Abstract
Patients with preexisting sleep disorders face a significantly increased risk of postoperative sleep disturbance (PSD) due to heightened sensitivity to surgical stress, anesthesia, and hospital-related environmental factors. There is a critical unmet need for effective prophylactic medications to prevent PSD, as current treatment options are limited and often inadequate. This study investigated the prophylactic effect of intraoperative esketamine (0.3 mg/kg/h) on PSD in 130 patients randomized into control and esketamine groups. Preoperative sleep quality was assessed using the Pittsburgh Sleep Quality Index, while postoperative sleep was evaluated using the Numerical Rating Scale (NRS) and Athens Insomnia Scale (AIS) on postoperative days (PODs) 1, 3, and 7. PSD was defined as an NRS or AIS score ≥6. Saliva samples were collected for 16S rRNA sequencing to analyze the oral microbiota. On POD 1, the esketamine group showed a significantly lower incidence of PSD (43.1 vs. 64.6%; OR, 0.414; P = 0.014) and reduced hydromorphone consumption. Preoperative oral microbiota profiles differed between patients with and without PSD, with specific bacterial taxa associated with sleep disturbance. These findings suggest that esketamine may alleviate postoperative sleep disruption, potentially through modulation of the oral microbiota.
Similar content being viewed by others
Introduction
Patients with preexisting sleep disorders are at significantly increased risk of postoperative sleep disturbance (PSD) due to heightened sensitivity to surgical stress, anesthesia, and hospital-related environmental disruptions. PSD, which may present as sleep deprivation, circadian rhythm disruption, or altered sleep architecture, affects approximately 15–72% of postoperative patients [1,2,3]. It is associated with adverse outcomes such as postoperative delirium, cognitive impairment, increased pain, and delayed recovery [2, 4,5,6,7]. Despite its high prevalence, treatment options remain limited in clinical practice, with pharmacologic interventions largely restricted to short-acting non-benzodiazepines like zolpidem and melatonin.
(S)-ketamine (esketamine), the enantiomer of (R,S)-ketamine, exhibits higher affinity for the N-methyl-D-aspartate receptor (NMDAR) compared to (R)-ketamine (arketamine) [8,9,10]. Esketamine is widely used as an anesthetic in several countries, including China. At subanesthetic doses (0.2–0.4 mg/kg, intravenous infusion), esketamine has shown rapid and robust antidepressant effects in patients with treatment-resistant major depressive disorder (MDD), although it is known to cause transient psychotomimetic and dissociative side effects [11, 12]. In 2019, esketamine nasal spray (developed by Johnson & Johnson) was approved in the United States and Europe for treatment-resistant depression. However, post-marketing surveillance using the U.S. FAERS database has raised concerns regarding its long-term efficacy, addiction potential, and risk of suicidal behavior [13]. Notably, a recent study demonstrated that intraoperative esketamine infusion could reduce the incidence of PSD in patients undergoing gynecological laparoscopic surgery [14].
Several factors contribute to PSD, including older age, female sex, postoperative pain and anxiety, nausea and vomiting, and poor adaptation to the hospital environment. Among these, preexisting sleep disorders and anxiety are considered major predictors of postoperative sleep disruption [15]. Thus, patients with high preoperative Pittsburgh Sleep Quality Index (PSQI) scores should receive special attention due to their elevated PSD risk.
Beyond these clinical factors, the oral microbiota—the second most diverse microbial community in the human body after the gut microbiota—has emerged as an important contributor to health and disease [16]. Growing evidence links oral microbiota dysbiosis to various neuropsychiatric disorders, including Alzheimer’s disease, autism spectrum disorder, adolescent anxiety and depression, and bipolar disorder [16,17,18,19,20,21,22]. Furthermore, alterations in the oral microbiota have been reported in individuals with sleep-related conditions such as insomnia and obstructive sleep apnea [23,24,25,26,27]. However, the specific role of oral microbiota in PSD, and how esketamine might modulate these microbial changes, remains poorly understood.
This study aimed to examine the prophylactic effect of intraoperative esketamine infusion on PSD in patients with preoperative sleep disorders and to explore the underlying mechanisms associated with oral microbiota.
Subjects and methods
Study design
This double-blinded, randomized, placebo-controlled clinical trial was approved by the Institutional Scientific Research and Clinical Trials Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2023-KY-1213). The trial was registered prior to patient enrollment in the Chinese Clinical Trial Registry: chictr.org.cn (ChiCTR2400079641). The study protocol was thoroughly explained to patients in advance, and written informed consent was obtained from all participants. This study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT).
The study enrolled patients who met the following criteria: 1) aged 18–65 years, 2) American Society of Anesthesiologists (ASA) Physical Status I–II, 3) preoperative PSQI > 5, and 4) scheduled to undergo colorectal, hepatobiliary, gastrointestinal, gynecological, or urological laparoscopic surgery between January 9, 2024, and May 31, 2024. The exclusion criteria were as follows: 1) refusal to participate in the study, 2) body mass index (BMI) > 30 kg/m², 3) a history of mental illness, 4) recent drug abuse, 5) contraindications or allergies to esketamine, and 6) cognitive dysfunction or inability to communicate.
One study-specific, unblinded physician prepared 130 uniquely numbered opaque randomization envelopes using a computer-generated sequence, assigning subjects in a 1:1 ratio (65 per group) to match the planned 130 subjects. Just before surgery, an unblinded nurse with specialized training—and with no direct contact with the participants—used the assigned envelope to prepare the study drug. Each envelope concealed the allocation for either esketamine (2.5 mg/ml; Jiangsu Hengrui Pharmaceutical Co., Ltd, China) or normal saline, and the drug was drawn into identical 20-mL syringes by the same nurse. The envelope was then resealed and securely stored, ensuring that all participants, healthcare workers, and study personnel (including monitors and sponsors) remained blinded until after data analysis. As previously reported [14], the esketamine group received an infusion of esketamine (0.3 mg/kg/h; Jiangsu Hengrui Pharmaceutical Co., Ltd, China), while the control group received an equivalent volume of saline.
Anesthesia and postoperative analgesia management
Routine intraoperative monitoring was established, including pulse oximetry, electrocardiography, and noninvasive blood pressure. The anesthesiologist had the discretion to place arterial puncture catheters for continuous arterial blood pressure monitoring as needed. Anesthesia was induced with etomidate (0.2 mg/kg), sufentanil (0.5 µg/kg), and rocuronium (0.9 mg/kg) to facilitate endotracheal intubation. Anesthesia was maintained with remifentanil (0.1–0.2 µg/kg/min), propofol (1–3 mg/kg/h), and 3% desflurane in 50% O2 (2 L/min), with the remifentanil and propofol infusion rates titrated to keep the heart rate and arterial blood pressure within 20% of baseline. Sufentanil (5-15 µg) and palonosetron (0.25 mg) were administered intravenously at the end of surgery for postoperative analgesia and to prevent postoperative nausea and vomiting. After surgery, the endotracheal tube was removed once adequate muscle strength was reestablished. Postoperative pain was managed using hydromorphone (0.2 mg/kg in 200 mL saline) via a patient-controlled intravenous analgesia (PCIA) pump: background infusion of 2 mL/h for 48 h, with a 2 mL bolus and a 10-min lockout time. Oxycodone was administered intravenously as a rescue analgesic if the pain score exceeded 4.
Outcome measurements
Postoperative sleep quality was evaluated using the Numerical Rating Scale (NRS) and Athens Insomnia Scale (AIS). Postoperative pain scores were also assessed using the NRS. Anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale (HADS) 1 day before surgery and on postoperative days (PODs) 1, 3, and 7. Postoperative fatigue was measured using the Christensen Fatigue Scale on PODs 1, 3, and 7. Oral saliva samples were collected from patients for subsequent analysis of oral microbiota to examine the correlation between differential microbiota and postoperative sleep quality as measured by NRS and AIS scores.
The primary outcome was the incidence of PSD on postoperative day 1 (POD 1). PSD was evaluated using two scales: Numerical Rating Scale (NRS): Ranges from 0 to 10, where 0 represents excellent sleep and 10 indicates the inability to sleep throughout the night. Athens Insomnia Scale (AIS): A self-rated psychometric questionnaire designed according to the International Classification of Diseases, 10th edition [28], consisting of 8 items (waking up at night, sleep induction, final awakening, total sleep duration, sleep quality, well-being, functional ability, and daytime sleepiness) with a total score ranging from 0 to 24. An AIS score of ≥6 indicates insomnia. PSD was defined as having an NRS score of ≥6 or an AIS score of ≥6, which signifies repeated sleep interruptions throughout the night or a worse condition [29, 30].
Secondary outcomes included the incidence of PSD, as well as NRS and AIS scores on PODs 3 and 7. Additionally, postoperative pain scores at rest and during movement were assessed at 24 and 48 h postoperatively and on PODs 3 and 7, along with postoperative hydromorphone consumption within the first 24 h and total consumption within 48 h. Anxiety and depression scores were measured on PODs 1, 3, and 7, and postoperative fatigue scores were also recorded on these days. Furthermore, we analyzed the correlation between differential microbiota and postoperative sleep quality and conducted post-hoc exploring subgroup analyses based on sex, BMI, age, baseline PSQI score, physical pain during the past month, and a STOP-Bang score of ≥3.
Postoperative pain at rest and during movement was assessed on PODs 1, 3, and 7 using the NRS, which ranges from 0 (no pain) to 10 (intolerable pain). Postoperative anxiety and depression were evaluated using the HADS [31], which consists of 14 questions with 7 items each for anxiety and depression. Each item is scored from 0 to 3, with scores ≥8 considered positive for both depression and anxiety. Postoperative fatigue was assessed using the Christensen Fatigue Scale [32], which consists of 5 scoring dimensions to evaluate the degree of fatigue: 1-2 points, normal; 3-4 points, feeling acceptable, with weakness only occurring with excessive activity and normal sleep; 5-6 points, able to maintain daily living activities, occasionally able to engage in slightly strenuous activities; 7-8 points, can only engage in some daily living activities, feeling effort when climbing stairs or walking, needing sleep; 9-10 points, unable to carry out daily activities or only very short activities, needing sleep. A total score of ≥6 points indicate the presence of fatigue, with higher scores indicating more severe fatigue.
Additionally, postoperative hydromorphone consumption, as well as postoperative complications including nausea and vomiting, dizziness, itching, and somnolence, were noted and treated accordingly. Other recorded data included surgery and anesthesia duration, intraoperative consumption of sufentanil and remifentanil, estimated infusion volume, blood loss, urine output, extubation time (the time from the end of surgery to the removal of the endotracheal tube), and length of hospital stay.
Measurement of oral microbiota
To reduce variability in oral microbiota, all participants adhered to a standardized perioperative diet provided by the hospital. As previously described, 2 mL of saliva was collected in the morning (8:00-10:00 AM) of the surgery day and POD 1 [33]. Participants were instructed to refrain from eating, drinking, and performing oral hygiene procedures half h before sampling to secure adequate saliva for analysis. All samples were immediately cooled to −18 °C and then stored at −80 °C within 8 h until further analysis. Patients with diabetes were excluded from the analysis since hyperglycemia and medication can markedly alter oral microbiota [34, 35].
DNA extraction and 16S rRNA analysis were performed at Metware Biotechnology Co., Ltd. (China). Total genomic DNA was extracted from the samples using the CTAB method. The bacterial 16S rRNA genes (V3–V4 region) were amplified using PCR with the forward primer 341 F (5′-CCTAYGGGRBGCASCAG-3′) and the reverse primer 806 R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR products were mixed with an equal volume of 1X loading buffer (containing SYBR Green) and run on a 2% agarose gel for detection. The products were then combined in equidensity ratios and purified using the Qiagen Gel Extraction Kit (Qiagen, Germany). After quantification and quality assessment, sequencing libraries were prepared using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) according to the manufacturer’s recommendations, with index codes added. Library quality was evaluated using a Qubit® 2.0 Fluorometer (Thermo Scientific) and an Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform to generate 250 bp paired-end reads. Raw reads were quality-filtered using fastp (v0.22.0; GitHub) to obtain high-quality clean tags. Paired-end reads were merged using FLASH (v1.2.11; FLASH Software). OTU clustering was performed using Uparse (v7.0.1001; Uparse Software), with sequences sharing ≥97% similarity assigned to the same OTU. OTU sequences were taxonomically classified in Mothur v1.48 against the SILVA 138.1 SSU rRNA database (https://www.arb-silva.de) using a confidence cutoff of 0.8–1. Taxonomic assignments for each sequence were then aggregated at the phylum, class, order, family, genus, and species levels to characterize community composition. Representative OTU/ASV sequences were aligned with MAFFT v7.520 (https://mafft.cbrc.jp/alignment/software/) to reconstruct phylogenetic relationships. Finally, to normalize sequencing depth across samples, each library was rarefied to the size of the smallest dataset.
Negative controls were included at the amplification stage and processed alongside samples using the identical protocol. Contamination was ruled out when negative controls showed no prominent peaks and no bands at the target position on a simulated gel; if these criteria were not met, the experiment was halted. Sequencing data processing proceeded as follows: Raw reads were demultiplexed by barcode and primer sequences, after which barcodes and primers were trimmed. High-quality reads were then filtered with fastp v0.22.0 (https://github.com/OpenGene/fastp) under these criteari: (1) automatic adapter detection and removal; (2) removal of reads containing ≥1 ambiguous “N” bases; (3) removal of reads in which low-quality bases (Q < 15) comprised >40% of the sequence; (4) sliding-window (4-base) trimming where average quality < 20; (5) removal of trailing poly-G stretches; (6) removal of reads shorter than 150 bp. Paired-end reads passing these filters were merged with FLASH v1.2.11 (http://ccb.jhu.edu/software/FLASH) to produce clean tags. These clean tags were screened for chimeras against the reference database using VSEARCH v2.22.1 (https://github.com/torognes/vsearch), and any chimeric sequences were discarded, yielding the final set of effective tags.
Sample size calculation and statistical analysis
The estimated sample size was calculated based on our preliminary study, where the incidence of PSD was 70% in control group, 45% in esketamine group on POD 1 in patients with preoperative sleep disorder following laparoscopic surgery. To detect reduction from 70% in control group to 45% in esketamine group in PSD incidence with a two-sided α = 0.05 and 80% power, 116 patients were required. Considering a 10% loss to follow-up rate, we included a total of 130 patients, with 65 cases in each group (n = 65 in control group. n = 65 in esketamine group).
Statistical analysis was performed using SPSS 25.0 (IBM SPSS, Chicago, Illinois, USA) and R statistical software, version 4.2.0 (R Project for Statistical Computing). Given the potential for type II errors in some normality tests (e.g., the Kolmogorov-Smirnov test), all continuous variables are reported as medians with interquartile ranges (IQRs). Differences between the two groups for continuous data were assessed using the Mann-Whitney U test. Categorical variables are presented as numbers and percentages and were compared using the chi-square or Fisher’s exact test, as appropriate.
All analyses were performed on the modified intention-to-treat (mITT) population, which included all randomized patients who received at least one dose of the intervention. The per-protocol (PP) analysis set comprised only those participants who completed both the intervention and the primary-outcome follow-up. For secondary outcomes, three instances of missing data occurred in both the control and esketamine groups. These secondary outcomes included the incidence of PSD, NRS and AIS scores on PODs 3 and 7; postoperative pain scores at rest and during movement at 48 h postoperatively and on PODs 3 and 7; postoperative hydromorphone consumption within 48 h; anxiety and depression scores on PODs 3 and 7; and postoperative fatigue scores on PODs 3 and 7. Missing data were managed by applying multiple imputation for variables with more than 10% missingness and median imputation for those with 10% or less missing.
Generalized estimating equations (GEE) were employed for statistical analysis, with days and day-by-intervention interactions treated as fixed effects. First, the treatment-by-time interaction was tested. If significant, between-group differences at each time point were evaluated and adjusted for multiple comparisons using the Bonferroni test; if not, only the main effect of treatment was tested. For PSD, NRS, AIS sleep quality, and HADS scores, time effects included preoperative day 1 and PODs 1, 3, and 7, while for postoperative pain NRS and fatigue scores, time effects included PODs 1, 3, and 7. We developed several models: 1) Model 0: No covariate adjustment. 2) Model 1: Adjusted for age, sex, and BMI. 3) Model 2: Further adjusted for diabetes, ASA classification, smoking status, preoperative PSQI score, STOP-Bang score ( ≥ 3), and whether physical pain was experienced in the past month. 4) Model 3: The time effects of all dependent variables were measured at three time points (POD1, POD3, and POD7) and further adjusted for preoperative NRS score, AIS score, and preoperative HADS score.
A post-hoc exploratory analysis was also conducted to evaluate disparities in primary outcomes within specific subgroups based on age, gender, BMI, physical pain condition, STOP-Bang score ( ≥ 3), and baseline sleep quality. We also applied a simple mediation analysis to determine whether 24-hour postoperative hydromorphone consumption mediates esketamine’s effect on sleep quality on POD 1.
α-diversity of oral microbiome was analyzed using Chao 1, Simpson, and observed species indices. β-diversity of oral microbiome was assessed using principal component analysis (PCA) and principal coordinates analysis (PCoA), with statistical significance evaluated using analysis of similarities (ANOSIM). Based on bacterial abundance, significant differential biomarkers between groups at different taxonomic levels were explored using linear discriminant analysis (LDA) effect size (LEfSe) (https://www.omicstudio.cn/tool). Only taxa with LDA scores >3 and p < 0.05 were considered significantly enriched. The results were visualized using taxonomic bar charts. Additionally, differential oral microbiota between groups were screened using the Mann-Whitney U test and displayed with violin plots. To account for multiple testing, two-sided p-values were adjusted using the Benjamini/Hochberg (B/H) method to control the false discovery rate (FDR), with q-values below 0.05 deemed statistically significant (FDR of 5%). Finally, Spearman’s rank correlation was used to assess the associations between differential oral microbiota and scores, with a p-value < 0.05 considered significant.
Results
Participants flow and baseline characteristics
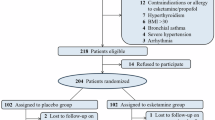
A total of 202 patients were assessed for eligibility between January 2024 and May 2024, and 72 were excluded based on the exclusion criteria. 130 patients were evenly divided into two groups: the control group (mean age: 51 years, n = 65) and the esketamine group (mean age: 52 years, n = 65) (Table 1). No data loss occurred on the POD 1 in either group. On POD 3, one instance of data loss was observed, while on POD 7, two instances occurred in the control group and three in the esketamine group (Fig. 1). All 130 randomized participants received the intervention, and no primary-outcome data were lost in either group. Therefore, all 130 participants were included in both the ITT and PP analysis sets. For secondary outcomes, missing data did not exceed 10%, and were imputed using the median.
The two groups were similar in terms of demographic data, including sex, ASA scores, height, weight, BMI, level of education, type of surgery (colorectal, hepatobiliary, gastrointestinal, gynecological, and urological laparoscopic surgery), comorbid conditions (hypertension, thyroid disease, liver disease, kidney disease, cerebrovascular disease), smoking and drinking status, malignancy, and preoperative hemoglobin levels. However, there were statistically significant differences in age (51 [36.5-54.5] vs. 52 [47–58], P = 0.009) and the prevalence of diabetes (3.1 vs. 12.3%, P = 0.048) between the control and esketamine groups. Additionally, there were no significant differences between the two groups regarding preoperative HADS scores, PSQI scores for sleep disorders, NRS and AIS scores for sleep quality, the proportion of STOP-Bang scores ≥ 3, or the incidence of physical pain in the past month (Table 1).
Intraoperative and postoperative clinical parameters
The consumption of intraoperative remifentanil and sufentanil, postoperative oxycodone usage, as well as the duration of surgery, anesthesia, hospital stay, infusion volume, estimated blood loss, and urine output, did not significantly differ between the two groups. However, the esketamine group had a longer extubation time compared to the control group (23 [18–31] min vs. 18 [11–26] min, P < 0.001) (Table S1).
Primary sleep outcomes
The esketamine group had a significantly lower incidence of PSD on the POD 1 compared to the control group (43.1 vs. 64.6%, odds ratio: 0.414 [95% CI, 0.204–0.840], P = 0.014). Additionally, on the POD 1, the esketamine group showed significantly lower NRS and AIS subjective sleep quality scores and a greater reduction in NRS scores from baseline compared to the control group (NRS: 5 [3–7] vs. 5 [4–7], P = 0.005; AIS: 5 [3–8] vs. 6 [4–9], P = 0.009; reduction of NRS: 0 [-2, 1] vs. -1 [-3, 1], P = 0.047) (Table 2 and Fig. 2).
Only the AIS subjective sleep quality scores on the POD 3 and the fatigue scores on the POD 7 were significantly lower in the esketamine group compared to the control group (AIS on the POD 3: 4 [2–7] vs. 4 [3–6], P = 0.025; fatigue score on the POD 7: 1 [0, 3] vs. 3 [1,5], P = 0.010). There were no significant differences between the two groups in PSD incidence, NRS scores on PODs 3 and 7, AIS scores on the POD 7, reduction of NRS scores from baseline on the PODs 3 and 7, reduction of AIS scores from baseline on the PODs 1, 3, and 7 (Table 2 and Table S2).
Postoperative pain, opioid consumption, postoperative anxiety and depression
There were no significant differences in NRS scores for pain at rest and during movement at 24 and 48 h postoperatively. However, median hydromorphone consumption in the first 24 h postoperatively (2.35 [2.00–3.29] mg vs. 2.60 [2.50–3.41] mg, P = 0.003) and total consumption over 48 h postoperatively (3.70 [3.00–5.65] mg vs. 4.52 [4.30–5.97] mg, P = 0.002) were significantly lower in the esketamine group compared to the control group (Table S2 and Table S3). The mediation analysis demonstrated that 24 h postoperative hydromorphone consumption accounted for only a modest proportion of the total effect (9.04% unadjusted; 13.35% adjusted) (Figure S1). Additionally, there were no significant differences in HADS-A and HADS-D scores between the two groups on PODs 1, 3, and 7 (Table 2).
The GEE analysis for NRS, AIS, HADS-A and D, fatigue, resting pain, and movement pain
The GEE analysis was conducted using four models: Model 0: No covariate adjustment. Model 1: Adjusted for age, sex, and BMI. Model 2: Further adjusted for diabetes, ASA classification, smoking status, preoperative PSQI score, STOP-Bang score ( ≥ 3), and whether the patient experienced physical pain in the past month. Model 3: The time effects of all dependent variables were measured at three time points (POD1, POD3, and POD7) and further adjusted for preoperative NRS, AIS, and HADS scores. The results demonstrated that the significant differences in the incidence of PSD on POD 1, as well as in the NRS and AIS subjective sleep quality scores, persisted in Models 1, 2, and 3. Additionally, the lower fatigue scores observed on POD1 and POD7 remained significant after adjustment. The significant difference in AIS scores on POD3 was maintained in Models 1 and 2 (Table S2).
Adverse events and subgroup analyses
The incidence of postoperative nausea and vomiting, dizziness, itching, and somnolence did not differ significantly between the two groups (Table S4). A stratified analysis based on six factors—sex, BMI, age, baseline PSQI score, past-month physical pain, and STOP-Bang score ( ≥ 3)—found no subgroup in which esketamine significantly reduced PSD incidence. (Fig. 3).
Oral microbiota analysis
Oral saliva samples were collected from 58 patients in the morning on POD 1. Significant differences of baseline characteristics of patients who the oral saliva collected from on the POD 1 were observed between the control and esketamine groups in terms of age (46 [35,54] vs. 53.5 [47.75, 58.25], P = 0.008), ASA classification (ASA I: 67.7 vs. 37.0%; ASA II: 32.3 vs. 63.0%, P = 0.019) and the presence of diabetes (0 vs. 5 [18.5], P = 0.018). Considering diabetes may affect the composition of oral microbiota, the oral microbiota data of patients with diabetes (n = 5) were excluded from the subsequent microbiome analysis. After the excluding, the ASA classification, but not the age of patients who the oral saliva collected from on the POD 1 were not significant difference between the two groups (Table S5). The composition (α- or β-diversity) of oral microbiota on POD 1 in patients were not significant differences between the control group (n = 22) and the esketamine group (n = 31) (Fig. 4A-E). In the esketamine group, the relative abundance of certain microbial communities was higher, including Streptococcus and Peptostreptococcus at the genus level, and Prevotella_nigrescens at the species level. Meanwhile, the relative abundance of Bacteroidota at the phylum level was lower (Fig. 4F-J and Table S6). Spearman correlation analysis revealed no significant association between the differential microbiota and NRS or AIS scores on POD 1 (Table S7).
A: α-diversity index of Observed_ASV (Mann-Whitney U test, U = 276, P = 0.2447). ns. not significant. B: α-diversity index of Shannon (Mann-Whitney U test, U = 300.5, P = 0.4708). ns. not significant. C: α-diversity index of Chao1 (Mann-Whitney U test, U = 270.5, P = 0.2067). ns. not significant. D: Principal component analysis (PCA) of beta-diversity based on the OTU level, where each point represents a single sample colored by group circle, indicated by the second principal component of 7.16% on the Y-axis and the first principal component of 42.71% on the X-axis (ANOSIM, R = 0.066, P = 0.392). E: Principal Coordinates Analysis (PCoA) plot based upon weighted (ANOSIM, R = 0.0099, P = 0.55), each dot represents a single sample indicated by a principal component of 18.35% on the X-axis and another principal component of 14.01% on the Y-axis. F: Microbiota composition with significant differences at the phylum level between the Control group and the Esketamine group on the POD1. *P < 0.05. G: Microbiota composition with significant differences at the genus level between the Control group and the Esketamine group on the POD1. *P < 0.05, **P < 0.01. H: Microbiota composition with significant differences at the species level between the Control group and the Esketamine group on the POD1. *P < 0.05. I: LEfSe algorithm of oral microbiota. Cladogram (LDA score > 3.0, P < 0.05) showed the taxonomic distribution difference among the Control group and the Esketamine group, indicating with different color region. Each continuous circle represents a differentially abundant taxonomic clades at phylum, class, order, family, genus and species level from the inner to outer rings. J: Histograms of the different abundant taxa based on the cutoff value of LDA score (log10) > 3.0 and P < 0.05 among. p, phylum; c, class; o, order; f, family; g, genus; s, species.
Next, we performed a stratified analysis based on age. In each age group, no significant differences were observed in the α-diversity on POD 1 between the two groups. In patients aged 18–49 years, and a significant difference in β-diversity was detected on POD 1 (PCA: R = 0.1425, P = 0.039) (Figures S2A-E). In patients aged 50–65 years, a significant difference in β-diversity was detected on POD 1 (PCA: R = 0.0784, P = 0.029; PCoA: R = 0.1171, P = 0.014) (Figures S3A-E).
For patients aged 18–49 years, the genera Granulicatella, Aggregatibacter, Peptostreptococcus, Peptoanaerobacter, the species Prevotella_sp_oral_clone_BU035, Prevotella_pleuritidis, Peptoanaerobacter_stomatis, Eikenella_corrodens, Ottowia_sp_oral_taxon_894, and Prevotella_saccharolytica showed significant differences between the two groups (Figure S2F-H and Table S8). Spearman correlation analysis revealed the genera Peptoanaerobacter, the species Prevotella_sp_oral_clone_BU035, Peptoanaerobacter_stomatis, and Ottowia_sp_oral_taxon_894 were positively or negatively correlated with NRS or AIS scores on POD 1 (Table S9). Similarly, for patients aged 50–65 years, significant differences were noted at multiple taxonomic levels on POD1: at the phylum level (Actinobacteria, Bacteroidota, and Firmicutes), at the genus level (Streptococcus, Porphyromonas, Rothia, unidentified_Saccharimonadales, Bergeyella, and unidentified_Clostridia), and at the species level (Streptococcus_pneumoniae, Haemophilus_parainfluenzae, Prevotella_pleuritidis, Clostridiales_bacterium_canine_oral_taxon_100, Streptococcus_anginosus, Gracilibacteria_bacterium_oral_taxon_873, Actinomyces_oris, Campylobacter_gracilis, Aggregatibacter_aphrophilus, Treponema_sp_I_T_AT24, Selenomonas_sp_oral_taxon_136, Leptotrichia_sp_oral_clone_IK040) (Figure S3F-J and Table S10). Among them, Bergenella at genus level Selenomonas_sp_oral_taxon_136 at species level was significantly positively correlated with NRS and AIS scores on the POD 1 (Table S11).
Additionally, oral saliva samples were collected from 59 patients on the morning of the surgical day. After excluding patients (n = 6) with diabetes, no significant differences were observed in the preoperative oral microbiota composition (α- or β-diversity) between the control group (n = 28) and the esketamine group (n = 25) (Figure S4).
After excluding diabetic patients, all the 53 patients were divided into No PSD group and PSD group basing on without or with PSD on the POD 1. We stratified the No PSD and PSD groups by treatment allocation (control vs. esketamine). In the control group, there were no significant differences in preoperative oral microbiota α- or β-diversity between the No PSD and PSD subgroups (Figure S5A–E). However, significant differences in oral microbiota were observed in the control group: Neisseria at the genus level differed significantly; Capnocytophaga_leadbetteri at the species level was significantly different (Figure S5F-I and Table S12). Moreover, the relative abundance of these two bacteria was significantly positively correlated with both NRS and AIS scores on the POD 1 (Table S13). In the esketamine group, no significant difference was found in preoperative oral microbiota α-diversity between the No PSD and PSD subgroups (Figure S6A–C). However, β-diversity differed significantly in the preoperative weighted PCoA (R = 0.1369, P = 0.044) (Figure S6D and E). Within the PSD subgroup of the esketamine group, the relative abundance of certain microbial communities was higher—specifically, Capnocytophaga and Haemophilus at the genus level, and Prevotella_aurantiaca, Capnocytophaga_sputigena and Haemophilus_parainfluenzae at the species level (Figure S6F–J and Table S14). In addition, the relative abundance of Haemophilus at the genus level and Haemophilus_parainfluenzae at the species level were significantly positively correlated with NRS and AIS scores on the POD 1. The relative abundance of Capnocytophaga_sputigena at the species level was significantly positively correlated with AIS scores on the POD 1 (Table S15).
Discussion
The main findings of this trial are summarized as follows. First, patients in the esketamine group showed a significantly lower incidence of PSD on POD 1 compared to the control group. Subjective sleep quality scores, assessed by the NRS and AIS, were significantly better in the esketamine group on POD 1, with a greater reduction in NRS scores from baseline. Improvements in AIS scores were also observed on POD 3, and fatigue scores were improved on POD 7. Second, while pain scores at rest and during movement at 24 and 48 h postoperatively did not differ significantly between groups, the esketamine group required significantly less hydromorphone during the first 24 and 48 h after surgery. Third, GEE models adjusted for multiple covariates—including age, sex, BMI, diabetes status, ASA classification, and others—confirmed that esketamine’s beneficial effects on PSD incidence and sleep quality remained significant after accounting for potential confounding factors. Finally, oral microbiota analysis revealed significant associations between specific microbial profiles and clinical sleep outcomes on POD 1. At the phylum level, the relative abundance of Proteobacteria was significantly correlated with sleep scores. At the genus level, unidentified Prevotellaceae, Capnocytophaga, Neisseria, and Haemophilus were associated with both NRS and AIS scores. At the species level, Capnocytophaga_leadbetteri, Selenomonas_sp. _oral_taxon_478, and Haemophilus_parainfluenzae also showed significant correlations with sleep quality measures. In summary, intraoperative esketamine infusion significantly improved early postoperative sleep quality and reduced opioid consumption. These beneficial effects persisted after adjusting for potential confounders. Moreover, differences in the oral microbiota—particularly in older patients and specific subgroups—highlight the potential role of microbial composition in postoperative sleep outcomes.
A previous study involving 102 female patients undergoing total laparoscopic hysterectomy compared propofol-based total intravenous anesthesia (TIVA) with sevoflurane-based inhalation anesthesia. The reported incidence of PSD in the TIVA group was 62%, 59%, and 41% on PODs 1, 3, and 7, respectively, while in the sevoflurane group it was 60%, 52%, and 22% on the corresponding days [36]. In our study, the incidence of PSD in the control group was 64.6%, 47.7%, and 56.9% on PODs 1, 3, and 7, respectively—findings generally consistent with the previous study, though we observed a rebound in PSD incidence on POD 7. This rebound may be explained by the inclusion of patients with preoperative sleep disorders in our trial. Both our findings and prior research suggest that the incidence of PSD on POD 1 is higher in patients with preexisting sleep disorders compared to those without (64.6 vs. 44%) [14]. A recent meta-analysis also supports this association, reporting that the pooled preoperative prevalence of sleep disturbances—defined by a PSQI score > 5—is 60.0% among surgical patients, with preexisting sleep disorders being a major risk factor for PSD [15]. Our previous clinical trial demonstrated that intraoperative esketamine infusion could reduce PSD incidence in patients without preoperative sleep disorders [14]. The current study extends those findings by showing that esketamine also significantly reduces PSD in patients with preexisting sleep disturbances. Taken together, our data suggest that perioperative esketamine infusion has a protective effect against PSD, regardless of baseline sleep status. Importantly, this trial specifically enrolled patients with preoperative sleep disorders and administered esketamine during anesthesia, thereby minimizing any placebo effects. These findings raise the possibility that esketamine may be beneficial for managing sleep disturbances in this population, though further studies are needed to confirm its broader therapeutic potential in sleep-related conditions.
There were significant differences in baseline characteristics between the groups, specifically in age (median 51 vs. 52 years) and the prevalence of diabetes (3.1 vs. 12.3%). While older age has been associated with poorer sleep quality [15], the relationship between diabetes and sleep quality remains less clearly defined. To account for these potential confounding factors, we performed GEE analyses in Models 1 and 2, adjusting for age and diabetes as covariates. Notably, even after these adjustments, the esketamine group continued to show significantly lower PSD incidence and improved NRS and AIS sleep quality scores. It is also worth noting that the esketamine group had a higher median age and greater prevalence of diabetes compared to the control group. Therefore, the observed differences in PSD outcomes cannot be attributed to these baseline imbalances, supporting the robustness of our findings. Moreover, our subgroup analyses were underpowered and did not detect significant treatment–subgroup interactions, despite observing significant esketamine effects in certain subgroups (e.g., patients aged 50–65). These findings may reflect chance, highlighting the need for future trials to incorporate pre-specified subgroup analyses to robustly assess differential protective effects.
Postoperative pain has a well-documented impact on both recovery and sleep quality in surgical patients [37]. In this study, although there were no significant differences in NRS pain scores—either at rest or during movement—between the esketamine and control groups, the esketamine group demonstrated significantly lower opioid consumption. Given esketamine’s known analgesic properties [8], its beneficial effects on postoperative sleep quality and reduction in PSD may, in part, be mediated by decreased opioid requirements. However, the precise relationship between perioperative opioid consumption and the development of PSD remains uncertain, as previous studies have reported inconsistent findings [38,39,40]. Although reduced opioid consumption may contribute partly to the observed improvement in PSD with esketamine, our mediation analysis shows this indirect pathway accounts for only a small fraction of the effect. The predominant benefit appears to arise from a direct action of esketamine on postoperative sleep. Further mechanistic studies are therefore warranted to elucidate the underlying biological pathways of esketamine’s sleep-modulating effects.
Prior research suggests that the oral microbiota plays a critical role in various psychiatric and neurological disorders [16, 18, 20, 41, 42]. However, as noted in the introduction, its relationship with sleep disorders remains largely underexplored [43]. Two clinical studies have reported significant alterations in the diversity and composition of the oral microbiota in patients with chronic insomnia compared to healthy controls [23, 44]. In the present study, preoperative saliva samples were collected from surgical patients for 16S rRNA sequencing. The results revealed significant differences in oral microbiota composition on POD 1 between patients with and without PSD. Specifically, the relative abundances of Actinobacteria and Bacteroidota at the phylum level were significantly lower in the PSD group compared to the non-PSD group. The oral microbial community is predominantly composed of Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, and Fusobacteria, which together account for approximately 96% of all detected species [45].
Previous studies have shown that in the gut, Actinobacteria is negatively associated with sleep disturbances in mobile phone–addicted college students and with aging-related inflammation in mice, while Bacteroidota has been reported to exert anti-inflammatory effects in the context of intestinal disease and chronic psychosocial stress [46, 47]. Although direct evidence in the oral microbiota is limited, these findings suggest that Actinobacteria and Bacteroidota may also be linked to sleep regulation, warranting further investigation [23, 48, 49]. At the genus level, higher relative abundances of Neisseria and Haemophilus, and at the species level, Haemophilus parainfluenzae, were positively correlated with poor sleep quality, as indicated by higher NRS and AIS scores. Notably, these bacteria have also been implicated in pediatric obstructive sleep apnea [50]; however, their role in postoperative or adult sleep disturbances remains unclear and requires further study. In summary, our findings suggest that dysbiosis of the oral microbiota may contribute to the development of PSD in surgical patients. Further research is needed to elucidate the specific microbial mechanisms involved and their potential as therapeutic targets.
The underlying mechanisms by which esketamine exerts its prophylactic effects on postoperative sleep quality and PSD remain unclear. It is plausible that postoperative physiological changes—such as elevated proinflammatory cytokine levels, endothelial dysfunction, glycocalyx degradation, and neutrophil activation—contribute to the development of PSD [51, 52]. Notably, both preclinical and clinical studies have demonstrated that esketamine possesses significant anti-inflammatory and immunomodulatory properties [53, 54]. Recently, we reported that repeated esketamine administration improved spatial working memory in a mouse model of chronic pain, an effect associated with modulation of the gut microbiota [55]. These findings suggest that esketamine’s beneficial impact on PSD may involve its anti-inflammatory and immunoregulatory actions, potentially mediated through alterations in host microbiota, including both the oral and gut microbial communities [16].
In this study, we identified several bacterial species that differed significantly between the esketamine and control groups, including Prevotella_nigrescens, Prevotella_pleuritidis, and Streptococcus_anginosus. However, the specific roles of these bacteria in sleep disorders remain poorly understood. Prevotella species are commonly found in mammal-associated microbial communities, including the skin, oral cavity, vagina, and gut. Prevotella nigrescens, in particular, is associated with periodontal disease and is prevalent in the oral cavity, where it may contribute to systemic inflammation [56]. Similarly, Prevotella_pleuritidis has been reported to be enriched in the oral microbiota of patients with early-onset rheumatoid arthritis [57]. Additionally, Streptococcus_anginosus has been implicated in promoting gastric inflammation [58]. Taken together, these findings suggest that specific members of the oral microbiota may influence postoperative sleep outcomes through inflammatory pathways. And esketamine’s prophylactic effect on PSD may, in part, be mediated by modulation of inflammation-related bacterial taxa. Nevertheless, further studies with larger cohorts are needed to clarify these associations and to better understand the role of the oral microbiota in postoperative sleep regulation.
Based on the current data, it remains unclear how esketamine infusion directly influences the oral microbiota in this study. However, preclinical research has demonstrated that esketamine and its enantiomer arketamine can modulate gut microbiota composition in mouse models of depression [59,60,61], pain [55, 62], and osteoporosis [63, 64]. Given the bidirectional relationship between the gut and oral microbiota [16], it is plausible that esketamine’s effects on autonomic nervous system activity and its anti-inflammatory properties may impact salivary composition and local immune responses, thereby indirectly altering the oral microbiota. Further studies are warranted to elucidate the dynamic interplay between gut and oral microbial communities and their potential contributions to the development and prevention of PSD.
This study has several limitations. First, it was conducted at a single center, which may limit the generalizability of the findings; future multi-center studies are needed to validate these results. Second, due to budget constraints, saliva samples were collected from only a subset of participants, potentially limiting the ability to fully elucidate the mechanisms underlying esketamine’s beneficial effects on PSD. Although we did not collect comprehensive preoperative dietary data using a food-frequency questionnaire, all patients consumed the same hospital-provided meals throughout the perioperative period, thereby minimizing diet-related variability in oral microbiota. We acknowledge, however, that habitual pre-hospital dietary patterns could still have influenced baseline microbial communities; future studies should include detailed dietary assessments to address this potential confounder. In addition, while 16S rRNA sequencing remains the gold standard for bacterial identification and is widely used to assign taxonomy via reference databases, its reliance on short reads covering only partial hypervariable regions (e.g., V3–V4) — approximately 30% of the full 1.5 kb gene — compromises both resolution and accuracy. Future work should therefore employ shotgun metagenomic sequencing to achieve species-level precision in characterizing oral microbiome dynamics. Third, although our GEE models adjusted for age and diabetes prevalence—both known to affect sleep quality and microbiota composition—residual confounding may remain. Future studies should implement stricter matching or stratification by these factors to enhance validity and interpretability. Fourth, bispectral index (BIS) monitoring was not employed during surgery, as BIS is not reliably correlated with ketamine-induced anesthesia states [65]. In addition, objective sleep assessments (e.g., polysomnography and electroencephalography) were not performed due to the logistical and technical challenges of implementing these methods in a large clinical cohort. Finally, the use of 16S rRNA sequencing presents inherent limitations, including reduced resolution at the species level and limited functional insights into microbial activity. This study was designed as an exploratory investigation to identify potential oral microbial biomarkers and their associations with PSD and esketamine intervention. Although our findings imply that the oral microbiota may mediate esketamine’s effects, they do not directly demonstrate this mechanism. To achieve a more comprehensive understanding of the relationship between oral microbiota, microbiome-derived metabolites, PSD, and esketamine’s mechanism of action, future studies should incorporate advanced sequencing techniques such as shotgun metagenomics, along with experimental validation.
In conclusion, this study demonstrates that intraoperative esketamine infusion significantly reduces the incidence of PSD and improves sleep quality in patients with preexisting sleep disorders. These benefits were accompanied by reduced opioid consumption and were associated with distinct changes in the oral microbiota. Together, these findings suggest that esketamine may exert its prophylactic effects on PSD, at least in part, through immunomodulatory and microbiota-mediated mechanisms.
Data availability
The 16S rRNA sequencing data have been deposited to the NCBI Sequence Read Archive and are available at the accession number PRJNA1208423.
Code availability
All codes related to this paper are available upon request from the corresponding authors.
References
Gögenur I, Wildschiøtz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100:45–9.
Chouchou F, Khoury S, Chauny J-M, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain?. Sleep Med Rev. 2014;18:273–82.
Su X, Wang DX. Improve postoperative sleep: what can we do?. Curr Opin Anaesthesiol. 2018;31:83–88.
Tang X, Li J, Yang B, Lei C, Dong H. Efficacy of sleep interventions on postoperative delirium: a systematic review and meta-analysis of randomized controlled trials. Anesthesiol Perioper Sci. 2023;1:29.
Wang X, Hua D, Tang X, Li S, Sun R, Xie Z, et al. The role of perioperative sleep disturbance in postoperative neurocognitive disorders. Nat Sci Sleep. 2021;13:1395–410.
O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135:1132–52.
Ibala R, Mekonnen J, Gitlin J, Hahm EY, Ethridge BR, Colon KM, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res. 2021;30:e13322.
Hashimoto K, Zhao M, Zhu T, Wang X, Yang J. Ketamine and its two enantiomers in anesthesiology and psychiatry: a historical review and future directions. J Anesth Transl Med. 2024;3:65–75.
Hashimoto K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharm. 2020;177:113935.
Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. 2022;27:559–73.
Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–31.
Zhang K, Yang Y, Yuan X, Zhang W, Han X, Lei C, et al. Efficacy and safety of repeated esketamine intravenous infusion in the treatment of treatment-resistant depression: A case series. Asian J Psychiatr. 2022;68:102976.
Jiang Y, Du Z, Shen Y, Zhou Q, Zhu H. The correlation of esketamine with specific adverse events: a deep dive into the FAERS database. Eur Arch Psychiatry Clin Neurosci. 2025;275:1373–81.
Qiu D, Wang X-M, Yang J-J, Chen S, Yue C-B, Hashimoto K, et al. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Netw Open. 2022;5:e2244514.
Butris N, Tang E, Pivetta B, He D, Saripella A, Yan E, et al. The prevalence and risk factors of sleep disturbances in surgical patients: a systematic review and meta-analysis. Sleep Med Rev. 2023;69:101786.
Hashimoto K. Emerging role of the host microbiome in neuropsychiatric disorders: overview and future directions. Mol Psychiatry. 2023;28:3625–37.
Dibello V, Lozupone M, Manfredini D, Dibello A, Zupo R, Sardone R, et al. Oral frailty and neurodegeneration in Alzheimer’s disease. Neural Regen Res. 2021;16:2149–53.
Olsen I, Hicks SD. Oral microbiota and autism spectrum disorder (ASD). J Oral Microbiol. 2019;12:1702806.
Baker JL, Mark Welch JL, Kauffman KM, McLean JS, He X. The oral microbiome: diversity, biogeography and human health. Nat Rev Microbiol. 2024;22:89–104.
Simpson CA, Adler C, du Plessis MR, Landau ER, Dashper SG, Reynolds EC, et al. Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol Behav. 2020;226:113126.
Maitre Y, Micheneau P, Delpierre A, Mahalli R, Guerin M, Amador G, et al. Did the brain and oral microbiota talk to each other? a review of the literature. J Clin Med. 2020;9:3876.
Cunha FA, Cota LOM, Cortelli SC, Miranda TB, Neves FS, Cortelli JR, et al. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: a case-control study. J Periodontal Res. 2019;54:63–72.
Liu M, Wang X, Wu F, Dai N, Chen M, Yu J, et al. Variations of oral microbiome in chronic insomnia patients with different tongue features. Am J Chin Med. 2020;48:923–44.
Chen Y, Chen X, Huang X, Duan Y, Gao H, Gao X. Analysis of salivary microbiome and its association with periodontitis in patients with obstructive sleep apnea. Front Cell Infect Microbiol. 2021;11:752475.
Gao Y, Wang H, Hu Y, Li J, Xu W, Zhao L, et al. Whole-genome metagenomic analysis of the oral microbiota in patients with obstructive sleep apnea. Sleep Breath. 2023;27:1383–98.
Ye J, Lv Y, Xie H, Lian K, Xu X. Whole-genome metagenomic analysis of the oral microbiota in patients with obstructive sleep apnea comorbid with major depressive disorder. Nat Sci Sleep. 2024;16:1091–108.
Lin W, Yang Y, Zhu Y, Pan R, Liu C, Pan J. Linking gut microbiota, oral microbiota, and serum metabolites in insomnia disorder: a preliminary study. Nat Sci Sleep. 2024;16:1959–72.
Song B, Li Y, Teng X, Li X, Yang Y, Zhu J. Comparison of morning and evening operation under general anesthesia on intraoperative anesthetic requirement, postoperative sleep quality, and pain: a randomized controlled trial. Nat Sci Sleep. 2020;12:467–75.
Cai J, Chen Y, Hao X, Zhu X, Tang Y, Wang S, et al. Effect of intraoperative dexmedetomidine dose on postoperative first night sleep quality in elderly surgery patients: a retrospective study with propensity score-matched analysis. Front Med (Lausanne). 2020;7:528.
Duan G, Wang K, Peng T, Wu Z, Li H. The effects of intraoperative dexmedetomidine use and its different dose on postoperative sleep disturbance in patients who have undergone non-cardiac major surgery: a real-world cohort study. Nat Sci Sleep. 2020;12:209–19.
Razavi D, Allilaire JF, Smith M, Salimpour A, Verra M, Desclaux B, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatr Scand. 1996;94:205–10.
Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg. 1982;69:417–9.
Lundtorp-Olsen C, Enevold C, Juel Jensen CA, Stofberg SN, Twetman S, Belstrøm D. Impact of probiotics on the salivary microbiota and salivary levels of inflammation-related proteins during short-term sugar stress: a randomized controlled trial. Pathogens. 2021;10:392.
Merkevičius K, Kundelis R, Maleckas A, Veličkienė D. Microbiome changes after type 2 diabetes treatment: a systematic review. Medicina (Kaunas). 2021;57:1084.
Guo XJ, Dai SX, Lou J, Ma XX, Hu XJ, Tu LP, et al. Distribution characteristics of oral microbiota and its relationship with intestinal microbiota in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1119201.
Niu Z, Gao X, Shi Z, Liu T, Wang M, Guo L, et al. Effect of total intravenous anesthesia or inhalation anesthesia on postoperative quality of recovery in patients undergoing total laparoscopic hysterectomy: a randomized controlled trial. J Clin Anesth. 2021;73:110374.
Luo M, Song B, Zhu J. Sleep disturbances after general anesthesia: current perspectives. Front Neurol. 2020;11:629.
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep. 2001;24:39–44.
Eacret D, Veasey SC, Blendy JA. Bidirectional relationship between opioids and disrupted sleep: putative mechanisms. Mol Pharm. 2020;98:445–53.
Ellis JD, Mayo JL, Gamaldo CE, Finan PH, Huhn AS. Worsening sleep quality across the lifespan and persistent sleep disturbances in persons with opioid use disorder. J Clin Sleep Med. 2022;18:587–95.
Sureda A, Daglia M, Argüelles Castilla S, Sanadgol N, Fazel Nabavi S, Khan H, et al. Oral microbiota and Alzheimer’s disease: do all roads lead to rome?. Pharmacol Res. 2020;151:104582.
McGuinness AJ, Loughman A, Foster JA, Jacka F. Mood disorders: the gut bacteriome and beyond. Biol Psychiatry. 2024;95:319–28.
Ni Y, Yu M, Liu C. Sleep disturbance and cognition in the elderly: a narrative review. Anesthesiol Perioper Sci. 2024;2:26.
Park SH, Shin NR, Yang M, Bose S, Kwon O, Nam DH, et al. A clinical study on the relationship among insomnia, tongue diagnosis, and oral microbiome. Am J Chin Med. 2022;50:773–97.
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17.
Ma J, Hong Y, Zheng N, Xie G, Lyu Y, Gu Y, et al. Gut microbiota remodeling reverses aging-associated inflammation and dysregulation of systemic bile acid homeostasis in mice sex-specifically. Gut Microbes. 2020;11:1450–74.
Zhu Z, Zhang J, Yuan G, Jiang M, Zhang X, Zhang K, et al. Association between mobile phone addiction, sleep disorder and the gut microbiota: a short-term prospective observational study. Front Microbiol. 2023;14:1323116.
Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–43.
Ishikawa D, Zhang X, Nomura K, Shibuya T, Hojo M, Yamashita M, et al. Anti-inflammatory effects of bacteroidota strains derived from outstanding donors of fecal microbiota transplantation for the treatment of ulcerative colitis. Inflamm Bowel Dis. 2024;30:2136–45.
Fang L, Tuohuti A, Cai W, Chen X. Changes in the nasopharyngeal and oropharyngeal microbiota in pediatric obstructive sleep apnea before and after surgery: a prospective study. BMC Microbiol. 2024;24:79.
Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56.
Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020;131:1693–707.
Wen Y, Xu J, Shen J, Tang Z, Li S, Zhang Q, et al. Esketamine prevents postoperative emotional and cognitive dysfunction by suppressing microglial M1 polarization and regulating the BDNF-TrkB pathway in ageing rats with preoperative sleep disturbance. Mol Neurobiol. 2024;61:5680–98.
Meshkat S, Ho RC, Cao B, Teopiz KM, Rosenblat JD, Rhee TG, et al. Biomarkers of ketamine’s antidepressant effect: an umbrella review. J Affect Disord. 2023;323:598–606.
Jiang Y, Wang X, Chen J, Zhang Y, Hashimoto K, Yang JJ, et al. Repeated (S)-ketamine administration ameliorates the spatial working memory impairment in mice with chronic pain: role of the gut microbiota-axis. Gut Microbes. 2024;16:2310603.
Ahmed HS. The impact of prevotella on neurobiology in aging: deciphering dendritic cell activity and inflammatory dynamics. Mol Neurobiol. 2024;61:9240–51.
Esberg A, Johansson L, Johansson I, Dahlqvist SR. Oral microbiota identifies patients in early onset rheumatoid arthritis. Microorganisms. 2021;9:1657.
Fu K, Cheung AHK, Wong CC, Liu W, Zhou Y, Wang F, et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell. 2024;187:882–.e17.
Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7:15725.
Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7:1294.
Ma L, Eguchi A, Liu G, Qu Y, Wan X, Murayama R, et al. A role of gut-brain axis on prophylactic actions of arketamine in male mice exposed to chronic restrain stress. Pharmacol Biochem Behav. 2024;238:173736.
Luan WW, Gu HW, Qiu D, Ding X, Liu PM, Hashimoto K, et al. Repeated administration of esketamine ameliorates mechanical allodynia in mice with chemotherapy-induced peripheral neuropathy: A role of gut microbiota and metabolites. Neurochem Int. 2025;185:105961.
Wan X, Eguchi A, Fujita Y, Ma L, Wang X, Yang Y, et al. Effects of (R)-ketamine on reduced bone mineral density in ovariectomized mice: a role of gut microbiota. Neuropharmacology. 2022;213:109139.
Wan X, Eguchi A, Chang L, Mori C, Hashimoto K. Beneficial effects of arketamine on the reduced bone mineral density in susceptible mice after chronic social defeat stress: Role of the gut-microbiota-bone-brain axis. Neuropharmacology. 2023;228:109466.
Hans P, Dewandre P-Y, Brichant JF, Bonhomme V. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br J Anaesth. 2005;94:336–40.
Acknowledgements
This study was supported by the grant from the National Natural Science Foundation of China (to Jian-Jun Yang, U23A20421 and to Xing-Ming Wang, 81901139). This study was also supported by The Programme of Introducing Talents of Discipline to Universities of Henan. Project name: Anesthesia and Brain Research. Project No. CXJD2019008 (to Jian-Jun Yang) and the Medical Technologies R&D Program of Henan province (to Xing-Ming Wang, LHGJ20230212). We used AI-tool ChatGPT to enhance the readability in the text.
Author information
Authors and Affiliations
Contributions
KH, MW, JY, XL designed the study. XL, DQ, and ND performed the experiments. XL analyzed the data. XL, MW, and JY drafted a significant portion of the manuscript. KH directed the discussion and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Hashimoto is the inventor of filed patent applications on “The use of R-Ketamine in the treatment of psychiatric diseases”, “(S)-norketamine and salt thereof as pharmaceutical”, “R-Ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder”, “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases”, “R-Ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder”, and “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases” by the Chiba University. Dr. K. Hashimoto has also received research support from Otsuka (Japan). Other authors declare no conflict of interest.
Ethics approval and consent to participate
This trial was approved by the Institutional Scientific Research and Clinical Trials Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2023-KY-1213). The study protocol was thoroughly explained to patients in advance, and written informed consent was obtained from all participants. The trial was registered prior to patient enrollment in the Chinese Clinical Trial Registry: chictr.org.cn (ChiCTR2400079641).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, XY., Qiu, D., Du, N. et al. Esketamine prevents postoperative sleep disturbance in patients with preoperative sleep disorders: a role for oral microbiota. Transl Psychiatry 15, 501 (2025). https://doi.org/10.1038/s41398-025-03705-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03705-9