Abstract
Convergent phenomenological and neuroimaging evidence support that the dysregulated segregation and integration between brain networks have been implicated in the neural mechanism of auditory verbal hallucination (AVH) in schizophrenia. Nevertheless, the macroscale topographical alterations of functional networks in patients with AVH remain elusive. Cortical gradient mapping provides a novel approach to characterize the distribution pattern of functional organization in a multidimensional connectivity space. Here, leveraging resting-state functional MRI data from 104 first-episode drug-naïve schizophrenia (64 with AVH, 40 without AVH) and 90 matched healthy controls (HC), we extracted intrinsic connectivity gradients and measured the range of each gradient, manifold eccentricity, global, within- and between-network dispersions in a three-dimensional gradient space to characterize the topographical structure of the brain. We then combined publicly available genetic and neurotransmitter distribution data to further unveil the potential molecular basis underlying the AVH-related topographical changes. Our analysis revealed a degradation of the principal unimodal-to-transmodal gradient in patients with AVH compared with those without AVH and HC, as well as the reduced 3D manifold dispersion, especially between high-order transmodal networks. The disrupted topographic pattern related to AVH was linked with expression maps of specific genes enriched for synaptic development and signaling and aligned with the distribution of the noradrenaline and acetylcholine systems. Our work depicts the comprehensive landscape of topographic abnormalities in AVH, underscoring the vital role of disrupted cortical hierarchy and network integration in its pathophysiology.
Similar content being viewed by others
Introduction
As one of the most prominent symptoms in schizophrenia, auditory verbal hallucination (AVH) is defined as the experience of perceiving voices in the absence of external auditory stimuli [1, 2]. Around 60-80% of schizophrenia patients report AVHs throughout their illness, and approximately 25% of them continue to suffer those distressing persistent AVHs even after receiving pharmacological therapy [3, 4]. The occurrence of AVHs could severely impair social and occupational functioning, diminish quality of daily life, and contribute to self-harm and suicidal behavior [5, 6]. Despite the serious effects, the precise mechanisms of AVH remain largely ambiguous, which impedes the development of novel therapeutic strategies.
Based on the abundant phenomenological descriptions and growing neuroimaging findings of AVH in the last few decades, several conceptual models have been proposed to establish the neurobiological framework of AVH. Classic “Spontaneous activity account” emphasized that AVH could be attributed to dysfunctions in sensory processing [7]. Unstable neuronal activities predispose the auditory and language-related cortex to endogenous activation without external stimulation, leading to abnormal hallucinatory experiences [7,8,9]. Another prevailing “source monitoring hypothesis” focused on impairments in cognitive functions related to self-referential processing. This model positions prefrontal and superior temporal regions monitoring inner speech as the hubs of the auditory hallucination circuit, in which the abnormal discharges from these areas cause a failure to dampen the response of the auditory cortex [10]. As a result, the hyperactive auditory cortex recognized inner speech as an external source and thus produced AVH. Recently, “Predictive processing theory” integrated the sensory-perceptual and cognitive features of AVH, and suggested that hallucinations result from the mismatch between the prior beliefs towards the external world generated by the associated cortex and the actual sensory information from the primary cortex [11,12,13]. Overall, the evidence from these models converges on the dysfunctions within networks and abnormal interactions between low-order perception-related networks and high-order cognition-related networks. However, there are limited studies to investigate macroscopic topographic reorganization and communication dysfunction of brain networks in schizophrenia patients with AVH from a whole-brain hierarchical perspective.
With advances in neuroimaging technologies and analytic methods, an emerging approach termed cortical gradient mapping has been proposed to characterize the topographical patterns of the large-scale network organization [14, 15]. Unlike conventional graph theory analysis or edge-based functional connectivity analysis, this approach leverages a non-linear decomposition technique to project high-dimensional functional connectome data into multiple low-dimensional manifolds (also called topological gradients), which could explicitly capture the alterations of continuous spatial cortical hierarchy along the functional axes [16]. Using resting-state functional MRI (rs-fMRI) data from the healthy population, this approach has identified the principal gradient anchoring low-order primary sensorimotor areas at one end and high-order transmodal association areas at another end, which spatially corresponds to the maps of synaptic distribution, myelination development and cognitive activation [14, 17,18,19]. Previous studies have revealed a reduced differentiation of the gradient, including schizophrenia [20], depression [21] and autism patients [22], indicating the abnormal interactions between lower and higher order brain systems in these diseases. To further characterize the functional organizations in multidimensional gradients, recent work calculated the Euclidean distance as dispersion to evaluate similarities between points within a manifold space [23]. Surpassing traditional graph theory metrics that estimate network integration and segregation at a single scale, dispersion reflects these network properties across multiple hierarchical manifold dimensions [23, 24]. Taken together, cortical gradient mapping provides a unique lens to understand how the disorganized hierarchical brain networks play a pivotal role in neurobiological mechanisms underpinning AVH in schizophrenia.
While numerous neuroimaging findings facilitated our understanding of the neural basis of AVH, the biological processes that shape the cortical reorganization in schizophrenia with AVH remain elusive. The emergence of publicly available transcriptomic and neurotransmitter brain maps could help bridge the gap between microscale genetic and molecular profiles and macroscale brain topography [25]. Combining neuroimaging data with transcriptomic [26,27,28,29,30] and neurotransmitter maps [31, 32] has provided insights into how microscale molecular abnormalities contribute to macroscale cortical organization alterations across different psychiatric disorders. Recent studies have revealed abnormal genetic expression [33] and neurochemical distribution [34] patterns linked with functional gradient changes in schizophrenia. Therefore, integrating this multiscale information would pave a promising avenue to comprehensively interpret the neurobiological substrates underlying AVH in schizophrenia and identify potential therapeutic targets.
Here, we aimed to elucidate the corresponding topographic changes of brain organizations and the associated biological basis in schizophrenia patients with AVH. Based on the rs-fMRI dataset containing first-episode drug-naïve schizophrenia with (AVH group) and without AVH (NAVH group) and matched healthy controls (HCs), we performed cortical gradient mapping analyses to investigate the disruptions in one-dimensional gradients and the dispersion of the functional communities within a multi-dimensional manifold space in patients with AVH. Utilizing transcriptomic data from Allen Human Brain Atlas (AHBA) [35] and neurotransmitter distribution data from JuSpace toolbox [36], we then decoded genetic signatures and molecular landscape of AVH-related gradient changes. We hypothesized that: (1) Compared with NAVHs and HCs, the AVH group exhibited a degradation of major cortical gradients and disrupted network topography; (2) the dispersion of functional gradients in AVHs was linked with gene expression patterns enriched in specific biological processes and spatial density distributions of different neurotransmitter systems.
Methods
Participants
In this study, a total of 104 first-episode drug-naïve schizophrenia patients who are aged between 18 to 60 and right-handed were recruited from the Mental Health Center at West China Hospital of Sichuan University. Additionally, 90 healthy controls (HC) matched in age, sex and education level were recruited from the local community. The diagnosis of schizophrenia was established by two experienced psychiatrists according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). All patients were experiencing their first episode of psychosis and had never received any antipsychotic treatment before. Duration of illness was determined by Nottingham Onset Schedule [37] based on the information provided by patients and their family members. The Positive and Negative Syndrome Scale (PANSS) [38] and Global Assessment of Functioning (GAF) [39] were used to evaluate the symptom severity. Participants with a history of neurological disease or head trauma, substance abuse, current pregnancy and MRI contraindications were excluded.
Following the criteria used in previous studies [40,41,42], schizophrenia patients were further categorized into the AVH group if they: (1) experienced AVH within the past four weeks, which was based on patients’ subjective report, medical records, and information from family members; (2) had a score ≥3 on P3 hallucination factor of PANSS. Whereas patients in the NAVH group did not report any hallucinations and had a PANSS P3 < 3. All participants gave written informed consent before participation, and this study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, following the Declaration of Helsinki.
MRI data acquisition and image preprocessing
The high-resolution T1-weighted images and resting-state functional MRI (fMRI) data were collected at West China Hospital on a 3.0 T GE scanner with an 8-channel coil. Acquired structural and functional data were preprocessed together using the fMRIPrep pipeline V20.2.0 based on Nipype v1.5.1 [43]. Following fMRIPrep preprocessing, the eXtensible Connectivity Pipeline- DCAN (XCP-D) was applied to postprocess the outputs [44]. Briefly, the following steps were conducted: skull stripping, co-registration with T1 image, slice-time correction, spatial normalization, linear detrending, nuisance regression, band-pass filtering and smoothing. Details of the imaging acquisitions and full description of data processing are summarized in Supplementary Methods.
To minimize the confounding effect of head motion, Subjects with a mean framewise displacement (FD) greater than 0.25 mm were excluded from further analysis. No participants were excluded based on this criterion. No significant differences in mean FD were found across AVH (mean = 0.083, SD = 0.027), NAVH (mean = 0.080, SD = 0.035) and HC group (mean = 0.087, SD = 0.031; F = 0.608, p = 0.545). Additionally, the mean FD did not significantly correlate with observed gradient measurement alterations (all p > 0.05, see supplementary Methods), indicating that these alterations are unlikely to be driven by head motion.
Gradient construction
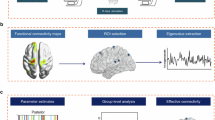
To construct a functional connectome for each participant, we parcellated the brain into 400 parcels based on the Schaefer atlas [45], a well-established parcellation scheme that provides coverage of whole cortical areas and is widely applied in functional neuroimaging studies. These 400 cortical parcellations were assigned into the pre-defined Yeo’s seven networks [46]: the visual network (VN), sensorimotor network (SMN), dorsal attention network (DAN), ventral attention network (VAN), limbic network (LIM), frontoparietal network (FPN) and default mode network (DMN). The pairwise Pearson correlation coefficients based on the mean time series between two regions were calculated to generate a functional connectivity matrix.
We used BrainSpace toolbox [47] to conduct functional gradient analysis. First, the individual functional connectivity matrix was z-transformed and thresholded at the sparsity of 90% (only maintain the top 10% of edges in each row of the matrix), and then a normalized cosine angle affinity matrix was calculated to capture the similarity of connectivity profiles between paired parcels [14]. The selected sparsity is in line with previous studies [14, 24, 48, 49], which could improve the reliability and validity of gradients by retaining important individual variations in connectome whilst removing noisy connections [50, 51]. In addition, to validate the robustness of our main results, we generated gradients based on connectivity matrix with different sparsity (50, 60, 70, and 80%) and evaluated the correspondence with those with the sparsity of 90%. Second, we ran a diffusion map embedding algorithm to identify gradient components that reflect the low-dimensional representations of the high-dimensional connectome data. The algorithm is mainly controlled by two key parameters: diffusion parameter α, which regulates the effects of sampling point density on the manifold (α = 0 maximal influence; α = 1 no influence), and time parameter t, which determines the eigenvalue scales of the diffusion operator [47, 48]. Consistent with the practice recommendation [14, 23, 47, 51], we set α = 0.5 and t = 0 to preserve global relations between data points within the embedded space. Finally, functional gradients across individuals were aligned to the group-level gradient derived from a subsample of HCP dataset (n = 217) in BrainSpace toolbox using iterative Procrustes rotation [52], which is commonly applied as a template for gradient alignment to ensure the stability and comparability of the gradients across participants [47, 53,54,55]. The first three gradients were included in the follow-up analyses on the aligned manifolds.
Functional gradient analyses
To depict the alteration of cortical hierarchy for the single gradient, we first calculated the range of each gradient, which indicates the distance between positive and negative extreme values within a given gradient. This metric could reflect the dispersion degree of the specific gradient distribution [48]. Additionally, several topographic measures were calculated for each participant to characterize the expansion and contractions of manifold in multidimensional spaces. The manifold eccentricity of each region was defined as the Euclidean distance between this region and the centroid in the 3D manifold space, and global dispersion was quantified as sum squared Euclidean distance of all brain regions to the global centroid [23, 24, 56]. At the network level, we calculated within-network dispersion as the sum squared Euclidean distance of regions in a specific network to the network centroid, and between-network dispersion as the Euclidean distance between network centroids [23, 56]. These metrics computed on individual aligned gradient space capture the similarity of connectivity patterns between cortical regions across multidimensional functional axes. Higher values of these metrics reflect greater functional segregation at the node, network or whole brain level [23, 24, 49, 56].
Genetic expression profiles underpinning manifold eccentricity alteration
We performed spatial correlation analysis with whole-brain gene expression data to unveil the potential genetic substrates specific to the AVH-related manifold alterations. The transcription profiles were derived from AHBA, in which the whole-brain microarray expression data were collected from 6 healthy human postmortem brains [35] (Table S1). Preprocessing was conducted using the abagen toolbox [57], which follows the recommended workflow for imaging-transcriptome analysis [58], to map the expression data to 400 brain regions in the Schaefer atlas. Briefly, the pipeline includes: (1) reannotate genetic probes; (2) intensity-based filtering with the background threshold of 50%; (3) select probes highest correlated to transcriptional data; (4) assign samples to the brain regions within 2 mm radius of a given parcel; (5) normalize sample expression values across genes with a robust sigmoid function; (6) normalize gene expression values across samples with identical procedures; (7) average expression profiles across donors and generate a single matrix, constructed by brain regions as rows and retained genes expression values as columns. Considering that the genetic data of the right hemisphere were available from only two donors, we exclusively included genetic data from the left hemisphere in the subsequent analysis. Due to the absence of gene expression data, eight regions of the left hemisphere were excluded. Finally, we obtained a 192×15631 regional transcriptional matrix.
Partial least squares (PLS) regression analysis was conducted to investigate the association between the gene expression profiles and AVH-related manifold alterations in schizophrenia [59]. We set the generated transcriptional matrix as predictor variables, and the t-statistics map of changes in manifold eccentricity between the AVH and NAVH groups as the response variables. Consistent with previous studies [21, 60], the first component of PLS (PLS1) is typically considered as the optimal representation that interprets the covariance of high-dimensional data matrix at a low-dimensional level. Accounting for the spatial autocorrelation effect, the statistical significance of the variance explained by PLS1 was examined using a spin permutation test (10,000 iterations) [61]. A bootstrapping method with 5000 iterations was additionally conducted to estimate the variability of each gene in PLS1 [60]. Z score of each gene weight on PLS1 was calculated as the ratio of the weight of each gene to its bootstrap standard error, and all genes were ranked based on their contribution to PLS1.
We performed gene enrichment analysis to further determine the biological functions of the AVH-related genes through an open online tool Metascape [62]. Significant genes with PLS1 weights of z > 3 or z < −3 (all PFDR < 0.005) were separately input into the Metascape website to conduct various enrichment analyses, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and WikiPathways. The significantly enriched biological processes were determined with a false discovery rate (FDR) corrected P < 0.05 threshold.
Neurotransmitter analysis
In order to identify the neurochemical architecture underlying the changes in manifold eccentricity induced by AVH, we conducted the spatial correlation analysis between the AVH-associated t-statistic maps in schizophrenia patients and the profiles of seven neurotransmitter systems available in the JuSpace toolbox (version 1.5) 36using Spearman correlation. The receptor/transporter maps were derived from positron-emission tomography in independent healthy populations, including the profiles of serotonin systems (5-HT1a receptor, 5-HT1b receptor, 5-HT2a receptor and serotonin transporter (SERT)), dopamine systems (D1 receptor, D2 receptor, dopamine transporter (DAT) and fluorodopa (FDOPA)), GABAergic system (GABA type A (GABAa) receptor), opioidergic system (μ-opioid (MU) receptors), noradrenergic system (norepinephrine transporter (NAT)), cholinergic system (vesicular acetylcholine transporter (VAChT)) and glutamatergic system (metabolic glutamate receptor 5 (mGluR5)). Detailed information was displayed in Table S2. Following the standard workflow by Dukart et al. the spatial correlation coefficient was calculated adjusting for the gray matter probability map, and the significance of spatial correlation was evaluated by comparison to a null distribution, which was obtained through 5000 spin-based permutations while accounting for spatial autocorrelation [36, 61]. P < 0.05 after Bonferroni correction for multiple comparisons was considered significant.
Statistical analysis
Two-sample t tests, chi-squared tests or one-way analysis of variance (ANOVA) were conducted to examine the differences in demographic and clinical characteristics between groups. To compare the differences in gradient topological metrics across the three groups, we performed an analysis of covariance (ANCOVA) with age and sex as covariates. FDR was applied to correct multiple comparisons (7 networks or 400 regions) and the significance level was defined as P < 0.05. For metrics showing a significant between-group difference, post-hoc pairwise t tests were further conducted with the same covariates.
Results
Demographics and clinical features
Finally, 194 participants (AVH = 64, NAVH = 40, HC = 90) were included in the current study. Details of demographic and clinical characteristics were displayed in Table 1. Briefly, the three groups showed no significant difference in sex, age and years of education, and AVH and NAVH patients did not differ in illness duration and GAF scores. Compared with the NAVH patients, AVH patients showed significantly higher PANSS total scores (p = 0.04), positive symptom scores (p < 0.01) and P3 scores (p < 0.01). However, no differences in terms of PANSS negative symptom scores and general scores were found between the two groups. After subtracting the P3 score, the PANSS total and positive scores in the AVH group revealed no statistical difference compared to the NAVH group.
Contracted connectome gradients in schizophrenia with AVH
Using the diffusion map embedding approach, the current study focused on the first three cortical gradients that could account for approximately 37.0% of variance in the functional connectome (Figure S1). In accordance with previous studies [14, 23, 48], the principal gradient (Gradient 1) was organized along a spatial axis from unimodal primary sensory areas including VN and SMN to transmodal areas (DMN). The second gradient (Gradient 2) anchored the visual cortex on one end and the somatomotor cortex on the other end. The third gradient (Gradient 3) dissociated visual and default-mode areas from areas involved in multiple-demand processing (Fig. 1A). Notably, the intergroup comparison showed no significant difference among the AVH, NAVH and HC groups in the explained variance of the first three gradients (Gradient 1: p = 0.08; Gradient 2: p = 0.10; Gradient 3: p = 0.58; Figure S2). Moreover, the first three gradients generated at different sparsity strategies exhibited significant correlations (r = 0.62-0.97, all p < 0.001, Figure S3) with those generated at the sparsity of 90%, confirming the robustness of our main results.
A The spatial topography of the first three functional cortical gradients in two-dimensional and three-dimensional space. B Violin plots (the box plots in the violin plots show median and quantiles) of the ranges in each gradient, and global dispersion across AVH (red), NAVH (blue) and HC (orange). For those showing a significant group difference in ANCOVA, post-hoc pairwise comparisons (two-tailed) were performed. Asterisk indicates p < 0.05. C F maps of the regional manifold eccentricities showed statistically significant regions after FDR corrections (p < 0.05). Abbreviation: ANCOVA analysis of covariance; FDR false discovery rate.
We firstly characterized the atypical hierarchical topographic pattern in schizophrenia with AVH through the range of each gradient, which reflects the differentiation of the entire cortex along the particular functional axis. The one-way ANCOVA with age and sex as covariates revealed significant group differences in the range of Gradient 1 (F = 3.14, p = 0.045), but not in Gradient 2 (F = 0.41, p = 0.66) or Gradient 3 (F = 2.18, p = 0.12) (Fig. 1B; Table S3). Post-hoc analysis further found that AVHs exhibited a narrower Gradient 1 range compared with both NAVHs (t = -2.25, p = 0.026) and HCs (t = -2.17, p = 0.031).
We then proceeded to quantify the global dispersion and nodal eccentricity in a three-dimensional framework that represents integration and segregation of the global brain organization across scales. Significant difference in global dispersion was found among the three groups (F = 4.75, p = 0.010), and post-hoc analysis revealed that AVHs had reduced global dispersion than NAVHs (t = -2.59, p = 0.011) and HCs (t = -2.90, p = 0.004) (Fig. 1B; Table S3). For nodal eccentricity, significant group differences were observed in the left superior temporal gyrus, left paracentral area, bilateral superior parietal lobule, right anterior cingulate gyrus and right middle occipital gyrus after FDR correction (Fig. 1C). Compared with HCs, AVHs showed a contracted manifold pattern (i.e., decreased nodal eccentricity) in the left superior temporal sulcus, left paracentral area, bilateral superior parietal lobule and right middle occipital gyrus, while NAVHs showed a contracted manifold pattern in the left paracentral area and bilateral superior parietal lobule and an expanded pattern in the anterior cingulate gyrus. We also found decreased nodal eccentricity in AVHs compared with NAVHs in the left superior temporal sulcus, right middle occipital gyrus and right anterior cingulate gyrus (Figure S4).
Furthermore, we estimated the within- and between-network dispersions to characterize the network-level functional geometry. No significant differences in within-network dispersion were observed among the three groups (Table S4). For between-network dispersion, ANCOVA and post-hoc tests indicated that AVH patients showed decreased dispersion between DAN-DMN (AVH vs NAVH: t = -2.32, p = 0.022; AVH vs HC: t = -3.34, p = 0.001), LIM-DMN (AVH vs NAVH: t = -2.47, p = 0.015; AVH vs HC: t = -3.44, p < 0.001), and LIM-FPN (AVH vs NAVH: t = -3.35, p = 0.001; AVH vs HC: t = -2.99, p = 0.003) compared with NAVHs and HCs, see in Fig. 2 and Table S5.
A The Violin plots (the box plots in the violin plots show median and quantiles) of between-network dispersion across AVH (red), NAVH (blue) and HC (orange). For those showing a significant group difference in ANCOVA, post-hoc pairwise comparisons (two-tailed) were performed. Asterisk indicates p < 0.05. B The F-value heatmap of the group difference. Asterisk indicates p < 0.05 after FDR correction. Abbreviation: VN visual network; SMN somatomotor network: DAN dorsal attention network; VAN ventral attention network; LIM limbic network; FPN frontoparietal network; DMN default-mode network; ANCOVA analysis of covariance; FDR false discovery rate.
Genetic expression profiles associated with contracted cortical gradients in AVH
To investigate the genetic underpinnings of the topographical patterns related to AVH in schizophrenia, we further conducted transcriptomic association analysis by correlating the AVH-related manifold eccentricity changes with the postmortem gene expression data from AHBA. The findings revealed that the PLS1 scores explained 12.1% of the variance in the eccentricity difference, significantly more than expected by chance (pspin < 0.001). The spatial distribution of PLS1 score-weighted map showed an anterior-posterior gradient of gene expression (Fig. 3A), which exhibited a positive correlation with the t-value maps in manifold eccentricity (Fig. 3B; r = 0.326, pspin < 0.001). After ranking the normalized weights of PLS1(Fig. 3C), we identified 1130 genes strongly contributed to PLS1 ( | Z | > 3), in which 383 genes had positive PLS1 weights (PLS1+ gene set) and 747 genes had negative weights (PLS1− gene set). Specifically, PLS1 + /- genes were overexpressed in brain regions with increased/decreased manifold eccentricity in AVH patients compared with the NAVH patients.
A The weighted gene expression map of regional first component of partial least square (PLS1) scores in the left hemisphere. B The Scatterplot which displayed a significant positive spatial correlation between regional PLS1 scores and between-group (AVH vs NAVH) t-value maps of manifold eccentricity (r = 0.326, pspin < 0.001). C The ranked gene weight list of PLS1. D,E. The top 10 terms from enrichment analysis of PLS1+ (D) and PLS1- (E) genes. The size of the bubble represents the number of genes involved in each term. The color of the bubble represents the significance level.
Applying Metascape platform to perform functional enrichment analysis, we found that the PLS1+ gene set was significantly enriched in the neurodevelopment-related biological processes of dendrite and synapse formation, remodeling and pruning, including “actin filament-based process”, “positive regulation of apoptotic process”, and “regulation of neurogenesis. Moreover, the PLS1− gene set is mainly enriched in biological processes linked to synaptic signaling and neurotransmitter transport, such as “modulation of chemical synaptic transmission”, “regulation of secretion by cell” and “regulation of membrane transport”. (Figure D,E; Table S6).
Molecular architecture underlying cortical topographic changes in AVH
Finally, we decoded molecular mechanisms underlying the altered manifold eccentricity in AVHs through spatial correlation analysis. After permutation test with Bonferroni correction, the manifold eccentricity alterations in AVHs compared with NAVHs (t-map) exhibited significantly positive correlations with NAT (r = 0.25, p < 0.001) and VAChT (r = 0.21, p < 0.001) (Fig. 4; table S7).
The Scatterplot showed significant positive correlations between between-group (AVH vs NAVH) t-value maps of manifold eccentricity and special neurotransmitter profiles after Bonferroni correction (p < 0.05), including NAT (left; r = 0.25, p < 0.001) and VAchT (right; r = 0.21, p < 0.001). Abbreviation: NAT, noradrenaline transporter; VAChT, vesicular acetylcholine transporter.
Discussion
Capitalizing on cortical gradient mapping technique, this study comprehensively delineated the altered hierarchical structure of the cortical topography in schizophrenia patients with AVH. The results demonstrated that compared with NAVH patients and HCs, AVH patients showed a degradation of the principal gradient ranging from unimodal to transmodal cortex. When projecting into multidimensional space, AVH patients showed notable decreased dispersion at global level and especially between LIM, DAN, FPN and DMN. By integrating neuroimaging data with transcriptomic and neurotransmitter profiles, we revealed that genes that were significantly correlated with the AVH-related manifold eccentricity alterations were mainly enriched in synaptic-related processes, including synaptic development and signaling. The atypical patterns of manifold eccentricity in AVHs were linked with the spatial distributions of noradrenaline and acetylcholine neurotransmitter systems. Together, these findings illuminated the neural mechanisms of AVH symptoms in schizophrenia from a multiscale perspective, which expands our knowledge about the psychopathology of auditory hallucination. More importantly, insights emerging from mapping brain connectome and its topography may provide a potential mechanistic framework to understand the biological substrates of human behavior and mental disorders in the future [63,64,65,66].
We observed that the AVH patients were characterized by a degradation of the principal unimodal-transmodal gradient at the unidimensional level. Our findings align with previous evidence of disrupted principal gradients in schizophrenia [20], both highlighting the central role of abnormal sensory-cognitive interactions in the development of psychotic symptoms, including AVH. The principal gradient presents the information processing route from perception of concrete stimuli to generate abstract conceptions, which could guide the directional signal flow across the cortex and integrate sensory and non-sensory information to shape comprehensive cognition [14, 16]. Constricted unimodal-transmodal gradient reflects a reduction of both physical distance and connectivity dissimilarity between sensorimotor network and high-order cognitive network and a blurring of functional network specialization, possibly corresponding to the increased crosstalk between auditory perception information and self-generated prior knowledge [12, 67]. This may result in decreased signal-to-noise ratio in the auditory processing system, and finally lead to the experience of AVH [8]. This result echoes the evidence from a recent work that the diminished principal gradients in schizophrenia could predict treatment outcomes of positive symptoms including hallucination [33]. Furthermore, the tethering hypothesis has pointed that the emergence of the unimodal-transmodal axis is driven and shaped by the brain development program [68, 69]. Thus, our findings are in support of the neurodevelopmental model of schizophrenia [70], indicating that the occurrence of AVH in patients may be led by the disruption of the cortical maturation process.
To further reflect the topographic abnormalities and cortical functional geometry alterations, we investigated the group difference of dispersion in the intrinsic coordinate system of the cortex. In AVH patients, we observed a decrease in global dispersion, which captures the reduced functional segregation at whole-brain level. This is congruent with previous work on the graph theoretical characteristics of AVH, indicating that the predisposing trait of AVH in schizophrenia may be attributed to the impaired distributed modular information processing ability of the connectome [71, 72]. Notably, when considering nodal eccentricity, the AVH-related alterations in schizophrenia were primarily characterized by the manifold contraction of the superior temporal sulcus (STS) and the middle occipital gyrus. The STS engages in auditory perception, language comprehension and multisensory integration [73, 74]. Converging evidence has emphasized the involvement of the STS in the hallucination network [75, 76], and TMS treatment targeting this area displayed effective alleviation of AVH in schizophrenia [77]. The involvement of the occipital gyrus is instrumental in phonological and semantic modulation, and the abnormal topographic pattern aligns with previous studies that found a deficit in the occipital-auditory pathway in patients with AVH [78, 79]. Therefore, the present results provide novel support for the pivotal roles these areas play in the connectome encoding AVH in schizophrenia.
Intriguingly, when we adopted a network-centered perspective exploring the AVH-related topographical alterations, we observed decreases in the dispersion values between distinct networks, including DMN, FPN, LIM and DAN. Despite the diffuse findings, they nevertheless indicated the abnormal patterns of integration and segregation across high-order networks. The findings of decreased differentiation between distinct networks support the “dysmodularity” hypothesis proposing that AVH in schizophrenia is invoked by a less modular brain which results in reduced information encapsulation and leakage of perceptual information into other systems [80]. Specifically, attenuated network segregation of DMN-LIM-FPN and DMN-DAN might drive the susceptibility to AVH in schizophrenia. As a pair of anticorrelated networks, DMN is involved in internally-directed cognitive processes including self-referential thought and inner speech monitoring while FPN is activated in cognitive control and predictive adjustment [81]. LIM is mainly responsible for emotional processing and generation of affective memories, and dysfunction of LIM has been implicated in the content and frequency of AVH experience [82, 83]. The compression of functional geometry in DMN-LIM and FPN-LIM suggests a neural mechanism whereby emotional signals from LIM intrude into information flow in DMN and FPN, resulting in excessive weight to the prior internal cognition and failure to adjust prediction errors, which may lead to a tendency toward illusory perception dominated by inner speech [11,12,13]. In addition, DAN plays a dominant role in allocating voluntary attention to external stimuli [84], and the reduced dispersion between DMN and DAN may reflect the penetration of internal beliefs into external input.
Another appealing contribution of the current study was to clarify the potential neurobiological substrates underpinning the disrupted cortical hierarchy in AVH. The neuroimaging-transcriptome association analysis identified AVH-specific gene expression patterns enriched in neurodevelopment and synaptic signaling. Basic biological processes associated with neurodevelopment encompass the formation, remodeling and pruning of axons and dendrites, and synaptic signaling is also crucial for synaptic stability and maturation [85]. Previous evidence demonstrated that reduced dendritic spine density and defects in synaptic plasticity were linked with the failure of corollary discharge, which was seen as the key mechanism of AVH [86]. Paralleled with our findings, a postmortem study also reported alterations in the expression of genes related to neurodevelopment and brain plasticity in schizophrenia patients with auditory hallucination [87]. Moreover, spatial association analysis revealed significant correlations between functional manifold distortion and distributions of noradrenergic and cholinergic systems. Disrupted noradrenergic activity has been associated with the impairment of sensory information filtering and hence may be implicated in the generation of AVH [88]. The cholinergic system plays an important role in memory, attention and language functions, of which dysfunctions could induce psychotic symptoms including delusions and hallucinations [89]. The recent studies further confirmed the involvement of acetylcholine in the neural mechanisms of AVH in schizophrenia, where the emerging M1/M4 agonists have been found to significantly relieve positive symptoms including hallucination in schizophrenia [90, 91]. These imaging-transcriptional and imaging-neurotransmitter markers advanced our understanding of how AVH in schizophrenia was supported by multiscale brain data and prompted development of potential therapeutic targets. However, it should be acknowledged that, in the absence of transcriptome profile and neurochemical data from the same patient sample, our speculative conclusions warrant further confirmation through additional preclinical and clinical evidence.
Although we recruited a cohort of first-episode drug-naïve schizophrenia patients to minimize the potential confounding effects of antipsychotic medication and illness chronicity on cortical topography, several limitations should be acknowledged. First, the patient with AVH was determined with interviews and the PANSS P3, which are rather rough and nonspecific. Future studies could provide additional insights by applying detailed rating scales specifically designed for AVH, such as PSYRATS [92]. Second, our study focused on the hallucination trait in schizophrenia, which took no account of patient’s experience during MRI scanning. It might be more interesting to investigate the connectome changes during the occurrence of an AVH. Third, we only investigated the AVH-related alterations in cortical functional organization, excluding the subcortical regions. Subcortical structures, including the thalamus and putamen, might make substantial contributions in shaping the whole-brain topography and play a vital role in the pathophysiology of AVH in schizophrenia [93, 94]. Subsequent studies should therefore examine subcortical gradients to provide a more comprehensive characterization of the topographic patterns associated with AVH. Fourth, the genetic expression data and neurotransmitter distribution maps were derived from healthy subjects. Accordingly, our findings should be considered as preliminary supportive evidence and further validations are necessary. Lastly, this study is a cross-sectional study with a moderate sample size, and large-scale prospective studies should be conducted to validate the functional gradient changes in schizophrenia patients with AVH.
In conclusion, we characterized the abnormal cortical topographic patterns associated with AVH in schizophrenia using gradient mapping technique within a multidimensional framework. Our findings revealed the degradation of the unimodal-transmodal gradient and decreased dispersion in functional communities, of which the neurobiological basis could map onto the dysregulation of neurodevelopment and dysfunctions in noradrenergic and cholinergic systems. Collectively, this study enriches our multiscale understanding of the pathophysiological mechanisms that underlie AVH.
Data availability
The human brain genetic data are freely available in the Allen Brain Atlas (https://human.brain-map.org/). The neurotransmitter data can be obtained from Juspace toolbox (https://github.com/juryxy/JuSpace/). All demographic, clinical and neuroimaging data used in the current study are not publicly available for download due to privacy or ethical restrictions of West China Hospital of Sichuan University. The data could be available from the corresponding authors upon reasonable request.
Code availability
The BrainSpace toolbox to run the functional gradient analyses is available from https://brainspace.readthedocs.io. The abagen toolbox used for AHBA data processing is available from https://github.com/rmarkello/abagen. Gene ontology enrichment analysis was performed using the Metascape software (https://metascape.org/gp/index.html#/main/step1). Neurotransmitter analysis was conducted in Juspace toolbox (https://github.com/juryxy/JuSpace/).
References
Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49.
Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–66.
Lim A, Hoek HW, Deen ML, Blom JD. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr Res. 2016;176:493–9.
Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–42.
Goghari VM, Harrow M, Grossman LS, Rosen C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol Med. 2013;43:1151–60.
Fujita J, Takahashi Y, Nishida A, Okumura Y, Ando S, Kawano M, et al. Auditory verbal hallucinations increase the risk for suicide attempts in adolescents with suicidal ideation. Schizophr Res. 2015;168:209–12.
Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127:202–14.
Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. British J psychiatry : J Ment Sci. 2007;190:57–62.
Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–75.
Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin Psychol Rev. 2007;27:140–54.
Fletcher PC, Frith CD. Perceiving is believing: a bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58.
Powers AR, Kelley III, Corlett M. PR. Hallucinations as top-down effects on perception. Biological psychiatry Cogn Neurosci neuroimaging. 2016;1:393–400.
Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR III. Hallucinations and strong priors. Trends Cogn Sci. 2019;23:114–27.
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA. 2016;113:12574–9.
Haak KV, Marquand AF, Beckmann CF. Connectopic mapping with resting-state fMRI. Neuroimage. 2018;170:83–94.
Huntenburg JM, Bazin PL, Margulies DS. Large-scale gradients in human cortical organization. Trends Cogn Sci. 2018;22:21–31.
Paquola C, Hong SJ. The potential of myelin-sensitive imaging: redefining spatiotemporal patterns of myeloarchitecture. Biol Psychiatry. 2023;93:442–54.
Sydnor VJ, Larsen B, Bassett DS, Alexander-Bloch A, Fair DA, Liston C, et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron. 2021;109:2820–46.
Haueis P. Multiscale modeling of cortical gradients: The role of mesoscale circuits for linking macro- and microscale gradients of cortical organization and hierarchical information processing. Neuroimage. 2021;232:117846.
Dong D, Yao D, Wang Y, Hong SJ, Genon S, Xin F, et al. Compressed sensorimotor-to-transmodal hierarchical organization in schizophrenia. Psychol Med. 2023;53:771–84.
Xia M, Liu J, Mechelli A, Sun X, Ma Q, Wang X, et al. Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes. Mol Psychiatry. 2022;27:1384–93.
Hong SJ, Vos de Wael R, Bethlehem RAI, Lariviere S, Paquola C, Valk SL, et al. Atypical functional connectome hierarchy in autism. Nat Commun. 2019;10:1022.
Bethlehem RAI, Paquola C, Seidlitz J, Ronan L, Bernhardt B, Consortium CC, et al. Dispersion of functional gradients across the adult lifespan. Neuroimage. 2020;222:117299.
Park BY, Bethlehem RA, Paquola C, Larivière S, Rodríguez-Cruces R, Vos de Wael R, et al. An expanding manifold in transmodal regions characterizes adolescent reconfiguration of structural connectome organization. eLife. 2021;10:e64694.
Markello RD, Hansen JY, Liu ZQ, Bazinet V, Shafiei G, Suárez LE, et al. neuromaps: structural and functional interpretation of brain maps. Nat Methods. 2022;19:1472–9.
Li J, Seidlitz J, Suckling J, Fan F, Ji GJ, Meng Y, et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun. 2021;12:1647.
Romme IA, de Reus MA, Ophoff RA, Kahn RS, van den Heuvel MP. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol Psychiatry. 2017;81:495–502.
Pretzsch CM, Arenella M, Lerch JP, Lombardo MV, Beckmann C, Schaefer T, et al. Patterns of brain maturation in autism and their molecular associations. JAMA psychiatry. 2024;81:1253–64.
Li J, Long Z, Sheng W, Du L, Qiu J, Chen H, et al. Transcriptomic similarity informs neuromorphic deviations in depression biotypes. Biol Psychiatry. 2024;95:414–25.
Li J, Chen H, Liao W. Biologically annotated heterogeneity of depression through neuroimaging normative modeling. Biol Psychiatry. 2025;S0006-3223:01307–1.
Li J, Long Z, Ji GJ, Han S, Chen Y, Yao G, et al. Major depressive disorder on a neuromorphic continuum. Nat Commun. 2025;16:2405.
Li J, Wang D, Xia J, Zhang C, Meng Y, Xu S, et al. Divergent suicidal symptomatic activations converge on somato-cognitive action network in depression. Mol Psychiatry. 2024;29:1980–9.
Yao G, Luo J, Li J, Feng K, Liu P, Xu Y. Functional gradient dysfunction in drug-naïve first-episode schizophrenia and its correlation with specific transcriptional patterns and treatment predictions. Psychol Med. 2024;54:1–13.
Yang, C. et al. More severe brain network hierarchy disorganization in treatment-naive deficit compared to non-deficit schizophrenia and underlying neurotransmitter associations. Schizophr. Bull. 2025;sbae231, https://doi.org/10.1093/schbul/sbae231. [Epub ahead of print].
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–9.
Dukart J, Holiga S, Rullmann M, Lanzenberger R, Hawkins PCT, Mehta MA, et al. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum Brain Mapp. 2021;42:555–66.
Singh SP, Cooper JE, Fisher HL, Tarrant CJ, Lloyd T, Banjo J, et al. Determining the chronology and components of psychosis onset: the nottingham onset schedule (NOS). Schizophr Res. 2005;80:117–30.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Aas IH. Guidelines for rating global assessment of functioning (GAF). Ann Gen Psychiatry. 2011;10:2.
Wei Y, Xue K, Yang M, Wang H, Chen J, Han S, et al. Aberrant cerebello-thalamo-cortical functional and effective connectivity in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Bull. 2022;48:1336–43.
Sone M, Koshiyama D, Zhu Y, Maikusa N, Okada N, Abe O, et al. Structural brain abnormalities in schizophrenia patients with a history and presence of auditory verbal hallucination. Transl Psychiatry. 2022;12:511.
Cui Y, Liu B, Song M, Lipnicki DM, Li J, Xie S, et al. Auditory verbal hallucinations are related to cortical thinning in the left middle temporal gyrus of patients with schizophrenia. Psychol Med. 2018;48:115–22.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Mehta K, Salo T, Madison TJ, Adebimpe A, Bassett DS, Bertolero M, et al. XCP-D: A robust pipeline for the post-processing of fMRI data. Imaging Neurosci. 2024;2:1–26.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral cortex (N. Y, NY : 1991). 2018;28:3095–114.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Vos de Wael R, Benkarim O, Paquola C, Lariviere S, Royer J, Tavakol S, et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Communications Biol. 2020;3:103.
Huang Z, Mashour GA, Hudetz AG. Functional geometry of the cortex encodes dimensions of consciousness. Nat Commun. 2023;14:72.
Gale DJ, Areshenkoff CN, Standage DI, Nashed JY, Markello RD, Flanagan JR, et al. Distinct patterns of cortical manifold expansion and contraction underlie human sensorimotor adaptation. Proc Natl Acad Sci USA. 2022;119:e2209960119.
Knodt AR, Elliott ML, Whitman ET, Winn A, Addae A, Ireland D, et al. Test-retest reliability and predictive utility of a macroscale principal functional connectivity gradient. Hum Brain Mapp. 2023;44:6399–417.
Hong SJ, Xu T, Nikolaidis A, Smallwood J, Margulies DS, Bernhardt B, et al. Toward a connectivity gradient-based framework for reproducible biomarker discovery. Neuroimage. 2020;223:117322.
Langs G, Golland P, Ghosh SS. Predicting activation across individuals with resting-state functional connectivity based multi-atlas label fusion. Medical Image Comput Computer-Assist Intervention : MICCAI Int Conf Med Image Comput Computer-Assist Intervention. 2015;9350:313–20.
Paquola C, Vos De Wael R, Wagstyl K, Bethlehem RAI, Hong SJ, Seidlitz J, et al. Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biol. 2019;17:e3000284.
Park BY, Larivière S, Rodríguez-Cruces R, Royer J, Tavakol S, Wang Y, et al. Topographic divergence of atypical cortical asymmetry and atrophy patterns in temporal lobe epilepsy. Brain : a J Neurol. 2022;145:1285–98.
Xie K, Royer J, Larivière S, Rodriguez-Cruces R, Frässle S, Cabalo DG, et al. Atypical connectome topography and signal flow in temporal lobe epilepsy. Prog Neurobiol. 2024;236:102604.
Li J, Zhang C, Meng Y, Yang S, Xia J, Chen H, et al. Morphometric brain organization across the human lifespan reveals increased dispersion linked to cognitive performance. PLoS Biol. 2024;22:e3002647.
Markello RD, Arnatkeviciute A, Poline JB, Fulcher BD, Fornito A, Misic B. Standardizing workflows in imaging transcriptomics with the abagen toolbox. eLife. 2021;10:e72129.
Arnatkeviciute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–67.
Abdi H, Williams LJ. Partial least squares methods: partial least squares correlation and partial least square regression. Methods Mol Biol (Clifton, NJ). 2013;930:549–79.
Morgan SE, Seidlitz J, Whitaker KJ, Romero-Garcia R, Clifton NE, Scarpazza C, et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc Natl Acad Sci USA. 2019;116:9604–9.
Váša F, Seidlitz J, Romero-Garcia R, Whitaker KJ, Rosenthal G, Vértes PE, et al. Adolescent tuning of association cortex in human structural brain networks. Cerebral cortex (N. Y, NY : 1991). 2018;28:281–94.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523.
Mo F, Zhao H, Li Y, Cai H, Song Y, Wang R, et al. Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2024;50:1326–36.
Zhang X, Xu R, Ma H, Qian Y, Zhu J. Brain structural and functional damage network localization of suicide. Biol Psychiatry. 2024;95:1091–9.
Cheng Y, Cai H, Liu S, Yang Y, Pan S, Zhang Y, et al. Brain network localization of gray matter atrophy and neurocognitive and social cognitive dysfunction in schizophrenia. Biol Psychiatry. 2025;97:148–56.
Xu R, Zhang X, Zhou S, Guo L, Mo F, Ma H, et al. Brain structural damage networks at different stages of schizophrenia. Psychol. Med. 2024;1–11, https://doi.org/10.1017/S0033291724003088.
Wang Y, Royer J, Park BY, Vos de Wael R, Larivière S, Tavakol S, et al. Long-range functional connections mirror and link microarchitectural and cognitive hierarchies in the human brain. Cerebral cortex (N. Y, NY : 1991). 2023;33:1782–98.
Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends Cogn Sci. 2013;17:648–65.
Reardon PK, Seidlitz J, Vandekar S, Liu S, Patel R, Park MTM, et al. Normative brain size variation and brain shape diversity in humans. Science. 2018;360:1222–7.
Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–48.
Xie Y, He Y, Guan M, Wang Z, Zhou G, Ma Z, et al. Low-frequency rTMS treatment alters the topographical organization of functional brain networks in schizophrenia patients with auditory verbal hallucination. Psychiatry Res. 2022;309:114393.
Gao Z, Xiao Y, Zhu F, Tao B, Yu W, Lui S. The whole-brain connectome landscape in patients with schizophrenia: A systematic review and meta-analysis of graph theoretical characteristics. Neurosci Biobehav Rev. 2023;148:105144.
Mesulam MM. From sensation to cognition. Brain : a J Neurol. 1998;121:1013–52.
Beauchamp MS. The social mysteries of the superior temporal sulcus. Trends Cogn Sci. 2015;19:489–90.
Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13:135–45.
Kim NY, Hsu J, Talmasov D, Joutsa J, Soussand L, Wu O, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2021;26:1299–309.
Hua Q, Wang L, He K, Sun J, Xu W, Zhang L, et al. Repetitive transcranial magnetic stimulation for auditory verbal hallucinations in schizophrenia: a randomized clinical trial. JAMA Netw open. 2024;7:e2444215.
Thoma RJ, Chaze C, Lewine JD, Calhoun VD, Clark VP, Bustillo J, et al. Functional MRI evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Front Psychiatry. 2016;7:39.
Xue K, Chen J, Wei Y, Chen Y, Han S, Wang C, et al. Altered dynamic functional connectivity of auditory cortex and medial geniculate nucleus in first-episode, drug-naïve schizophrenia patients with and without auditory verbal hallucinations. Front Psychiatry. 2022;13:963634.
David AS. Dysmodularity: a neurocognitive model for schizophrenia. Schizophr Bull. 1994;20:249–55.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–93.
Ćurčić-Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F, et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. 2017;148:1–20.
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–51.
Chuhma N, Mingote S, Kalmbach A, Yetnikoff L, Rayport S. Heterogeneity in dopamine neuron synaptic actions across the striatum and its relevance for schizophrenia. Biol Psychiatry. 2017;81:43–51.
Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–27.
Gilabert-Juan J, Sáez AR, Lopez-Campos G, Sebastiá-Ortega N, González-Martínez R, Costa J, et al. Semaphorin and plexin gene expression is altered in the prefrontal cortex of schizophrenia patients with and without auditory hallucinations. Psychiatry Res. 2015;229:850–7.
Oranje B, Glenthøj BY. Clonidine normalizes sensorimotor gating deficits in patients with schizophrenia on stable medication. Schizophr Bull. 2013;39:684–91.
Carruthers SP, Gurvich CT, Rossell SL. The muscarinic system, cognition and schizophrenia. Neurosci Biobehav Rev. 2015;55:393–402.
Kaul I, Sawchak S, Walling DP, Tamminga CA, Breier A, Zhu H, et al. Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA psychiatry. 2024;81:749–56.
Krystal JH, Kane JM, Correll CU, Walling DP, Leoni M, Duvvuri S, et al. Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part, randomised, double-blind, placebo-controlled, phase 1b trial. Lancet (Lond, Engl). 2022;400:2210–20.
Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879–89.
Zhuo C, Zhu J, Qin W, Qu H, Ma X, Yu C. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. British J psychiatry : J Ment Sci. 2017;210:209–15.
Perez-Rando M, García-Martí G, Escarti MJ, Salgado-Pineda P, McKenna PJ, Pomarol-Clotet E, et al. Alterations in the volume and shape of the basal ganglia and thalamus in schizophrenia with auditory hallucinations. Prog Neuropsychopharmacol Biol Psychiatry. 2024;131:110960.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project Nos. 82441007, 82120108014 and 81901705); Sichuan Science and Technology Program (Project No. 2024NSFSC1773, 2025ZNSFSC1771); Chengdu Science and Technology Office, major technology application demonstration project (2022-GH03-00017-HZ) ; 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Project No. ZYGD23003); Xiamen Medical and Health Key Project (3502Z20244008). SL acknowledges support from the Humboldt Foundation Friedrich Wilhelm Bessel Research Award and Chang Jiang Scholars (program T2019069).
Author information
Authors and Affiliations
Contributions
Conception and study design: ZG, HS, YX and SL; Methodological development and statistical analysis: ZG, HS, FZ and QZ; Data collection, acquisition and interpretation: BT, FZ, WY, SL and QG; Manuscript draft: ZG, HS, MPD and SL; Funding acquisition: ZG, YX and SL; Critical revisions of the manuscript and final approval of this version to be published: all authors.
Corresponding authors
Ethics declarations
Competing interests
Melissa P. DelBello has received research support from NIH, PCORI, Acadia, Alkermes, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, Sage, Sunovion, and Vanda. She is also a consultant or on the advisory board for Alkermes, Allergan, Janssen, Johnson and Johnson, Lundbeck, Merck, Myriad, and Sage. The remaining authors declare no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Z., Sun, H., Zhu, F. et al. Abnormal cortical topographic patterns associated with auditory verbal hallucination in first-episode schizophrenia. Transl Psychiatry 16, 8 (2026). https://doi.org/10.1038/s41398-025-03748-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03748-y