbfox119del larvae travelling greater distances than rbfox1+/+ siblings (p rbfox1+/+ vs rbfox1+/19del < 0.05, p rbfox1+/+ vs rbfox119del/19del < 0.05) (Fig. 1H). On transition from dark to light (analysed at 1 s time bin resolution from sec 240 to sec 360 as the startle response during the transition is visible only at this resolution) we found a significant main effect of time [Effect of time: χ2(118) = 1051.3482, p < 0.001] and genotype [Effect of genotype: χ2(2) = 8.9155, p < 0.05], and a significant two-way interaction between time and genotype [Effect of time by genotype: χ2(236) = 514.8308, p < 0.001] on the amplitude of response, whereby rbfox119del/19del larvae startled more than rbfox1+/+ (p < 0.05) and rbfox1+/19del (p < 0.05) siblings (Fig. 1H). As there was a significant difference in basal locomotion, we also examined the amplitude of response on transition from dark to light normalizing the data against baseline, as reported previously [34]. We observed similar results as in absence of normalisation: significant main effect of time [Effect of time: χ2(118) = 1249.918, p < 0.001] and genotype [Effect of genotype: χ2(2) = 37.716, p < 0.001], and a significant two-way interaction between time and genotype [Effect of time by genotype: χ2(236) = 4532.966, p < 0.001] whereby rbfox119del/19del larvae startled more than rbfox1+/+ (p < 0.0001) and rbfox1+/19del (p < 0.001) siblings.
Anxiety-like behaviour in adult animals was assessed using the NTD assay. When introduced to a novel tank, zebrafish will first dive to the bottom of the tank, to seek protection, and then gradually increase their swimming over time [35]. Over the entire duration of the NTD assay, we found no significant differences between rbfox1 genotypes (p > 0.05). However, during the first minute of the assay, we found a significant two-way interaction between genotype and the time spent at the bottom of the tank [Effect of genotype by proportion at the bottom tank: χ2(10) = 22.3333, p < 0.05], such that rbfox119del/19del fish spent less time at the bottom zone of the tank than rbfox1+/+ fish (p < 0.001) (Supplementary Figure 2E). In line with previous findings [2], we observed no significant differences in distance travelled (p > 0.05) (Supplementary Figure 3F), nor in the number of the transitions to the top area of the tank between rbfox1 genotypes (p > 0.05) (Supplementary Figure 3E).
Thus, our data showed that loss of rbfox1 resulted in behavioural changes in zebrafish that are relevant to core domains often altered in human psychiatric disorders (such as hyperactivity, impulsivity, reduced habituation and heightened arousal) and suggest that adaptation occurs as the animal develops.
rbfox1 is expressed in the developing neural and cardiac tissues and in adult brain regions involved in stress, social and emotional behaviours, reward and learning
Consistent with our behavioural results and as seen previously [2, 3], when we assessed rbfox1 mRNA distribution in rbfox1+/+ larvae and adults (Tübingen strain), we found that rbfox1 was expressed in regions of the brain involved in the response to stress, in social and emotional behaviour, and in reward and learning (Fig. 1A, B), in agreement with data in rodents [36] and humans [37].
In wild type larvae rbfox1 is expressed in the spinal cord and hindbrain lateral neurons (Fig. 1A, 28 hpf), whereas at later developmental stages, rbfox1 expression was detected in the mid- and hindbrain (Fig. 1A, 2–5 dpf) and in the heart (Fig. 1A, 5dpf), as described previously [38].
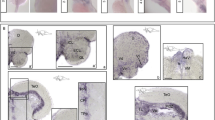
In adult fish, rbfox1 is expressed along the entire rostro-caudal brain axis. In the forebrain, rbfox1 was detected in the glomerular (GL), internal (ICL) and external (ECL) cellular layers of the olfactory bulbs (Fig. 1B a, a’). More caudally, rbfox1 expression was detected in the medial zone of the dorsal telencephalic area (Dm), and in the dorsal (Vd), lateral (Vl) and ventral (Vv) nuclei of the ventral telencephalic area (Fig. 1B b). In the diencephalon, rbfox1 expression was detected in the ventral habenular nucleus (HaV), in the anterior (A) and ventromedial (VM) thalamic nuclei (Fig. 1B c). In the midbrain, rbfox1 was detected in the posterior part of the parvocellular preoptic nucleus (PPp, Fig. 1B d), in the ventral zone of the periventricular hypothalamus (Hv, Fig. 1B d, e’’), in the periventricular gray zone (PGZ,) and in the central zone (CZ) of the optic tectum (Fig. 1B e, e’), in the torus longitudinalis (TL) (Fig. 1B e, e’, g), in the periventricular nucleus of posterior tuberculum (TPp), in the anterior tuberal nucleus (ATN, Fig. 2B e, e”), in the posterior tuberal nucleus of the hypothalamus (PTN, Fig. 1B f) and in the paraventricular organ (PVO, Fig. 1B f). In the hindbrain, rbfox1 was observed in the lateral division of the valvula cerebelli (Val) (Fig. 1B g).
A rbfox1 whole mount in situ hybridisation (ISH) on zebrafish larvae at 28 h post fertilisation and 3-4-5 days post fertilisation. B rbfox1 ISH on adult zebrafish (a - d) forebrain and (e-g) mid-/hind-brain transverse sections. Black boxes represent the region of the brain showed in higher magnification panels. Schematic depictions of the lateral view of the zebrafish brain indicate position of levels illustrated by ISH. Scale bars: 200 µm in A, 100 µm in B a-c, e, f); 50 µm in B) a’, d, e’, e”, g. Abbreviations: A, anterior tuberal nucleus; ATN, anterior thalamic nucleus; CZ, central zone of the TeO; D, dorsal area of dorsal telencephalon; DIL, diffuse nucleus of the inferior lobe of the hypothalamus; Dm, dorsal area of medial telencephalon; ECL, external cellular layer; GL, glomerular layer; HaV, ventral area of the habenula; Hv, ventral hypothalamus; ICL, internal cellular layer; PGZ, periventricular gray zone; PPp, parvocellular preoptic nucleus, posterior part; PTN, posterior tuberal nucleus of the hypothalamus; PVO, paraventricular organ; TeO, optic tectum; TL, longitudinal tori; TPp, periventricular nucleus of the posterior tuberculum; Val, valvula cerebelli; Vd, ventral area of dorsal telencephalon; Vl, central area of lateral telencephalon; VM, ventromedial nucleus; Vv, ventral area of ventral telencephalon.
rbfox1 mutant larvae show elevated cortisol levels and altered expression of crhb, nr3c2, bdnf and trkb2
As RBFOX1 has been linked to regulation of HPA axis and BDNF/TRKB gene expression [4, 15, 16], we examined the expression of components of the HPI axis and of bdnf/trkb2. In parallel, we measured whole-body cortisol levels, in resting physiological conditions, at both larval and adult stages, to assess physiological outcomes. Since we did not observe significant differences in behaviour between wild type and heterozygous animals, we employed wild type and homozygous animals only.
We performed qPCR experiments in 5dpf zebrafish larvae to assess changes in the expression levels of the HPI axis markers corticotropin releasing hormone (crhb), mineralocorticoid receptor (nr3c2), glucocorticoid receptor (nr3c1), corticotropin releasing hormone receptor 1 (crhr1), corticotropin releasing hormone receptor 2 (crhr2), mineralocorticoid receptor 1 (mc1r), proopiomelanocortin a (pomca) and Krüppel-like factor 9 (klf9), as well as of bdnf and trkb2. In teleosts, the duplication of the genome gave rise to two CRH genes, crha and crhb, and two TRKB genes, trkb1 and trkb2 [39, 40]. Among these, crhb and trkb2 are most commonly referred to as the functional orthologues of mammalian CRH and TRKB respectively, whereas crha and trkb1 function appear more divergent and remain less well understood [39, 40]. Zebrafish also possess two pomc paralogues (pomca and pomcb), but only pomca is functionally relevant for the activation of the HPI axis [41], and six melanocortin receptors (mc1r, mc2r, mc3r, mc4r, mc5ra, mc5rb). Among these, we selected mc1r as representative of melanocortin signalling. Then, unlike other teleosts, zebrafish possess a single copy of the MR (nr3c2), GR (nr3c1) and KLF9 (klf9) genes, and a single copy of the BDNF gene (bdnf) [40, 42]. In mammals, several TRKB splicing isoforms are present, but the most abundant ones are the full-length (TRKB.FL/TK+) and the truncated (TRKB.T1/TK-), this latter lacking the catalytic tyrosine kinase (TK) domain [43]. As in zebrafish the presence of both trkb2 full-length and truncated forms has been demonstrated [44], here we used a pair of trkb2 TK+/TK- common primers, and another pair targeting only TK+ to distinguish effects on the expression of the two isoforms.
In rbfox119del/19del larvae, we observed a significant upregulation of bdnf (p < 0.05) and TK+ (p < 0.05), and a significant downregulation of TK- (p < 0.05) expression levels (Fig. 3A). Regarding HPI axis, in rbfox119del/19del larvae we found a significant upregulation of crhb (p < 0.0001) and a significant upregulation of nr3c2 (p < 0.01) (Fig. 2B). We observed no significant changes in the expression levels of the other genes examined (p > 0.05) (Fig. 3B).
A Expression levels of bdnf and trkb2 full-length (TK+) and truncated (TK-) in 5 days post fertilisation (dpf) larvae (rbfox1+/+ and rbfox119del/19del). trkb2 expression is shown in a stacked bar format, where the directly measured TK+ (long form) is shown as the lower portion and the inferred TK- (short form) is shown on top. B Expression levels of HPI axis genes crhb, nr3c2, nr3c1, crhr1, crhr2, mc1r, pomca and kf19 in 5dpf larvae (rbfox1+/+ and rbfox119del/19del). C Whole-body cortisol measurement of 5dpf larvae (left panel) and adult fish (right panel) in resting physiological conditions. Cortisol values are normalised per whole-body homogenate (g) (rbfox1+/+ and rbfox119del/19del). D, E Expression levels of crhb and nr3c2 in presence or absence of the TRKB selective D antagonist ANA-12 and E agonist 7,8-DHF in 5dpf larvae (rbfox1+/+ and rbfox119del/19del). F–I Behavioural assays in presence or absence of the TRKB agonist 7,8-DHF in rbfox1 larvae (rbfox1+/+ and rbfox119del/19del): F mean distance travelled, G average speed, H startle response to white light stimulus and I proportion of responders over time in the response and habituation to acoustic startle assay. J–N Whole mount in situ hybridisation (ISH) for trkb2 anti-sense riboprobe in J rbfox1+/+ and K rbfox119del/19del, and L trkb2 sense riboprobe in rbfox1+/+ 5dpf larvae (lateral view in the main boxes and dorsal view in the smaller boxes on the top right corner). M, N Sagittal cryosection of trkb2 ISH in M rbfox1+/+ and N rbfox119del/19del 5 dpf larvae. Black boxes in M-a and N-a represent the region of the brain showed in higher magnification panels in M-a’ and N-a’. Scale bars: 200 µm in J), K) and L); 100 µm in M-a) and N-a); 50 µm in M-a’) and N-a’). O Schematic depiction (sagittal) of zebrafish larval brain indicating position of levels illustrated by ISH on sagittal cryosections. P trkb2 ISH intensity mean in the hypothalamus of 5dpf zebrafish larvae, rbfox1+/+ versus rbfox119del/19del. N = 4 larvae x genotype. Q Expression levels of pac1-hop and pac1-short in rbfox1+/+ and rbfox119del/19del 5 dpf larvae. For qPCR experiments, reference genes were actin – β 2 (actb2) and ribosomal protein L13a (rpl13a). Each green dot/pink triangle in A-D, K-L represents a pool of 15 larval heads (eyes and jaw removed), while yellow ones represent larvae exposed to TRKB drugs. Where indicated, we used Log10 transformation to normalise the data facilitating a clearer visualisation of trends within the dataset. All larvae employed were progeny of rbfox1+/19del in-cross and were genotyped after experiments and prior to data analysis. For cortisol measurement (G), each dot/triangle represent a pool of 12 larvae in the left panel (larvae) and a single whole zebrafish in the right panel (adult fish). In all graphs: bars represent standard error of the mean (SEM); * p < 0.05; ** p < 0.01; **** p < 0.0001.
To assess rbfox1 LoF effects on zebrafish larvae during early developmental stages, we performed crhb, bdnf and trkb2 qPCR experiments also in 3dpf larvae. Similarly to 5dpf larvae, in 3dpf rbfox119del/19del larvae we observed a significant upregulation of crhb (p < 0.01), bdnf (p < 0.05) and TK+ (p < 0.05), and a significant downregulation of TK- (p < 0.05) (Supplementary Figure 4A).
We measured whole-body cortisol levels under resting physiological conditions in both larvae and adult zebrafish to evaluate the physiological impact of rbfox1 loss and observed changes in crhb expression. We found a significant increase in cortisol levels in rbfox119del/19del larvae (p < 0.05) (Fig. 3C). In contrast, we observed no significant differences in adult fish (p > 0.05) (Fig. 3C).
These findings showed that rbfox1 LoF led to alterations in crhb, nr3c2, bdnf and trkb2 and cortisol levels in larvae, in line with our behavioural data and implicating RBFOX1 as a critical regulator of stress response mechanisms.
Pharmacological treatment targeting TrkB signalling restores crhb and nr3c2 gene expression and disrupted hyperactivity, startle response and habituation in rbfox1 LoF larvae
To assess whether molecular and behavioural changes seen in rbfox1 LoF larvae were mediated by bdnf/trkb2 signalling, we repeated qPCRs for the dysregulated genes in rbfox1 LoF mutants (crhb, nr3c2, bdnf, trkb2) and behavioural experiments in 5dpf zebrafish larvae following chronic exposure (from 5hpf to 5dpf) to the TRKB agonist 7,8-DHF or antagonist ANA-12. Given that in our line rbfox1 expression was knocked down throughout development, we initiated TRKB drug treatment from gastrulation.
In rbfox1+/+ larvae, neither TRKB agonist 7,8-DHF or antagonist ANA-12 had a significant effect on crhb and nr3c2 gene expression (p > 0.05) (Fig. 3D, E). In rbfox119del/19del larvae, chronic exposure to either TRKB agonist or antagonist significantly reduced crhb and nr3c2 expression levels relative to untreated mutant larvae (p < 0.001), restoring gene expression to levels comparable with wild type controls (p > 0.05) (Fig. 3D, E). Following identification and removal of outliers (1 outlier in the rbfox1+/+ + ANA-12 group, 1 outlier in the rbfox1+/+ + 7,8-DHF group and 1 outlier in the rbfox119del/19del + 7,8-DHF group), we observed a significant downregulation of nr3c2 expression levels in rbfox119del/19del + ANA-12 versus rbfox1+/+ un-exposed controls (p < 0.01) (Fig. 3D) and a significant upregulation of nr3c2 expression levels in rbfox119del/19del + 7,8-DHF versus both exposed (p < 0.01) and un-exposed (p < 0.05) controls (Fig. 3D, E). Interestingly, while having the same effect on crhb and nr3c3 expression, ANA-12 and 7,8-DHF had different outcomes on bdnf expression. Chronic treatment with ANA-12 reduced bdnf expression in rbfox1+/+ (p < 0.05) but had no effect on rbfox119del/19del (Supplementary Figure 4C, left panel). On the other hand, chronic treatment with 7,8-DHF increased bdnf levels in both rbfox1+/+ and rbfox119del/19del larvae (Supplementary Figure 4C, right panel).
Then, we examined the effects of ANA-12 and 7,8-DHF on behaviour to assess which behavioural alterations observed in rbfox1 mutant were influenced by bdnf/trkb2 signalling. In presence of 7,8-DHF we found a significant two-way interaction between genotype and drug on distance travelled [Effect of genotype by drug: χ2(2) = 47.3782, p < 0.05] and on swimming velocity [Effect of genotype by drug: χ2(2) = 31.3951, p < 0.05] such that treatment with 7,8-DHF restored hyperactive behaviour observed in rbfox119del/19del larvae (p < 0.01) (Fig. 3F, G). Further, following chronic exposure to 7,8-DHF there was no significant difference between rbfox119del/19del and rbfox1+/+ larvae in response to the white light-induced startle (Fig. 3H) or in the proportion of responders to the acoustic startle (p > 0.05) (Fig. 3I). ANA-12 had no significant effects on rbfox119del/19del behavioural alterations.
As the hypothalamus is the primary player in the stress response and Rbfox1 had only previously been shown to affect TRKB expression within the hippocampus [16], we used ISH to determine whether trkb2 mRNA expression was reduced within the hypothalamic area. In agreement with previous findings [45], in rbfox1+/+ we found that trkb2 is widely expressed in the brain of 5dpf larvae, whereas in rbfox119del/19del larvae, consistently with our qPCR experiments, we found a significant overall reduction of trkb2 mRNA in the whole brain (Fig. 3J–L), including in the hypothalamus (p < 0.01) (Fig. 3M, N, P, Supplementary Figure 5).
Our findings showed that the dysregulation in crhb and nr3c2 expression caused by rbfox1 LoF was prevented by TRKB agonists and antagonists. Further, we showed that hyperactivity, startle hypersensitivity and heightened proportion of responders to the acoustic startles observed in rbfox1 mutants are at least partially mediated by disrupted bdnf/trkb2 signalling.
rbfox1 LoF alters pac1a expression levels of zebrafish larvae
As RBFOX proteins have been shown to be able to regulate Pac1 alternative splicing to include the hop cassette [7] and PAC1 has been shown to regulate Bdnf transcription and potentiate TRKB activity [17], we examined expression levels of pac1a, the zebrafish homologue of the mammalian PAC1, in rbfox1+/+ and rbfox119del/19del 5dpf larvae by qPCR.
In mammals there are several PAC1 isoforms and their role in the regulation of stress is poorly understood [9]. In the brain, the predominant isoforms are PAC1-hop (long isoform) and PAC1-short [9]. Zebrafish possess two pac1 genes, pac1a and pac1b, but only pac1a contains the hop cassette [9]. PAC1-short enhances CRH transcription, while PAC1-hop reduces CRH synthesis during late stress recovery phase [4]. We measured expression levels of both pac1a-short and -hop isoforms and we observed no differences in pac1a-short expression, but we found a significant upregulation of pac1a-hop in rbfox119del/19del mutant larvae (p < 0.05) (Fig. 3Q).
This finding shows that elevated pac1a-hop/Pac1a-hop alone is not sufficient to counteract crhb increase and strengthens previous data [7] suggesting that RBFOX1 is not the main regulator, or at least not the sole regulator of, PAC1 alternative splicing.
rbfox1 mutants undergo adaptive mechanisms and allostatic overload during development
The HPA axis possesses a vital role in the maintenance of allostasis, the process by which the body achieves stability in response to stress or environmental challenges. Dysregulation of the HPA axis often leads to disrupted allostasis during later life, also termed as allostatic load (i.e., the physiological consequence resulting from the cumulative “wear and tear” of the body in response to chronic stress) [22]. As we observed dysregulation of the HPI axis gene expression in rbfox1 mutant larvae and altered behavioural responses in rbfox1 mutant larvae but not in adults, we assessed expression of HPI axis components in adult zebrafish, in the presence and absence of acute stress (NTD), to explore the possibility of adaptation that may contribute to differences in allostatic load.
We first measured crhb, nr3c2, nr3c1, bdnf and trkb2 mRNA expression levels. In physiological resting conditions, we observed no differences in HPI nor bdnf/trkb2 expression levels (p > 0.05) (Fig. 4A, B). However, after stress exposure, regarding the HPI axis, in rbfox119del/19del adults we observed the same dysregulation seen in rbfox1 LoF larvae: we found significant upregulation of crhb (p < 0.001) and mr (p < 0.0001) and no changes in nr3c1 expression levels (p > 0.05) (Fig. 4A), and significant upregulation of bdnf (p < 0.01) and TK+ (p < 0.05), and significant downregulation of TK- (p < 0.05) (Fig. 4B, C).
Expression levels of A) HPI axis genes corticotropin releasing hormone b (crhb), mineral corticoid receptor (mr) and glucocorticoid receptor (gr) and of B) bdnf and trkb2 truncated/full-length (TK-/TK+) (common primers) in adult zebrafish brain (rbfox1+/+ and rbfox119del/19del) in normal resting conditions and after exposure to a stressor (novel tank diving). C) Expression levels of the trkb2 TK+ and TK- in adult zebrafish brain (rbfox1+/+ and rbfox119del/19del) after exposure to a stressor. trkb2 expression is shown in a stacked bar format, where the directly measured TK+ (long form) is shown as the lower portion and the inferred TK- (short form) is shown on top. D) Expression levels of proliferating cell nuclear antigen (pcna) in 5 days post fertilisation zebrafish larvae and adult zebrafish brain (rbfox1+/+ and rbfox119del/19del) in normal resting conditions. E) Fertility rate and F) survival rate of rbfox1+/+ and rbfox119del/19del. Each green dot/pink triangle in A-B) represents a single adult rbfox1+/+ or rbfox119del/19del brain under resting physiological conditions respectively, while yellow dots/triangles represent rbfox1+/+ or rbfox119del/19del brain after stress exposure respectively. In B) each green dot/pink triangle represents single adult rbfox1+/+ or rbfox119del/19del brain after stress exposure respectively. In D) for larvae each green dot/pink triangle represents a pool of rbfox1+/+ or rbfox119del/19del 15 larval heads (eyes and jaw removed) respectively; for adults each green dot/pink triangle represents a single adult rbfox1+/+ or rbfox119del/19del brain respectively. In E) each dot/triangle represents average fertility of 3–5 trios (1 male and 2 females) assessed over 2–3 petri dish (50 embryos per dish) per trio. Each trio belonged to a different tank (for each genotype for each batch) to avoid tank effect). In F) each dot/triangle represents the percentage of survival of a single fish stock comprising 50 larvae. For qPCR experiments, reference genes were actin – β 2 (actb2) and ribosomal protein L13a (rpl13a). Where indicated, we used Log10 transformation to normalize the data facilitating a clearer visualization of trends within the dataset. All larvae employed were progeny of rbfox1+/19del in-cross and were genotyped after experiments and prior to data analysis. In all graphs: bars represent standard error of the mean (SEM); * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Excess glucocorticoid (GC) exposure during early developmental stages or early life stress (ELS) can lead to vulnerability to allostatic overload in the long-term [19, 22]. One of the effects caused by GC- or ELS-induced allostatic overload is reduced cell proliferation during adulthood [19]. This effect has been suggested to be developmentally dynamic, since it is often preceded by increased cell proliferation during early stages [19]. Further, BDNF signalling has been shown to regulate neural stem cell proliferation through TK-, suggesting that rbfox1 LoF fish showing altered levels of TK- expression, may show altered proliferation across the life course [46]. We therefore assessed the rate of proliferation at both larval and adult stages using qPCR for proliferating cell nuclear antigen (pcna). We observed a significant upregulation of pcna expression levels in 5dpf rbfox119del/19del zebrafish larvae (p < 0.05) and a significant downregulation of pcna expression levels in the brains of adult rbfox119del/19del fish (p < 0.01) when compared to rbfox1+/+ siblings (Fig. 4D).
In adult zebrafish, other effects caused by GC-induced allostatic overload include reduced fertility and survival rates [19]. Therefore, we assessed fertility and survival of rbfox119del/19del zebrafish and rbfox1+/+ siblings. We found that both fertility (p < 0.05) and survival (p < 0.001) rates of rbfox119del/19del fish were significantly reduced compared to rbfox1+/+ siblings (Fig. 4E, F).
Consistent with our findings (disrupted HPI axis gene expression and increased cortisol in rbfox1 mutant larvae under resting physiological conditions and in rbfox1 adult mutant only following stress exposure), these results suggest that rbfox1 mutants may engage compensatory mechanisms during development similar to those triggered by early-life GC exposure. While these mechanisms may transiently restore homeostasis, they likely lead to enduring alterations in stress responsivity and neurodevelopmental trajectories.
Discussion
In this study we generated a CRISPR-Cas9 LoF rbfox1 zebrafish line (rbfox119del) to investigate the mechanisms by which RBFOX1 loss increases susceptibility to psychiatric disorders.
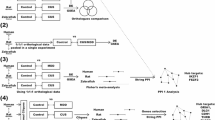
Allostatic load has been linked with several cognitive disorders including depression, schizophrenia, anxiety and PTSD [47]. HPA axis overactivity is a key factor in the onset of the allostatic load [22]. RBFOX1 has been shown to influence expression of stress-related genes such as PAC1 and BDNF/TRKB. Therefore, alterations in RBFOX1 function may increase psychiatric disorder vulnerability through alterations in stress response systems, such as the HPA axis, pre-disposing to allostatic overload vulnerability in later life (Fig. 5).
A In response to stressful challenges (yellow flash), BDNF expression levels increase, stimulating CRH transcription via TRKB.FL/TK+ (yellow pathway). At the same time, stress triggers also PACAP transcription (red pathway) and OTP-mediated transcription of RBFOX1 and CRH (blue pathway). PACAP stimulates CRH transcription via PAC1-short and inhibits CRH transcription via PAC1-hop. PACAP binding to PAC1-short also increases BDNF transcription (red line) and enhances TRKB.FL/TK+ activity (red line). RBFOX1 functions as a “switch” maintaining the balance between full-length/truncated TRKB isoforms (promoting TRKB mRNA stability and/or alternative splicing): during the stress recovery phase, RBFOX1 switches the balances in support of TRKB.T1/TK- isoform, decreasing CRH levels and turning off the HPA axis. B RBFOX1 deletion leads to an increase in BDNF expression levels (yellow arrow and white box) and an unbalance between TRKB.FL/TK+ (increased) and TRKB.T1/TK- (decreased) isoforms. Such dysregulation ultimately leads to an increase in CRH transcription (and possible hyperactivation of the HPA axis) and increase in PAC1-hop levels as possible compensatory mechanism. Our data suggest that RBFOX1-mediated regulation of TRKB.T1/TK- mRNA stability and/or alternative splicing is necessary to promote neuroplasticity and stress resilience.
Similarly to Rbfox1 deficient mice [6] and as seen in previous studies employing rbfox1 mutant zebrafish [2, 23], we found that rbfox119del/19del mutants were hyperactive and impulsive. These findings are consistent with studies implicating common and rare RBFOX1 genetic variants as risk factors for psychiatric disorders like ASD, ADHD, schizophrenia and anxiety/stress disorders [5, 6, 48]. At larval stages rbfox119del/19del were hyperactive and had increased burst swimming, indicative of increased impulsivity. In the response and habituation to acoustic startle assay, rbfox119del/19del larvae startled more and had a higher response rate over time compared to rbfox1+/+ siblings, consistent with previous findings in rbfox1 mutant zebrafish [30]. In a forced light-dark assay, although baseline hyperactivity of rbfox119del/19del larvae makes interpretation difficult, rbfox119del/19del larvae showed an increased startle response upon dark to light transition, consistent with heightened anxiety. These results suggest that rbfox1 LoF leads to heightened arousal, aligning with studies linking RBFOX1 to mood and anxiety disorders, including PTSD [49]. Interestingly, rbfox1 19del/19del adults showed reduced bottom dwelling only during the first minute of the novel tank diving assay (an adult measure of anxiety-like behaviour) while there were no significant differences during the remaining 4 min of the assay This latter result suggests behavioural adaptation possibly coupled with increased impulsivity or impaired threat assessment (decreased bottom dwelling during the first minute of assay). This aligns with the broader rbfox119del/19del behavioural profile (hyperactivity, heightened startle and poor habituation at larval stages) indicative of dysregulated arousal rather than reduced anxiety. Similarly, in the 5-CSRTT we found that rbfox119del/19del were more impulsive than rbfox1+/+ siblings, consistent with RBFOX1 variants being associated with impulsivity and related disorders such as ADHD and ASD [6].
In rbfox1 LoF larvae, under resting physiological conditions, we observed increased cortisol levels and dysregulation of crhb and nr3c2, both key components of fish HPI axis, which is analogous to the HPA axis in mammals. The HPA axis is crucial for managing stress, with CRH triggering cortisol secretion, affecting metabolism, immunity and behaviour [50, 51]. In zebrafish larvae under resting physiological conditions, we found increased cortisol, crhb and nr3c2, with no changes in the expression levels of the other HPI axis genes assessed (nr3c1, crhr1, crhr2, mc1r, pomca, kf19). In adults, although resting levels were unchanged, rbfox1 LoF showed an exaggerated molecular response to stress. Notably, both CRH and MR have important roles in the brain beyond their canonical endocrine functions via the HPA axis. CRH regulates anxiety and arousal acting as a neuromodulator in limbic regions such as the amygdala and hippocampus [52]. Similarly, hippocampal MRs play a key role in early stress responses by supporting cognitive appraisal of novel situations and promoting behavioural flexibility, thus shaping the initial reaction to stress [53]. Elevated cortisol and crhb levels as seen in rbfox119del/19del larvae under resting physiological conditions, suggest a chronic anxiety state. Increased cortisol, CRH and MR levels are linked to mood disorders like depression and PTSD [54,55,56,57]. These findings support a role for RBFOX1 in CRH regulation and suggest that RBFOX1 LoF may contribute to mood disorders and stress resilience via HPA axis dysregulation and/or non-canonical brain-specific effects of CRH and MR.
BDNF and TRKB are also key regulators of the stress response [58]. BDNF levels often increase during stress to promote neuronal survival and plasticity, buffering negative effects of stress on the brain via TRKB [10, 11, 13, 58,59,60]. Dysfunction of BDNF/TRKB signalling is linked to several stress-related disorders [13]. The increased bdnf expression we observed in rbfox119del/19del larvae may suggest a compensatory response to cope with crhb increase and maintain homeostasis. Given BDNF’s established role in modulating neuronal plasticity and stress adaptation, its upregulation may serve to stabilise or buffer hypothalamic circuits disrupted by excessive crhb transcription [58]. This response could represent an intrinsic attempt to mitigate possible overactivation of the HPI axis or the reduced activity within the trkb2 signalling (a suggestion supported by finding that incubation in the TRKB agonist 7,8-DHF rescues the behaviour as well as the expression of crhb) and maintain functional equilibrium in the face of impaired regulatory input caused by rbfox1 loss. The observed increase in bdnf expression could also result from either dysregulation of bdnf repressors or an imbalance in trkb2 isoform expression caused by rbfox1 loss, where upregulation of the full-length isoform may enhance bdnf expression through a positive feedback mechanism [40, 61]. At 3 and 5dpf, in rbfox1+/+ larvae we detected both trkb2 truncated (TK-) and full-length (TK+) forms, and in rbfox119del/19del larvae we observed a significant downregulation of TK- and a significant up-regulation of TK+, which is predicted to lead to an increased activation of TRKB signalling, consistent with the increased crhb expression as seen here [62]. Studies in rodents showed that Rbfox1 upregulation led to increased TrkB.T1 (TK-) expression in the hippocampus and no changes were observed following Rbfox1 deletion [16]. In contrast, we observed downregulation of trkb2 TK- expression in rbfox119del/19del larvae in resting physiological conditions. The difference between these studies may be due to i) differences in the tissue examined (zebrafish larvae whole head versus rodent hippocampus), ii) the developmental stage of the animals (larval versus adult stage), iii) the genotype of the animals studied (rbfox119del/19del in our study versus Rbfox1+/- in the study in rodents), or iv) presence of compensatory mechanisms (in the hippocampal mouse model Rbfox1 knockdown resulted in upregulation of Rbfox2). As for this latter hypothesis, as in previous studies in zebrafish [2], we did not see any changes in rbfox2 expression upon rbfox1 LoF (Supplementary Figure 4B) suggesting that this compensatory mechanism does not occur in larval fish.
Consistent with the possibility that RBFOX1 LoF may contribute to dysregulation of the HPA axis through a direct or indirect effect on BDNF/TRKB pathway, chronic exposure to TRKB agonist (7,8-DHF) or antagonist (ANA-12) restored crhb and nr3c2 gene expression to that seen in rbfox1+/+ larvae. ANA-12 is a selective non-competitive antagonist of TRKB exerting central TRKB blockade and producing rapid and long-lasting anxiolytic and antidepressant effects [63]. Mice treated with ANA-12 showed reduced anxiety-like behaviour [63] and in stressed rats, ANA-12 blocked Crh increase in the hypothalamus and amygdala [64]. 7,8-DHF is a potent selective agonist of TRKB used in the treatment of several disorders, including depression and schizophrenia, and has been shown to enhance memory consolidation and emotional learning in healthy rodents [65]. In a similar fashion to the effects of ANA-12 seen in rats (blocking Crh increase) [57], after chronic treatment with ANA-12, we observed that the expression of HPI axis genes in rbfox119del/19del larvae was restored to rbfox1+/+ levels [66]. Interestingly, ANA-12, despite restoring crhb and nr3c2 levels to wild type, did not rescue rbfox119del/19del larval behaviour, indicating that blockade of TRKB can normalise some aspects of HPI axis output but is insufficient to correct the behavioural phenotype (perhaps because it further disrupts necessary TRKB-mediated signalling in the brain). In contrast, 7,8-DHF, normalises crhb and nr3c2 expression and rescues some behavioural aspects altered in rbfox119del/19del larvae (i.e., hyperactivity, startle response to white light stimulus and proportion of fish responding to acoustic stimuli), suggesting that enhancing TRKB signalling through the full-length receptor may restore functional signalling balance and downstream pathways (e.g., synaptic plasticity, habituation mechanisms). Finally, the fact that 7,8-DHF increases bdnf expression in rbfox119del/19del larvae while ANA-12 has no effect points to the positive feedback loop whereby TRKB activation enhances BDNF expression [67, 68], helping restore adaptive neuroendocrine and behavioural responses. Future studies employing acute or temporally restricted treatments (e.g., 3–5 dpf), or employing different TRKB agonist/antagonist, will help delineate the specificity of TRKB signalling effects. Interestingly, rbfox119del/19del larvae were differentially sensitive to the effect of TRKB drugs on nr3c2 expression, most likely due to the unbalanced TK+/TK- levels. These findings warrant further investigations. It is of note that neither TRKB agonist nor antagonist had a significant effect on crhb nor nr3c2 expression in rbfox1+/+ larvae. One possible explanation is that, as the larvae were not exposed to any stressors, any effect on Trkb2 signalling had limited effect on HPI axis activity.
In rbfox119del/19del larvae, we also observed upregulation of pac1a-hop with no changes in pac1a-short levels. Previous work in zebrafish has shown that Pac1-short gain of function caused persistent crh increase, while overexpression of Pac1-hop prevented stress-induced crh transcription activation [4]. The same study also suggests that RBFOX1 regulates the alternative splicing of PAC1 promoting the formation of PAC1-hop [4]. However, the increase in pac1-hop seen in our study argues against this suggestion and suggests an adaptive response to the increased expression of crh either through rbfox2 [7] (whose expression was not altered by rbfox1 LoF (Supplementary Figure 4B)) or through an unknown mechanism. Elevated pac1a-hop/Pac1a-hop alone might be not sufficient to counteract crhb upregulation, given the concurrent increase in bdnf levels and the shift in trkb2 isoform expression (upregulation of the excitatory long form and downregulation of the inhibitory short form). This interpretation is further supported by the decreased in crhb levels observed following chronic TRKB modulation. Another option is that the upregulation of pac1a-hop mRNA may not reflect increased functional Pac1a-hop receptor activity, particularly in the context of disrupted regulation following rbfox1 loss, suggesting that RBFOX1 might be involved in PAC1 mRNA stability rather than alternative splicing.
In contrast to larvae, differences in crhb, nr3c2, bdnf and trkb2 gene expression and cortisol levels were not seen in adult animals under resting conditions. However, rbfox119del/19del adults showed increased crhb and nr3c2 on challenge with a stressor, aligning with the previous suggestion that Rbfox1 is required for the termination of the acute endocrine stress response [4] and that chronic stress during development leads to adult HPI axis adaptation, often at a cost in later life (e.g., long-lasting disruption in stress-response circuits associated with adult behavioural deficits, reduced fertility and survival, decreased pcna expression) [19, 69]. This interpretation is consistent with previous work by Eachus et al., which demonstrated that early-life exposure to excess GC can lead to long-term changes in stress-regulatory circuits and adult behavioural dysfunction [19]. Supporting this, rbfox1 LoF adults show heightened crhb, nr3c2 and bdnf expression, and imbalance in trkb2 isoform expression, specifically in response to a stressor (the novel tank diving assay). Together, these findings suggest that while baseline homeostasis may be restored through developmental adaptation, stress responsivity remains dysregulated, pointing to an allostatic shift toward a passive coping style for rbfox1 mutants [70, 71]. Passive (cautious, risk-averse, high CRH) and active (bold, exploratory, low CRH) coping styles refer to behavioural strategies that animals employ to respond to stress and that are underpinned by distinct neuroendocrine pathways, providing a valuable framework for interpreting stress susceptibility, resilience and the behavioural consequences of genetic mutations [70, 71]. The shift toward a passive coping style of rbfox1 mutants support a role for RBFOX1 in shaping stress responsivity and behavioural strategies. In rbfox119del/19del adults we observed also reduced fertility and reduced survival rates, two long-term effects often associated with excess GC-exposure/early life stress [19]. Another long-term effect of excess GC-exposure/early life stress is reduced neural stem cell proliferation in adult animals, often accompanied by increased proliferation at early stages [19]. Our findings show that rbfox119del/19del showed increased pcna levels at larval staged but reduced pcna levels during adulthood, corroborate this latter hypothesis of a dynamic developmental effect of early life stress. Consistent with the crhb increase observed in stressed rbfox119del/19del adults, and as seen in larvae, after acute stress, rbfox119del/19del adults also showed unbalanced trkb2 expression, with upregulation of TK+ and downregulation of TK-.
In conclusion, given the conservation between fish HPI axis and the mammalian HPA axis, our data unveils a pivotal new finding in CRH regulation, revealing an interplay between RBFOX1 and BDNF/TRKB in the context of chronic stress and stress resilience and suggests that RBFOX1 plays a crucial role in adaptive stress mechanisms. In response to stressful challenges, RBFOX1 functions as a “switch” regulating the balance between short/long isoforms of TRKB receptors: during the stress recovery phase, RBFOX1 switches the balance towards TRKB.T1/TK- isoform, decreasing CRH levels and turning off the HPA axis (Fig. 5). In rbfox119del/19del, this regulatory switch appears disrupted (increased cortisol, crhb, nr3c2, bdnf and full-length trkb2 expression, alongside reduced truncated trkb2) suggesting a failure to terminate the stress response. Behaviourally, mutants exhibited hyperactivity, impulsivity, heightened arousal and reduced habituation, phenotypes that parallel core symptoms seen in several human psychiatric disorders. Pharmacological experiments support a functional consequence of this dysregulation. Although both ANA-12 (TRKB antagonist) and 7,8-DHF (TRKB agonist) normalised crhb and nr3c2 levels, only 7,8-DHF rescued behavioural abnormalities. These findings indicate that, at least at larval stages, changes in behaviour are not solely due to disrupted crhb and nr3c2 but also reflect disrupted TRKB signalling. The differential effects of these compounds also point to the positive feedback role of TRKB-FL signalling on BDNF expression [67, 68], which may be essential for behavioural homeostasis. Together these results suggest that RBFOX1 contributes to the liability of psychiatric disorders through CRH, MR and BDNF/TRKB dysregulation, which leads to disrupted development and vulnerability to allostatic overload in later life. The suite of behavioural effects seen in rbfox1 LoF animals coupled with altered response on exposure to a stressor are reminiscent of reported traits in passive versus active coping styles [70] suggesting that RBFOX1 activity may also play a key role in individual differences in coping strategies. Although we show a key role for RBFOX1 in regulation of the HPA axis, consistent with this being a primary mechanism by which RBFOX1 variants pre-disposes to psychiatric disorders, RBFOX1 regulates the splicing of a wide range of additional genes [15] which may also play a part of disease vulnerability.
Methods
Animal maintenance
All fish were maintained in a recirculating system (Tecniplast, UK) with a 14 h:10 h light/dark cycle and a constant temperature of 28 °C. Fish were fed with ZM-400 fry food (Zebrafish Management Ltd.) in the morning and brine shrimps in the afternoon. Breeding was set up in the evening, in sloping breeding tanks (Tecniplast) provided with dividers for timed mating. The following morning, dividers were removed to allow spawning. Eggs were collected in Petri dishes (max 50 eggs/dish). Infertile eggs were removed, and fertile ones were incubated at 28 °C. Petri dishes were checked daily to ensure consistent developmental stage across groups. If reared, larvae were moved to the recirculating system at 5 days post fertilisation (dpf) and fed as stated above.
All procedures were carried out under license in accordance with the Animals (Scientific Procedures) Act, 1986 and under guidance from the Local Animal Welfare and Ethical Review Board at Queen Mary University of London.
Generation of rbfox1 loss of function zebrafish line
The zebrafish rbfox1 loss of function (LoF) mutant line was generated as described previously using Tübingen strain as background [72]. CRISPR RNA (crRNA) (Merck) was designed to target rbfox1 exon 2 (CCCAGTTCGCTCCCCCTCAGAAC, PAM sequence in bold, MwoI recognition site underlined). A 3 µL injection mix containing 1 µL (FC 83 ng/µL) crRNA, 1 µL (FC 83 ng/µL) tracrRNA (Merck, #TRACRRNA05N) and 1 µL (FC 1.67 µM) Cas9 protein (New England Biolabs, #M0646) was freshly prepared on the morning of the injection procedure. Then, 1 nL of the injection mix was injected into one-cell stage zebrafish embryos (~100–150 embryos). Injection efficacy was assessed at 24 h post fertilisation (hpf) by polymerase chain reaction (PCR) from genomic DNA (rbfox1_Forward, 5′-TAATCAAGACGCCCCAGCAC–3′; rbfox1_Reverse, 5′- GTACTCAGCAGGAATGCCGT- 3′) followed by MwoI (New England Biolabs, #R0573S) restriction enzyme digestion. Successful injections will introduce indel mutations disrupting the recognition site of the restriction enzyme, preventing this latter from cutting the PCR amplicon. Once reached sexual maturity (at ~ 3 months of age) injected fish (F0) were outcrossed with wild type to generate F1 embryos. The progeny may carry different mutations due to the mosaic nature of the F0 parents. F1 fish were therefore screened for mutations leading to premature termination codon (PTC) via cloning into pGEM-T Easy vector (Promega, #A1360), followed by transformation, colony PCR, DNA purification (Monarch® Plasmid Miniprep Kit, New England Biolabs, #T1010) and sequencing (Source BioScience PLC). Quantitative real time PCR (qPCR) was used to confirm reduction of rbfox1 mRNA expression.
Although our previous studies examined two distinct rbfox1 mutant lines (rbfox1del19 vs rbfox1sa15940) [2], we chose to focus on the rbfox1del19 line for the current study due to its more pronounced phenotype.
Fish breeding and genotyping
For adult experiments, mixed sexes were used. When possible, animals were associated with an identification number and genotype was assigned after data analysis. For larval experiments, fish were generated by heterozygous in-cross and genotyped prior to data analysis. Genomic DNA was extracted from fins and using the HotSHOT method. Briefly, samples were incubated at 95 °C in 50 mM NaOH for 30 min, followed by 1 min at 10 °C. Reaction was stopped using 1 M Tris HCl (1/10 of the initial NaOH volume), pH 8.00. Genotyping primers were the same as the ones used to identify founder carriers.
Drug treatment
Zebrafish embryos of each genotype (rbfox1+/+ and rbfox119del/19del) were treated from 5hpf to 5dpf with 20 μM TRKB antagonist ANA-12 (abcam, Cat.ab146196), or 20 μM TRKB agonist 7,8-dihydroxyflavone (7,8-DHF) (abcam #ab120996), or dimethylsulfoxide (DMSO, vehicle 0.01%) (Merck #34869). Treatment was performed in Petri dishes (max 30 embryos x dish) and drugs or vehicle were dissolved in fish water. Solutions were replaced daily.
Quantitative real-time PCR
Quantitative Real-time PCR (qPCR) of target RNA was performed on 5dpf rbfox1 wild type (rbfox1+/+) and rbfox1 homozygous (rbfox119del/19del) larvae, using Luna® Universal One-Step RT-qPCR Kit (New England Biolabs #M3005) and a Bio-Rad 96-well qPCR machine (CFX96 Touch Real-Time PCR Detection System). Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific) following manufacturer’s instructions. Briefly, after homogenization, RNA was isolated by precipitation, rinsed in ethanol and resuspended in RNase free water. Total RNA was then quantified using BioDrop (Biochrom Ltd.) and up to 1 μg was reverse transcribed to cDNA using the ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs, #E6560) following manufacturer’s instructions. The resulting cDNA yield and quality were evaluated using BioDrop (Biochrom Ltd.). As for zebrafish trkb2 TK+ and TK- isoforms quantification, Ct values resulting from the amplification of the TK+ specific product were subtracted from the Ct values resulting from the amplification of the TK+/TK- common product. All reactions included 5 biological replicates and 3 technical replicates. For experiments in larvae, each biological replicate consisted of 15 larval heads (eyes and jaw removed). For experiments in adults, each biological replicate consisted of a single brain. Actin – β 2 (actb2) and ribosomal protein L13a (rpl13a) were employed as reference genes. For assessing rbfox1 mRNA expression, rbfox1 primers were designed upstream of the CRISPR deletion. Accession numbers, primer sequences and amplification efficiencies for all the reference and target genes can be found in Supplementary Table 1.
In situ hybridisation
In situ hybridisation (ISH) was carried out on whole mount zebrafish larvae and on larval (sagittal) and adult brain (transverse) sections as described previously [2]. For rbfox1 ISH, the original plasmid used to generate the riboprobe was provided by Dr William Norton (University of Leicester). The plasmid used to produce the trkb2 riboprobe was generated in our laboratory (Forward primer: 5’-GTTCGTGGAATGGCTTGCTG-3’, Reverse primer: 5’-TCTGGCCCACGATGTTTTCA-3’) using the pGEM®-T Easy Vector System (Promega, #A1360) and in-house generated E. coli DH5α competent cells. Riboprobes to identify rbfox1 (NM_001005596) and trkb2 (NM 01197161.2) mRNA were synthetised by in vitro transcription (IVT) using MAXIscript™ SP6/T7 kit (Invitrogen by Thermo Fisher Scientific, #AM1322), following manufacturer’s instructions and using a DIG RNA Labeling Mix, 10× conc (Roche, #11277073910) containing digoxigenin labeled uracil.
In situ hybridisation on whole mount zebrafish larvae
In situ hybridisation was carried out on 28hpf, 2- 3- 4- and 5dpf rbfox1+/+ and rbfox119del/19del larvae. To prevent skin pigmentation, embryos were incubated in 0.2 mM 1-phenyl 2-thiourea (PTU) (Sigma, #S527335) from 24hpf. When they reached the desired age, larvae were fixed in 4% paraformaldehyde (PFA) (Merck, #158127) overnight (ON) at 4 °C. The following day, larvae were rinsed in 1x phosphate buffered saline (PBS) (Thermo Fisher Scientific, #18912014) supplemented with Tween 20 (Sigma, #P1379) (0.05% v/v), dehydrated in ascending methanol series (25%, 50%, 70%, 80%, 90%, 100% methanol, 5 min each) and stored in 100% methanol at −20 °C. To perform ISH experiments, larvae were rehydrated in descending methanol series (100%, 90%, 80%, 70%, 50%, 25% methanol), 5 min each, and washed in 1xPBS, 5 min. Larvae were permeabilised using proteinase K (PK) (ITW Reagents, #A3830) (stock 20 μg/mL in 1xPBS) as follows: 28hpf larvae were permeabilised in PK 1:2000 in 1xPBS for 20 min at room temperature (RT), older stages were permeabilised in PK 1:1000 at 37 °C for at least 30 min. Then, larvae were post fixed in 4% PFA for 20 min and washed in 1xPBS at RT, 5 × 5 min. Prehybridisation was carried out in hybridisation solution (HB) containing 50% formamide, 5% saline sodium citrate buffer (SSC), 50 µg/mL heparin, 0.05 mg/mL yeast RNA, 0.1% Tween 20, and 0.92% citric acid at 68 °C for 2 h. Thereafter, larvae were incubated in HB containing rbfox1 riboprobe (500 pg/µL), ON at 68 °C. Post hybridisation washes were performed at 68 °C with a gradient of 2xSSC and formamide (50%, 25% and 0% formamide), 10 min each, and then twice with 0.02xSSC, 30 min each. Subsequently, larvae were blocked in blocking solution (BS) containing 10% normal sheep serum (Gibco, #16070096) and 2 µg/µL bovine serum albumin, for 1 h at RT. After blocking step, larvae were incubated in anti-digoxigenin Fab fragments conjugated with alkaline phosphatase (Roche, #11093274910), 1:2000 in BS, 1 h at RT and then ON at 4 °C. The following day, larvae were washed in 1xPBS, 6 ×15 min each, and then in alkaline phosphatase (100 mM NaCl, 100 mM Tris HCl, 50 mM MgCl2, 0.1% Tween 20) (NTMT) buffer, 3 × 5 min each. The chromogenic reaction was carried out by incubating the larvae in BCIP/NBT solution (Merck, #203790) in NTMT buffer, at RT in the dark, and were observed every 20 min until the signal detection. After reaching the desired staining, larvae were washed in 1xPBS at RT, post fixed in 4% PFA for 2 h, cleared and stored in 80% glycerol at 4 °C. For sagittal cryosections, larvae were embedded in 1.5% low gelling temperature agarose (Scientific Laboratories Supplies, #A9414) supplemented with 5% Sucrose in 1xPBS. Sections (5 μm) were collected on adhesive microscope slides Superfrost® Plus Gold (Epredia).
In situ hybridisation on sections
ISH was conducted on paraffin embedded rbfox1+/+ and rbfox119del/19del adult brains (transverse sections) and 5dpf rbfox1+/+ and rbfox119del/19del zebrafish larvae (sagittal sections). Fish were culled by an overdose of tricaine prior to head removal. Dissected adult brains and larvae were fixed in 4% PFA in 1xPBS, ON at 4 °C. Tissues were then rinsed in 1xPBS and dehydrated in ascending ethanol series (15 min in each of 30%, 50%, 70%, 80%, 90%, 100% ethanol) and embedded in paraffin. Transverse (adult brains) or sagittal (5 days old larvae) sections of 12 µm (adult brains) or 7 µm (5 days old larvae) thickness were cut using a microtome (Leica). To perform ISH, slides were de-waxed in xylene (twice, 10 min each), rehydrated in descending ethanol series (2 ×5 min in absolute ethanol, then 90%, 80%, 70%, 50% and 25% ethanol, 5 min each), and rinsed in 1xPBS for 5 min. Then, sections were permeabilised using PK (0.05 μg/μL) for 8 min at RT, washed with 2 mg/mL glycine twice (5 min each), post fixed in 4% PFA for 20 min and washed in 1xPBS at RT. Prehybridisation was carried out in HB, for 1 h at 68 °C. Thereafter, sections were incubated in HB containing rbfox1 riboprobe (500 pg/µL), ON at 68 °C. Post hybridisation washes were performed at 68 °C twice for 20 min in 1xSSC, twice for 20 min in 0.2xSSC, and several washes were performed in 1xPBS, 5 min each at RT. Then, sections were blocked in BS for 30 min at RT and incubated in a 1:2000 dilution of anti-digoxigenin Fab fragments conjugated with alkaline phosphatase in BS, ON at 4 °C. The following day, sections were washed in 1xPBS, 5 × 10 min each. The chromogenic reaction was carried out by incubating the slides in BCIP/ NBT solution in NTMT buffer, at RT in the dark, and were observed every 20 min until the signal detection. When the desired staining was obtained, sections were washed in 1xPBS at RT, dehydrated in ascending ethanol series (25%, 50%, 70%, 80%, 90%, 100% ethanol, 5 min each), cleared in xylene (twice, 5 min each) and mounted with dibutyl phthalate polystyrene xylene mounting medium (Sigma, #06522).
Image acquisition and processing
Pictures of whole mount ISH on zebrafish larvae were acquired by Leica MZ75 microscope. For ISH on sections, pictures were acquired using a Leica DMRA2 upright epifluorescent microscope with colour QIClick camera (Leica) and processed with Velocity 6.3.1 software (Quorum Technologies Inc). Quantification of the ISH staining signal intensity was performed as described previously [73], using Fiji software [74]. Adult anatomical structures were identified according to the Neuroanatomy of the Zebrafish Brain by Wullimann [75].
Behavioural assays
For larvae, all behavioural experiments were conducted on the progeny of a rbfox1+/19del in-cross. Larvae were genotyped prior to data analysis. For adults, where the genotype of the animals was known, fish were pseudorandomised across testing systems with all trials having an approx. equal number of each genotype. The adult fish were weight- and age-matched, with approximately equal numbers of both sexes. Sex was not treated as a biological variable in the statistical analyses.
Larval behavioural experiments
Patterns of locomotor activity of 5dpf rbfox1+/+, rbfox1+/19del and rbfox119del/19del mutant zebrafish larvae were investigated as described previously [24, 29, 33]. Tests were conducted between 9 a.m. and 4 p.m. At 5dpf, larvae were placed in individual wells of a 24-well plate. To reduce stress due to manipulation, larvae were acclimatised for at least 1 h before testing. Then, plates were placed into the DanioVision observation chamber (Noldus). Locomotion parameters such as distance travelled and swimming velocity were recorded using EthoVision XT software (Noldus). Data were exported in 1 min and 1 s time bins and analysed with R programming language [76]. For the hyperactivity assay, larval basal locomotion was tracked for 15 min in dark conditions. Larval swimming burst was assessed as described previously [24] and peaks were considered as the acceleration events when larvae travelled > 5 mm in <12 s. The forced light-dark transition assay was performed as described previously [77, 78] with modifications: after an initial 5 min period of dark (baseline), larvae were exposed to one light/dark cycle of 1 min light (Noldus white light device) followed by 5 min dark. The response and habituation to acoustic startle stimuli was performed as described previously [29]: after 10 min of baseline (no stimuli, dark conditions), larvae were subjected to 10 sound/vibration stimuli (Noldus tapping device) over 20 s (2 s intervals between each stimulus).
5-Choice serial reaction time task
We measured impulsive action using a zebrafish version of the 5-Choice Serial Reaction Time Task (5-CSRTT) [25]. Adult rbfox1+/+, rbfox1+/19del and rbfox119del/19del zebrafish, 7 months old, mixed sexes, were singly housed for a week prior to experiment and remained singly housed for the whole duration of the assay (9 weeks). Fish were tested using the Zantiks AD units. Each unit was provided with a small tank with five apertures and a food hopper insert. The five apertures created five different entry points for the fish, acting like the five nose poke holes of the rodent version of the assay. The food hopper was placed at the opposite side of the five apertures and formed an area for the fish to enter and collect food reward. Below the testing tank there was an integrated screen, used to display white light (stimulus) into the five apertures. Responses were detected when a fish entered these apertures and recorded with an integrated camera placed at the top of the tank. The experiment consisted of five training stages: i) habituation, ii) initiator training, iii) stimulus light training, iv) 5-CSRTT/no delay, v) 5-CSRTT/variable-delay. Details of each stage are provided in Supplementary Table 2.
Novel tank diving
Novel tank diving is a behavioural test to assess anxiety-like behaviour in adult fish. Response to novel tank was assessed in rbfox1+/+, rbfox1+/19del and rbfox119del/19del 9 months old zebrafish, mixed-sexes, as described previously [33]. Fish were singly housed for a week prior to performing the experiment and acclimatized for at least 1 h in the behavioural room on the testing day. Behavioural assays were conducted between 9 a.m. and 2 p.m. During the test, fish were individually placed into a 1.5 L tank and their behaviour was tracked and recorded using EthoVision system (Noldus). Data were exported in 1 min time bin and analysed as previously described [33]. Experimental groups were randomised during testing. We analyzed three behaviours in response to the novel tank: i) time spent in the bottom of the tank, ii) total distance traveled, and iii) number of transitions to the top–bottom area of the tank.
Whole-body cortisol extraction
Larvae
Larval cortisol was extracted from pools of 12 larvae x sample using a modified protocol based on Baiamonte et al. [35]. Briefly, larvae were snap-frozen upon collection and stored at −20 °C until extraction was performed. On the day of extraction, frozen samples were thawed on ice in and homogenised in 400 μl of ice-cold 1×PBS for 30 s using a Bioruptor (MP Biomedicals). Cortisol was extracted by adding 500 μl of ethyl acetate (Sigma) to each homogenate. Samples were vortexed for 30 s, centrifuged at 5000 rpm for 10 min and then snap frozen at −80 °C. The organic phase was carefully decanted into glass vials (VWR). The extraction was repeated twice and the combined organic phases were pooled. The pooled ethyl acetate extracts were evaporated at 60 °C using a rotary evaporator (IKA). The dried cortisol was resuspended in 200 μl of 1xPBS, vortexed for 30 s and stored at −20 °C until cortisol quantification using a human salivary cortisol ELISA kit (Salimetrics).
Adults
Adult cortisol was extracted from 4 adult zebrafish whole-body. Fish were weighed and homogenised in 2× volumes (w/v) of 1xPBS using a Bioruptor (MP Biomedicals). Cortisol was extracted using the same ethyl acetate procedure as for larvae. However, after evaporation, to prevent any interference with the assay, residual lipids were eliminated by partitioning the dried extracts between 500 μl 1xPBS and 500 μl hexane (Sigma). The upper organic layer was discarded and the aqueous phase was retained and stored at −20 °C until cortisol quantification using a human salivary cortisol ELISA kit (Salimetrics).
Cortisol measurement
Samples were thawed on ice and 50 μl of each was assayed using the Salimetrics human salivary cortisol ELISA kit, following the manufacturer’s protocol. Cortisol values for adults were normalised to body weight (ng/g), while larval and juvenile values were normalised to total tissue protein (ng/mg). This assay was validated for use with zebrafish whole-body extracts previously [35, 79]. Standards, high and low controls and zebrafish samples (25 μL) were added to the plate provided with the Salimetric kit, along with Assay Diluent (25 μL) for the zero standard. The Enzyme Conjugate was diluted 1:1600 in Assay Diluent and 200 μL was dispensed into each well. After mixing for 5 min at 500 rpm, the plate was incubated at RT for 1 h and washed four times with 1× wash buffer (300 μL x well x wash). Next, 200 μL of TMB Substrate Solution was added, followed by a 30-min dark incubation with first 5 min mixing. The reaction was stopped with 50 μL of Stop Solution, mixed until wells turned yellow, and absorbance was read at 450 nm within 10 min using an OMEGA plate reader (BMG Labtech).
Statistical analysis
For qPCR, relative mRNA expressions were calculated using the Pfaffl method [80]. Outliers were identified and removed using Dixon’s test (α = 0.05) [81]. Differences in gene expression were assessed using a one-way ANOVA followed by Tukey’s post-hoc test using GraphPad Software (Prism). For behavioural analysis, all data were analysed with R programming language [76]. Scripts used for analysis are available on GitHub (https://github.com/AdeleLeg). For models where distance moved, distance to zone, velocity, or top halves visits were the response variable, we fitted data to mixed linear models using the “lme4” package, and where proportion of responders or proportion of time spent in the bottom third were our response variable, we fitted data to beta regression models using the “betareg” package. In all instances, for all experiments, we used genotype as fixed effect, and fish ID and day of experiment as random effects. In the response and habituation to acoustic startle, we used also the stimulus number as fixed effect. As in García-González et al. [33], we reported significant fixed effects as Type II Wald χ2 from models using the package “car,” post hoc Tukey’s tests were also conducted as necessary with the package “emmeans”. For the 5-CSRTT, overall correct responses (learning) and anticipatory responses (impulsivity) were assessed using the formulas in Supplementary Table 2. Statistical analysis of mean intensity in in situ hybridisation images of zebrafish larval sections was performed using ImageJ. Mean intensity values were quantified across specified regions of interest, indicated in Supplementary Figure 5, ensuring that comparable areas of similar dimensions were used for each sample to calculate differences in expression. The resulting values were compared across groups using a paired t-test to determine significant differences using GraphPad Software (Prism). For all experiments, sample size needed to achieve adequate statistical power for detecting a significant effect was determined based on data from previous research or pilot studies. Accepted α level and power were respectively ≤ 0.05 and ≥ 0.80. All experiments were independently replicated in the laboratory at least three times to ensure reproducibility and reliability of the results.
Fertilisation and survival analysis
Fertilisation and survival rates were measured as described previously [19]. For fertilisation rate we measured the percentage of fertilised eggs when pairing rbfox119del/19del and compared it with fertilisation rate of rbfox1+/+ siblings. Data were collected upon 3 different mating trials, each trial comprising 3–5 trios (1 male and 2 females). Trials were performed on different weeks, using different trios for each test. Eggs were collected in different Petri dishes, properly labelled to distinguish between trios. Fertilisation rate was determined by averaging across Petri dishes from the same trio and calculated at 6-8hpf. For survival analysis, we measured the percentage of surviving animals (across 3 tanks for each genotype for each batch, to avoid tank effect) from when larvae were added to the nursery (at 5dpf) until 2 months of age, when fish were transferred into adult aquarium.
Data availability
All data supporting the findings of this study are available within the article and its supplementary information files. R scripts used for analysis are deposited on GitHub (https://github.com/AdeleLeg). Any additional materials, including plasmids generated in this study, are available from the corresponding author on reasonable request.
References
Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address: plee0@mgh.harvard.edu, Cross-disorder group of the psychiatric genomics consortium. genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–1482.e11.
Antón-Galindo E, Adel MR, García-González J, Leggieri A, López-Blanch L, Irimia M, et al. Pleiotropic contribution of rbfox1 to psychiatric and neurodevelopmental phenotypes in two zebrafish models. Transl Psychiatry. 2024;14:1–11.
Thisse B, Thisse C. ZFIN Publication: Thisse et al., 2004 [Internet]. ZFIN. [cited 2020 Aug 10]. Available from: http://zfin.org/ZDB-PUB-040907-1.
Amir-Zilberstein L, Blechman J, Sztainberg Y, Norton WHJ, Reuveny A, Borodovsky N, et al. Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron. 2012;73:279–91.
Abi-Dargham A, Moeller SJ, Ali F, DeLorenzo C, Domschke K, Horga G, et al. Candidate biomarkers in psychiatric disorders: state of the field. World Psychiatry. 2023;22:236–62.
O’Leary A, Fernàndez-Castillo N, Gan G, Yang Y, Yotova AY, Kranz TM, et al. Behavioural and functional evidence revealing the role of RBFOX1 variation in multiple psychiatric disorders and traits. Mol Psychiatry. 2022;27:4464–73.
Lee JA, Tang ZZ, Black DL. An inducible change in Fox-1/A2BP1 splicing modulates the alternative splicing of downstream neuronal target exons. Genes Dev. 2009;23:2284–93.
Shaughnessy CA, Myhre VD, Hall DJ, McCormick SD, Dores RM. Hypothalamus-pituitary-interrenal (HPI) axis signaling in Atlantic sturgeon (Acipenser oxyrinchus) and sterlet (Acipenser ruthenus). Gen Comp Endocrinol. 2023;339:114290.
Biran J, Gliksberg M, Shirat I, Swaminathan A, Levitas-Djerbi T, Appelbaum L, et al. Splice-specific deficiency of the PTSD-associated gene PAC1 leads to a paradoxical age-dependent stress behavior. Sci Rep. 2020;10:9559.
Linz R, Puhlmann LMC, Apostolakou F, Mantzou E, Papassotiriou I, Chrousos GP, et al. Acute psychosocial stress increases serum BDNF levels: an antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology. 2019;44:1797–804.
Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA. 2012;109:1305–10.
Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–84.
Miao Z, Wang Y, Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int J Mol Sci. 2020;21:1375.
Suliman S, Hemmings S, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. 2013;7:55.
Fogel BL, Wexler E, Wahnich A, Friedrich T, Vijayendran C, Gao F, et al. RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum Mol Genet. 2012;21:4171–86.
Tomassoni-Ardori F, Fulgenzi G, Becker J, Barrick C, Palko ME, Kuhn S, et al. Rbfox1 up-regulation impairs BDNF-dependent hippocampal LTP by dysregulating TrkB isoform expression levels. eLife. 2019;8:e49673 Chao MV, Westbrook GL, editors.
Solés-Tarrés I, Cabezas-Llobet N, Vaudry D, Xifró X. Protective effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide against cognitive decline in neurodegenerative diseases. Front Cell Neurosci [Internet]. 2020 [cited 2024 Jul 16];14. Available from: https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2020.00221/full.
Mcewen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N. Y Acad Sci. 2004;1032:1–7.
Eachus H, Choi MK, Tochwin A, Kaspareit J, Ho M, Ryu S. Elevated glucocorticoid alters the developmental dynamics of hypothalamic neurogenesis in zebrafish. Commun Biol. 2024;7:1–14.
Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39.
Bornstein SR, Steenblock C, Chrousos GP, Schally AV, Beuschlein F, Kline G, et al. Stress-inducible-stem cells: a new view on endocrine, metabolic and mental disease?. Mol Psychiatry. 2019;24:2–9.
Kinlein SA, Wilson CD, Karatsoreos IN Dysregulated hypothalamic–pituitary–adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry [Internet]. 2015 [cited 2024 Jul 10];6. Available from: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2015.00031/full.
Adel MR, Antón-Galindo E, Gago-Garcia E, Arias-Dimas A, Arenas C, Artuch R, et al. Decreased brain serotonin in rbfox1 mutant zebrafish and partial reversion of behavioural alterations by the SSRI fluoxetine. Pharmaceuticals. 2024;17:254.
Lange M, Norton W, Coolen M, Chaminade M, Merker S, Proft F, et al. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol Psychiatry. 2012;17:946–54.
Parker MO, Ife D, Ma J, Pancholi M, Straw C, Smeraldi F, et al. Development and automation of a test of impulse control in zebrafish. Front Syst Neurosci [Internet]. 2013. https://www.frontiersin.org/articles/10.3389/fnsys.2013.00065/full#h5 [cited 2021 Mar 25];7. Available from:
Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: opposite influences of dopamine D1 and D2-family receptors. Neurobiol Learn Mem. 2009;92:243–8.
Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci J Soc Neurosci. 2007;27:4984–94.
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–18.
Leggieri A, García-González J, Torres-Perez JV, Havelange W, Hosseinian S, Mech AM, et al. Ankk1 loss of function disrupts dopaminergic pathways in Zebrafish. Front Neurosci [Internet]. 2022 [cited 2022 May 27];16. Available from: https://www.frontiersin.org/article/10.3389/fnins.2022.794653.
Zhong Y, Zhang N, Zhao F, Chang S, Chen W, Cao Q, et al. RBFOX1 and working memory: from genome to transcriptome revealed posttranscriptional mechanism separate from attention-deficit/hyperactivity disorder. Biol Psychiatry Glob Open Sci. 2023;3:1042–52.
Davies MN, Verdi S, Burri A, Trzaskowski M, Lee M, Hettema JM, et al. Generalised anxiety disorder – a twin study of genetic architecture, genome-wide association and differential gene expression. PLOS ONE. 2015;10:e0134865.
Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines. 2019;7:E23.
García-González J, de Quadros B, Havelange W, Brock AJ, Brennan CH. Behavioral effects of developmental exposure to JWH-018 in wild-type and disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish. Biomolecules. 2021;11:319.
Hillman C, Kearn J, Parker MO. A unified approach to investigating 4 dpf zebrafish larval behaviour through a standardised light/dark assay. Prog Neuropsychopharmacol Biol Psychiatry. 2024;134:111084.
Baiamonte M, Parker MO, Vinson GP, Brennan CH. Sustained effects of developmental exposure to ethanol on Zebrafish anxiety-like behaviour. PLoS ONE. 2016;11:e0148425.
Gene Detail:: Allen Brain Atlas: Mouse Brain [Internet]. [cited 2024 Jul 22]. Available from: https://mouse.brain-map.org/gene/show/92602.
Brain tissue expression of RBFOX1 - Summary - The Human Protein Atlas [Internet]. [cited 2024 Jul 22]. Available from: https://www.proteinatlas.org/ENSG00000078328-RBFOX1/brain.
Thisse, B, Thisse, C Fast Release Clones: A High Throughput Expression Analysis. 2004; Available from: https://zfin.org/ZDB-PUB-040907-1.
Grone BP, Maruska KP. Divergent evolution of two corticotropin-releasing hormone (CRH) genes in teleost fishes. Front Neurosci. 2015;9:365.
Sahu MP, Pazos-Boubeta Y, Pajanoja C, Rozov S, Panula P, Castrén E. Neurotrophin receptor Ntrk2b function in the maintenance of dopamine and serotonin neurons in zebrafish. Sci Rep. 2019;9:2036.
Shi C, Lu Y, Zhai G, Huang J, Shang G, Lou Q, et al. Hyperandrogenism in POMCa-deficient zebrafish enhances somatic growth without increasing adiposity. J Mol Cell Biol. 2020;12:291–304.
Faught E, Vijayan MM. The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci Rep. 2018;8:18081.
Tessarollo L, Yanpallewar S. TrkB Truncated Isoform Receptors as Transducers and Determinants of BDNF Functions. Front Neurosci [Internet]. 2022. [cited 2022 Jun 15];16. Available from: https://doi.org/10.3389/fnins.2022.847572.
Martin SC, Marazzi G, Sandell JH, Heinrich G. Five Trk Receptors in the Zebrafish. Dev Biol. 1995;169:745–58.
Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69.
Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP Kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009;6:42–53.
Finlay S, Rudd D, McDermott B, Sarnyai Z. Allostatic load and systemic comorbidities in psychiatric disorders. Psychoneuroendocrinology. 2022;140:105726.
Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15:637–46.
van der Kolk B. Posttraumatic stress disorder and the nature of trauma. Dialogues Clin Neurosci. 2000;2:7–22.
Karin O, Raz M, Tendler A, Bar A, Korem Kohanim Y, Milo T, et al. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol Syst Biol [Internet]. 2020 [cited 2023 Feb 23];16(7). Available from: https://onlinelibrary.wiley.com/doi/10.15252/msb.20209510.
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–21.
Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharm Toxicol. 2004;44:525–57.
Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501.
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12.
Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N. Y Acad Sci. 2009;1179:56–69.
Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, et al. The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front Behav Neurosci [Internet]. 2021 [cited 2023 Feb 26];14. Available from: https://www.frontiersin.org/articles/10.3389/fnbeh.2020.601939.
Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74.
Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Eckert A, Karen S, Beck J, Brand S, Hemmeter U, Hatzinger M, et al. The link between sleep, stress and BDNF. Eur Psychiatry. 2017;41:S282.
Nakagomi A, Okada S, Yokoyama M, Yoshida Y, Shimizu I, Miki T, et al. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. Npj Aging Mech Dis. 2015;1:1–11.
Fenner BM. Truncated TrkB: Beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24.
Cazorla M, Prémont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low–molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–57.
Azogu I, Plamondon H. Blockade of TrkB receptors in the nucleus accumbens prior to heterotypic stress alters corticotropin-releasing hormone (CRH), vesicular glutamate transporter 2 (vGluT2) and glucocorticoid receptor (GR) within the mesolimbic pathway. Horm Behav. 2017;90:98–112.
Bollen E, Vanmierlo T, Akkerman S, Wouters C, Steinbusch HMW, Prickaerts J. 7,8-Dihydroxyflavone improves memory consolidation processes in rats and mice. Behav Brain Res. 2013;257:8–12.
Haapasalo A, Koponen E, Hoppe E, Wong G, Castrén E. Truncated trkB.T1 is dominant negative inhibitor of trkB.TK+-mediated cell survival. Biochem Biophys Res Commun. 2001;280:1352–8.
Tuvikene J, Pruunsild P, Orav E, Esvald EE, Timmusk T. AP-1 transcription factors mediate BDNF-positive feedback loop in cortical neurons. J Neurosci. 2016;36:1290–305.
Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM. A Positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J Neurosci. 2014;34:12547–59.
Eachus H, Ryu S, Placzek M, Wood J. Zebrafish as a model to investigate the CRH axis and interactions with DISC1. Curr Opin Endocr Metab Res. 2022;26:100383.
Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–21.
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35.
Keatinge M, Tsarouchas TM, Munir T, Porter NJ, Larraz J, Gianni D, et al. CRISPR gRNA phenotypic screening in zebrafish reveals pro-regenerative genes in spinal cord injury. PLOS Genet. 2021;17:e1009515.
Dobrzycki T, Krecsmarik M, Monteiro R. Genotyping and quantification of in situ hybridization staining in Zebrafish. J Vis Exp. 2020;155:e59956. https://doi.org/10.3791/59956.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Wullimann MF, Rupp B, Reichert H The brain of the zebrafish Danio rerio: a neuroanatomical atlas. In: Wullimann MF, Rupp B, Reichert H, editors. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Basel: Birkhäuser; 1996 [cited 2021 Aug 10]. pp. 19–87. Available from: https://doi.org/10.1007/978-3-0348-8979-7_5.
R Development Core Team. R: A language and environment for statistical computing. [Internet]. 2008 [cited 2020 Jun 8]. Available from: https://stat.ethz.ch/pipermail/r-help/2008-May/161481.html.
Gutiérrez HC, Vacca I, Schoenmacker G, Cleal M, Tochwin A, O’Connor B, et al. Screening for drugs to reduce zebrafish aggression identifies caffeine and sildenafil. Eur Neuropsychopharmacol. 2020;30:17–29.
Parker MO, Brock AJ, Sudwarts A, Brennan CH. Atomoxetine reduces anticipatory responding in a 5-choice serial reaction time task for adult zebrafish. Psychopharmacology (Berl). 2014;231:2671–9.
Alderman SL, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol. 2009;164:61–9.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45.
Dean RB, Dixon WJ. Simplified statistics for small numbers of observations. Anal Chem. 1951;23:636–8.
Acknowledgements
CHB, AL, SH, PA and WH were supported by the NIH grant U01 DA044400-03. JGG was supported by a 2022 NARSAD Young Investigator Grant (Number #30749) by the Brain & Behavior Research Foundation. NFC and BC were supported by the grants PID2021-127776OB-I100, 202218 31 and 2021-SGR-01093 from the Ministry of Science, Innovation and Universities (Spain), Fundació La Marató de TV3, and AGAUR-Generalitat de Catalunya, respectively, and by ICREA Academia 2021. Authors would like to acknowledge SciDraw (https://scidraw.io/) for images used to create the graphical abstract. We thank Prof. Marina Resmini for kindly providing the equipment used to dry cortisol extracts.
Author information
Authors and Affiliations
Contributions
AL conceived the project, designed and conducted the experiments, analysed the data and wrote the manuscript. JGG designed and performed the CRISPR/Cas9 experiment generating the line. SH contributed to the experiments. PA contributed to the experiments. SA contributed to the experiments. XW contributed to the experiments. WH contributed to the experiments. NFC edited the manuscript. BC edited the manuscript. CHB directed the study, designed the experiments, edited the manuscript and secured funding. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Leggieri, A., García-González, J., Hosseinian, S. et al. rbfox1 LoF mutants show disrupted bdnf/trkb2 and crhb/nr3c2 expression and increased cortisol levels during development coupled with signs of allostatic overload in adulthood. Transl Psychiatry 15, 519 (2025). https://doi.org/10.1038/s41398-025-03703-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03703-x
This article is cited by
-
circ-Ttc3 alleviates LPS-induced neuronal injury via stabilizing RBFOX1 to inhibit inflammation and oxidative stress
Molecular Biology Reports (2026)