Abstract
Autism Spectrum Disorder (ASD) is characterized by deficits in social interaction, alongside abnormal sensory reactivity that often manifests as avoidance or repetitive behaviors. This review proposes that these core features may stem from somatosensory system dysfunction responsible for processing sensory information driven by an underlying excitatory-inhibitory (E/I) imbalance, a common finding in ASD models, which could drive such sensory impairments and ultimately contribute to the core social and behavioral deficits. We explore how recent advancements in hiPSC-derived assembloid models, which integrate multiple components of the human somatosensory pathway, provide a powerful platform to investigate these mechanisms. Crucially, this review not only highlights the promise of these models but also provides a critical evaluation of their inherent limitations, including cellular immaturity and the absence of key non-neuronal components. By examining the ongoing strategies to overcome these challenges, such as advanced co-culture systems, xenotransplantation, and bioengineering, this review offers a comprehensive outlook on the future of assembloid technology in elucidating ASD pathophysiology and developing novel therapeutic strategies.
Similar content being viewed by others
Introduction
Every organism, including humans, constantly interacts with its environment from birth, and the somatosensory system serves as the gateway for these interactions. External stimuli trigger internal responses that shape our perceptions, emotions, and behaviors, influencing survival, development, learning, and self-actualization. Thus, the interplay between individuals and their surroundings critically impacts cognitive, emotional, and physical growth, ultimately defining human identity. When infants perceive a parent’s affectionate gaze or gentle voice, they develop a sense of security and comfort, encouraging them to explore further and engage in complex behaviors. As children grow, interactions with peers teach them linguistic and non-linguistic communication, aiding in understanding social roles, rules, and the development of social skills.
External stimuli are equally important for emotional development. Positive interactions, such as comfort, support, and praise from family and peers, help children express emotions healthily and develop empathy, cooperation, and conflict resolution skills. Proper interaction with the environment also significantly influences physical and neurological development in infancy and childhood. The somatosensory system continuously matures and refines itself through exposure to external stimuli. For instance, tactile feedback from grasping objects helps infants enhance hand-eye coordination, strengthening neural connections involved in motor control [1]. Similarly, visual stimuli aid in establishing and refining neuronal pathways between the eyes and brain, improving visual processing. Auditory stimuli from the environment support the development of hearing and language abilities [2,3,4].
Overall, interactions with external stimuli, especially in early childhood, lay the foundation for individual growth. These early experiences shape cognitive, emotional, and physical development, profoundly influencing who we become.
Somatosensory abnormalities in autism spectrum disorder patients
According to diagnostic criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision (DSM-5-TR) and the International Classification of Diseases, 11th Revision (ICD-11), autism spectrum disorder (ASD) is identified based on the following core deficits: impairments in social communication and interaction, restricted and repetitive behaviors/interests/activities [5, 6]. One of the representative characteristics of ASD is the atypical response to external stimuli, manifesting as heightened or diminished sensitivity, which significantly impairs social interaction [7]. For instance, a person with auditory hypersensitivity might avoid noisy outdoor activities. Similarly, tactile sensitivities can lead to an insistence on wearing specific clothing, while discomfort with certain tastes or textures may result in the refusal of unfamiliar foods. From this perspective, many of the deficits observed in individuals with ASD may stem from problems in sensory integration.
Sensory integration, a concept first introduced in the 1970s by American educational psychologist and occupational therapist Dr. Jean Ayres, refers to the process by which the brain integrates sensory information received from various sensory organs and orchestrates appropriate physical responses [8]. Abnormal sensory integration in ASD has been reported in numerous studies. For example, children with ASD demonstrated significantly longer reaction times and lower stimulus recognition than typically developing controls when exposed to multimodal sensory stimuli, including auditory and visual inputs [9]. Such abnormalities in sensory integration make it difficult to respond appropriately to sensory inputs, ultimately complicating social communication and interaction. Since the concept of sensory integration became appreciated and widely accepted, studies have revealed that 90–96% of individuals diagnosed with ASD experience sensory abnormalities, which are now considered part of the diagnostic criteria for ASD [10,11,12].
Among the sensory abnormalities experienced by individuals with ASD, approximately 60% are known to be related to changes in tactile sensitivity [13]. The somatosensory system delivers diverse types of tactile information, including mechanical signals such as pressure, vibration, and texture from the peripheral nervous system (PNS) to the central nervous system (CNS) via an ascending pathway. In turn, the brain processes this sensory input and sends appropriate signals back to the PNS through the descending pathway to elicit corresponding responses. Tactile sensation is likely the first sensory modality to emerge, shaping early brain development, bonding with family members, and forming social relationships [14,15,16]. Thus, abnormalities in tactile sensitivity can significantly impact the formation of social relationships. For example, if abnormalities occur in sensory reception or in the organizational processes within the CNS, individuals may avoid physical contact or external stimuli, refrain from outdoor activities, and exhibit obsessive behavior toward stimuli that provide stability. Conversely, individuals with hyporesponsiveness to tactile stimuli may repetitively touch objects, seek more substantial pressure, and demonstrate insensitivity to injuries to their bodies or objects. Impaired tactile discrimination can result in difficulties in tool use, recognizing physical properties, and performing simple but organized tasks such as dressing. These behavioral abnormalities are frequently observed in individuals with ASD.
Accordingly, this review will center on the tactile system to enhance our understanding of how altered sensory integration and processing contribute to the social and functional challenges experienced by individuals with ASD.
The sensory integration therapy in ASD patients
Sensory Integration Therapy (SIT) is an intervention method designed to alleviate sensory abnormality symptoms by promoting effective interactions between children and their environments through various sensory stimuli. This therapeutic approach was first developed by Dr. Jean Ayres, who proposed the theory of sensory integration as mentioned, and is based on the premise that the brain maintains synaptic plasticity even beyond critical developmental periods [17]. Synaptic plasticity refers to the brain’s ability to strengthen or weaken synaptic connections as neural activity changes in response to a range of sensory experiences, including visual, auditory, and tactile stimuli [18,19,20,21].
The goal of SIT is to improve the sensory integration systems in children with sensory processing disorders, enabling them to adapt to sensory stimuli. The therapy is designed in a personalized manner by licensed occupational therapists, ensuring that children can actively participate, and incorporates methods that address various senses, including visual, auditory, tactile, proprioceptive, and vestibular modalities [22, 23]. Typically, the therapy is conducted in indoor play environments equipped with trampolines, ball pits, and ladders, and employs play-based programs to encourage active participation and a wide range of sensory-motor experiences for children with ASD [24]. During these interventions, the occupational therapist gradually increases the difficulty of activities to support the improvement of the child’s performance abilities [25]. For example, in children with hypersensitivity, the therapy may initially begin with gentle stimulation of the skin using soft objects like feathers to reduce anxiety and aversion, and gradually increasing intensity within the child’s tolerance using media such as sand and fabrics. Ultimately, the intervention aims to generalize improvements to daily adaptive activities, such as playing in sand or dressing.
SIT is widely applied to children with ASD. Results from parent surveys indicate that SIT is among the most highly preferred and frequently used therapeutic approaches for children with ASD [26, 27], and more than 95% of pediatric occupational therapists report implementing SIT in practice [28, 29]. The therapeutic efficacy of SIT has been demonstrated in numerous previous studies. For example, a study conducted at Nagasaki University administered SIT for 8-10 months to 8 children with high-functioning ASD (mean age: 56.8 ± 9.0 months) diagnosed according to DSM-4 criteria [30]. Comparison of scores on the Japanese version of the Miller Assessment for Preschoolers [31, 32] before and after therapy showed significant improvements in total score as well as in foundation, coordination, non-verbal, and complex subdomains. Similarly, a study from a clinic in Jakarta included 72 children aged 2-5 years diagnosed with ASD according to DSM-5 criteria [33]. These children participated in SIT sessions twice a week for 12 weeks, and as a result, showed significant improvements relative to the control group in communication, daily living skills, and receptive and expressive domains. Nevertheless, the paucity of preceding studies with long-term follow-up of more than 6 months constitutes a limitation to determining whether SIT possesses consistent long-term efficacy. Furthermore, negative findings regarding the efficacy of SIT have been reported as being attributable to interventions that focus solely on the provision of sensory stimuli without sufficiently incorporating standardized principles of SIT (e.g., therapist-child relationship, just-right challenge) [25, 34].
In conclusion, while the efficacy of SIT in children with ASD remains a subject of debate, the evidence suggests that the controversy stems from inconsistent application rather than a lack of inherent effectiveness. Studies reporting negative outcomes often point to interventions that fail to incorporate the standardized principles of SIT. This implies that when properly implemented, SIT can be a powerful therapeutic tool. Therefore, future research is crucial for establishing clear guidelines for SIT delivery and for building a bridge between its foundational neurobiological mechanisms and clinical outcomes. This will allow us to fully realize the therapeutic potential of SIT.
Structure of the somatosensory system
To understand the sensory abnormalities observed in individuals with ASD, it is essential first to grasp the nature and developmental process of the somatosensory system. The human somatosensory system facilitates the perception of three major sensory domains: exteroception, interoception, and proprioception. Exteroception encompasses sensations such as touch, temperature, pain, and itch. Interoception refers to the perception of internal states of the body, including heartbeat and gastrointestinal activity. Proprioception involves the awareness of the position and movement of body parts [35]. Given that atypical tactile processing is a prominent feature in individuals with ASD, this review will focus specifically on the tactile modality, which falls within the domain of exteroception.
The flow of information within the somatosensory system is bidirectional, involving distinct functional roles for its two primary routes. The ascending pathway, carrying signals toward the brain, is primarily responsible for sensory perception. In contrast, the descending pathway, which transmits signals away from the brain, modulates the body’s responses to these stimuli. Tactile information transmitted via the ascending pathway involves a three-step relay system, ultimately reaching the primary somatosensory cortex for processing. The reception and transmission of sensory information at each stage are as follows:
Primary sensory neurons extend to peripheral sensory organs, including the skin, muscles, and visceral tissues, initially collecting sensory information and transmitting it through the dorsal root ganglia (DRG) to the spinal cord within the CNS. For example, primary sensory neurons in the skin capture stimuli through specialized receptors distributed across various layers of the skin. During this process, mechanical energy from external stimuli is converted into receptor potentials, a form of membrane potential that encodes the sensory information for further transmission [36, 37]. Receptors expressed on primary sensory neurons are classified into low-threshold mechanosensitive receptors (LTMRs) and high-threshold mechanosensitive receptors (HTMRs) based on mechanical sensitivity thresholds. LTMRs, with relatively low thresholds, allow the detection of fine tactile stimuli such as stretch, vibration, and light brushing. In contrast, HTMRs, which have higher thresholds, primarily mediate the perception of nociceptive signals [38]. Information detected by LTMRs and HTMRs is transmitted through nerve fibers classified into Aβ, Aδ, and C fibers, depending on their axonal diameter and the presence of myelination. For tactile stimuli detected by LTMRs, signals are conveyed through either myelinated Aβ fibers (axon diameters: 6-12 µm) or unmyelinated C-fiber LTMRs (axon diameters: 0.2-1.5 µm). In contrast, nociceptive stimuli detected by HTMRs are transmitted via myelinated Aδ fibers (axon diameters: 1-5 µm) or unmyelinated C-fiber HTMRs [38].
Tactile stimuli applied to the skin are received by various mechanoreceptors distributed across the epidermis and dermis, including Merkel cells, Meissner corpuscles, Pacinian corpuscles, and Ruffini endings [36]. Signals from these mechanoreceptors are then transmitted primarily via Aβ fibers or through free-nerve endings that utilize C-fiber LTMRs. Mechanoreceptors exhibit differential response patterns to stimuli. For instance, Meissner and Pacinian corpuscles rapidly adapt receptors that respond quickly to stimuli but cease to respond if the stimulus persists. In contrast, Merkel cells and Ruffini endings have slowly adapting receptors that react more gradually and continue responding to sustained stimuli. Free-nerve endings exclusively receive nociceptive signals transmitted via Aδ fibers and C-fiber HTMRs. This intricate organization enables the perception of a wide range of tactile stimuli [36].
Primary sensory neurons connect to the DRG and extend into the spinal cord, forming synapses with secondary sensory neurons that relay signals to the thalamus. During this process, tactile and nociceptive information ascend to the thalamus via different routes within the spinal cord [39, 40]. Tactile information is conveyed via the dorsal column-medial lemniscal (DCML) pathway. Primary sensory neurons carrying tactile signals enter the spinal cord through the dorsal column, a white matter tract, and project to the dorsal column nuclei in the medulla, where they synapse with secondary neurons. These secondary neurons then decussate at the medial lemniscus and ascend to the ventral posterolateral (VPL) nucleus of the thalamus. In contrast, nociceptive information is conveyed via the spinothalamic pathway. Primary sensory neurons transmitting nociceptive signals enter the dorsal horn, a region of gray matter in the spinal cord, and form synapses with secondary neurons. These secondary neurons decussate within the spinal cord as they traverse the ventral horn, eventually ascending to the VPL nucleus of the thalamus [39, 40].
In the thalamus, secondary sensory neurons synapse with tertiary neurons, which relay signals to the primary somatosensory cortex (S1). S1, located in the postcentral gyrus of the parietal lobe, is subdivided into Brodmann’s Areas 1, 2, 3a, and 3b. Area 1 and 3b primarily process cutaneous stimuli, Area 3a processes proprioceptive inputs, and Area 2 integrates both tactile and proprioceptive information [41]. While S1 serves as the primary region for sensory information processing and responding to the PNS, it also interacts extensively with the secondary somatosensory cortex (S2) for integration, sensory memory formation, and interpretation. S2 also connects to limbic structures, such as the hippocampus and amygdala, which play key roles in memory and emotional responses. These interconnections underscore the role of the somatosensory system in higher-order cognitive functions and social behaviors beyond simple sensory perception [42].
In addition to the three-step transmission system of sensory information from the PNS to the CNS, inhibitory interneurons also play a crucial role as regulators in sensory signal transmission. These interneurons are distributed throughout the sensory system, including the spinal cord, thalamus, and cortex. They enhance contrast for more explicit stimulus discrimination and maintain neural balance by regulating the intensity and scope of stimuli [43, 44]. Through this modulation, information on sensory signals can be transformed at each synapse along the pathway. Thereby, these synaptic points may represent potential sites of sensory processing abnormalities observed in individuals with ASD. Notably, many studies have reported an imbalance in excitatory and inhibitory neuronal activity (E/I imbalance) in ASD, which may underlie sensory reception or processing deficits [45, 46]. This imbalance and its impact on somatosensory processing will be discussed in detail later.
The intricate pathway of the somatosensory system, from peripheral receptors to cortical areas and their regulation by the balance of excitatory and inhibitory neurons, highlights how this balance plays a critical role in essential sensory functions and complex behaviors. Understanding these mechanisms will offer insights into the pathogenesis of ASD, where sensory processing abnormalities contribute to the clinical manifestations of the disease (Fig. 1).
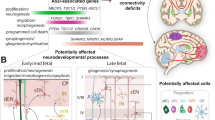
Schematic representation of the ascending pathway transmitting touch or pain information from peripheral mechanoreceptors to the primary somatosensory cortex (S1). The regions where the E/I balance is established are highlighted (black: excitatory, red: inhibitory) [68,69,70, 108, 114, 129,130,131,132]. Created with BioRender.com.
Development of the somatosensory system
The developmental process of the somatosensory system is highly intricate. Although many aspects remain poorly understood, research has shown that the formation and specification of neurons within the somatosensory system, spanning from the peripheral to the CNS, are tightly regulated by specific transcription factors.
Studies on embryonic development have shown that multipotent neural crest progenitors originating from the dorsal neural tube migrate to form the DRG [47]. These progenitors subsequently differentiate into sensory neuron precursors. Initially present as unspecialized sensory neurons, they later undergo subtype specification through the regulated expression of transcription factors, as revealed by single-cell transcriptomic analyses in mouse models [47]. Furthermore, interactions with cells in the PNS have been shown to facilitate synaptic terminal formation in primary sensory neurons [48]. For instance, in mice, the activation of BDNF signaling by Merkel cells in the skin is critical for the maturation of slowly adapting type I (SA1) afferent neurons [49]. Similarly, in C. elegans, the interaction between chemotaxin-2 in muscle cells and L1CAM in the skin is essential for the specialization of sensory neurons [50].
Primary sensory neurons connected to the PNS must extend their axons through the DRG toward the brain. Although the precise mechanisms governing this process remain only partially understood, several studies have highlighted the critical role of specific proteins in directing proper axonal pathfinding. For instance, studies on chick spinal cords have demonstrated that axons of sensory neurons rely on the secreted protein Semaphorin for accurate projection within the spinal cord. Semaphorin functions as a short-range inhibitory cue, modulating axonal guidance by differentially regulating the projection of nerve growth factor-dependent axons and neurotrophin 3-dependent axons, thereby determining distinct projection patterns [51, 52]. Similarly, several transcription factors are also known to be essential for establishing neuronal connectivity from the spinal cord to the brain. Frizzled-3, a critical polarity protein involved in axon development, plays a pivotal role in establishing proper neuronal connectivity. Studies on mouse embryos have revealed that the absence of Frizzled-3 results in a substantial reduction in ascending axons targeting the brainstem, with a complete absence of axonal projection in the midbrain or thalamus [53, 54]. In a mouse model where Phox2a, a transcription factor transiently expressed in spinal neurons during embryonic stages, was knocked out, defects in axonal migration and formation of the anterolateral system and subsequent impaired nociception were observed [48, 55]. Since the temporal and spatial expression patterns of Phox2a in mice and humans are similar, it is likely that its role is conserved across species.
In the CNS, during early developmental stages, the brain prepares to receive and process sensory stimuli, even before actual signals are transmitted from the PNS. Notably, spontaneous neural signaling exchanges between the thalamus and cortex are observed before the sensory inputs arrive from the PNS. The circuit transmitting signals from the thalamus to the cortex is referred to as the thalamocortical neuronal circuit (TC). During development, TC activity plays a crucial role in the spatial organization of sensory areas within the cortex [56]. The formation of the TC circuit is known to be influenced by the transcription factor Btbd3, which regulates the directionality of dendrite growth. In neurons of the mouse somatosensory cortex, neuronal activation triggers Btbd3 to translocate to the nucleus, where it modulates dendritic morphology via transcriptional regulation [57]. Specifically, Btbd3 prunes dendrites that extend in directions not aligned with the active signal source, thereby orienting dendritic growth toward the activated neurons. For the TC circuit to form and cortical arealization during brain development, input signals from the thalamus are essential. Before receiving sensory stimuli from the PNS, the TC exhibits unstable and sporadic bursts of activity. As inhibitory circuits mature, this activity becomes finely tuned, enabling the cortex to precisely differentiate and process sensory inputs [58]. The formation of cortical areas is also influenced by calcium waves originating in the thalamic nuclei. Changes in the pattern of thalamic waves were shown to result in corresponding changes in cortical arealization [59]. Overall, this “pre-sensory period,” during which thalamocortical axonal connections are established, is considered crucial for preparing the brain to process future sensory stimuli (Fig. 2).
The upper timeline indicates major developmental milestones of the human somatosensory system. The proteins listed at the bottom of each stage are characteristically expressed during that specific period. The lower section highlights region-specific organoid models that recapitulate the developmental stages of the human somatosensory system [85, 87, 133,134,135,136,137,138,139,140,141,142,143,144,145,146]. Created with BioRender.com.
In summary, the development of the somatosensory system is shaped by both intrinsic factors, such as transcription factor regulation, and external experiences. These mechanisms underscore the dynamic interplay between genetic programming and sensory experience in establishing a functional sensory system. Nowakowski and colleagues proposed the “proto-regions” hypothesis, which suggests that during early development, molecular differences drive arealization, and subsequent activity-dependent changes finely tune the functions of each region [60]. This perspective highlights that while neuronal fate in the mammalian brain is predetermined early on, it remains amenable to modification through plasticity. This viewpoint also underscores the importance of further investigating how genetic factors interact with external sensory inputs to shape the development of the somatosensory system, a critical area of study for advancing ASD research.
ASD-associated genetic factors and sensory abnormalities
According to the National Institute of Mental Health, ASD is classified as a neurodevelopmental disorder because its symptoms are typically observable within the first two years of life, and abnormal brain growth patterns can be detected as early as six months of age [61,62,63]. Diagnostic tools such as facial expression response analysis and electroencephalography (EEG) have been reported to help identify ASD symptoms [5, 6]. This suggests that intrinsic factors may play a fundamental role in the onset of ASD, rather than the classical view that its symptoms are primarily a consequence of deficits in social activity. In fact, studies involving monozygotic twins have shown that the manifestation of ASD symptoms is influenced by genetic factors, with heritability estimates ranging from 40 to 90% [64]. Beyond twin studies, recent findings from Genome-Wide Association Studies (GWAS) have identified genetic factors associated with ASD development [65]. In addition to genetic contributions, environmental factors such as parental age, maternal health during pregnancy, and perinatal conditions are also known to increase the risk of ASD [64]. As a result, understanding the interaction between genetic and environmental factors, and the mechanisms underlying their interplay, has become a critical area of ASD research.
Many of the diverse ASD-associated genetic factors identified through GWAS are not absolute causes of the disorder on their own. However, by analyzing the convergence of the cellular mechanisms influenced by these genetic variants, it is possible to uncover the core pathogenic processes underlying ASD. Such analyses reveal that the majority of ASD-related genetic variants are associated with synaptic structure and function (e.g., SHANK2, SHANK3, CNTNAP2, FMRP, SYNGAP1, KATNAL2, ANK2, NRXN1) or chromatin/transcriptional regulation (e.g., CHD8, ARID1B, POGZ, TBR1, FOXP1, MECP2) [64]. These findings suggest that abnormalities in neural network structure/function, together with dysregulation of gene expression required for neural information processing and storage, are key contributors to ASD pathology. A recent study employing brain organoids with ASD-associated genetic mutations such as SUV420H1, ARID1B, and CHD8 deficiency provides strong support for this hypothesis [66]. The study found that while each model had distinct gene expression patterns, they shared common cellular and functional abnormalities, including impairments in inhibitory neuronal differentiation and function, as well as in the differentiation and activity of deep-layer projection neurons. Further evidence comes from animal studies. Selimbeyoglu et al. investigated social deficits in Cntnap2-deficient mice by utilizing optogenetics to manipulate the balance between excitatory and inhibitory neurons in the prefrontal cortex [67]. Enhancing Parvalbumin (PV) neuron activity while simultaneously reducing the excitability of pyramidal neurons led to increased interaction times with littermates and greater social exploration behaviors, even in CNTNAP2-knockout mice. This study demonstrated that the E/I balance is crucial for proper social interactions. Another study explored sensory abnormalities in the SHANK3B-knockout mouse, a model of ASD-related sensory processing deficits [68]. This research demonstrated that mice with SHANK3B deficiency exhibited heightened sensitivity to fine vibrational stimuli compared to wild-type mice. Calcium imaging revealed increased responsiveness of pyramidal neurons in layers II/III of the somatosensory cortex, accompanied by reduced inhibitory neuron activity. The E/I imbalance is further proposed to be a key driver of ASD pathophysiology by many studies from mouse models for ASD: In NLGN3 R451C knock-in mice, increased GABAergic signaling was observed in the sensory cortex, and mice with MECP2 deletion in inhibitory neurons exhibited reduced GABA synthesis and release, along with sensory integration deficits. Likewise, CNTNAP2-deficient models displayed a reduction in inhibitory neuron populations, and in BTBR mouse models, a decrease in inhibitory postsynaptic current (IPSC) frequency in the hippocampus was associated with impaired social behavior, which could be rescued by treatment with the GABA A receptor agonist clonazepam [45, 46].
Furthermore, the concept of E/I imbalance as a core mechanism extends beyond the cortical circuits in the brain. A compelling body of evidence suggests that disruptions across the entire somatosensory pathway, including the PNS, are critical contributors to ASD pathophysiology. For example, the role of MECP2 along different segments of the somatosensory pathway has been extensively explored using mouse models where the gene was selectively deleted in specific regions, including the spinal cord, DRG, and forebrain [69, 70]. Mice with MECP2 deletion in the spinal cord and DRG exhibited heightened sensitivity in the Air Puff Response test and showed significantly reduced preference for novel textures in the Textured Novel Object Recognition Test (NORT). In contrast, deletion of MECP2 specifically in the excitatory neurons of the forebrain did not lead to notable behavioral changes [69]. These findings suggest that the pathological role of ASD-associated genetic mutations may not be confined to the brain but could also involve disruptions in the reception and transmission of sensory stimuli at earlier stages of the somatosensory pathway, contributing to a broader excitatory and inhibitory imbalance across the entire somatosensory system. Mechanistic investigations revealed that MECP2 deletion caused reduced expression of GABRB3, a subunit of the GABA A receptor expressed from the dorsal horn of the spinal cord to the nerve terminals of LTMRs. As GABA A receptor-mediated inhibitory signaling is crucial for regulating sensory input, its downregulation likely contributes to impaired sensory modulation, leading to excessive sensory perception. This dysregulation correlates with anxiety-like behaviors and social deficits observed in the MECP2-deficient mouse models [69]. Further reinforcing the significance of peripheral contributions, subsequent research demonstrated that restoring GABRB3 expression in peripheral sensory neurons of MECP2-deleted mice ameliorated their heightened Air Puff Response and social deficits, although impairments in memory and motor function persisted [70].
Taken together, these findings underscore the importance of understanding how ASD-associated genetic factors influence not only brain regions that process sensory information but also the entire somatosensory pathway. They emphasize the need for further research into the mechanisms by which these genetic factors affect sensory input reception, transmission, and integration in ASD.
Advantages and limitations of non-human animal models for ASD research
Investigating the neurological mechanisms underlying ASD is challenging due to technical and ethical limitations associated with studying developmental stages of human brains, especially in infants aged 1-2 years, a critical period when ASD pathology typically emerges through genetic or prenatal environmental factors. It is nearly impossible to manipulate or control ASD-related genetic or environmental factors. Furthermore, recruiting a sufficiently large cohort for meaningful analysis poses significant difficulties. Although GWAS have identified numerous genetic factors associated with ASD, critical questions remain unanswered, such as whether these factors are necessary or sufficient for disease onset, the mechanisms through which they exert their effects, and how they interact with environmental factors to increase the risk of ASD. Addressing these questions is crucial for advancing current research.
One of the most significant advantages of non-human animal (hereafter referred to as ‘animal’) models in ASD research is their utility in behavioral experiments. Many representative symptoms of ASD, such as anxiety, deficits in social interactions, and altered responsiveness to auditory or tactile stimuli, can be quantitatively analyzed in animal models [45]. Moreover, these models allow researchers to observe behavioral changes caused by ASD-related genetic factors identified by GWAS [69] (Table 1). However, there are inherent limitations due to the biological differences between animal models and humans, making it challenging to generalize findings. For instance, early childhood is a period of rapid brain development in humans, particularly involving the formation of distinct cortical structures, including the ventricular zone (VZ), inner subventricular zone (iSVZ), outer subventricular zone (oSVZ), intermediate zone (IZ), subplate (SP), cortical plate (CP), and marginal zone (MZ). In mice, which are widely used in ASD research, the iSVZ and oSVZ are significantly underdeveloped compared to humans, and brain development occurs over a much shorter time frame (within just a few days) [66, 71]. Notably, the oSVZ is a characteristic structure of primates, known to contain neural progenitor cells and differentiating cells that contribute to the size and complexity of the primate brain. This distinction is particularly relevant when studying ASD, as abnormalities in cortical development are one of the key features of the disorder [72]. These differences can complicate the analysis of developmental processes and pathological mechanisms. In the context of somatosensory system research, comparative studies between human and mouse DRG using single-cell transcriptome analyses have revealed differences in the expression patterns of receptors and ion channels involved in somatosensory processing between the two species [73]. This underscores the importance of validating molecular findings from animal models in human systems, as discrepancies may impact the translational relevance of the results.
Overall, while animal models provide valuable insights, particularly in behavioral and genetic studies, their biological and developmental differences highlight the necessity of complementing animal research with human-based studies to achieve a comprehensive understanding of ASD mechanisms (Table 1).
Human induced pluripotent stem cells for ASD research
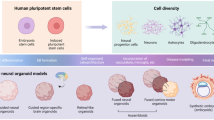
As human models, brain cells differentiated from human induced pluripotent stem cells (hiPSCs) are actively utilized in ASD research. A primary advantage of this model is its ability to use cells derived directly from individuals with ASD or related neurodevelopmental disorders, thereby capturing the patient’s unique genetic characteristics [74,75,76,77,78]. By obtaining cells from ASD patients and comparing them with cells from non-ASD family members, such as parents or siblings, researchers can minimize variables such as genetic diversity and environmental differences. This enables researchers to investigate how disease-specific genetic mutations influence cellular development and physiological functions. For instance, comparisons between cells from ASD patients and their relatives provide valuable insights into genotype-phenotype relationships in ASD pathogenesis [79].
A particularly promising approach in this field is the utilization of brain organoid models, where cells self-organize into three-dimensional structures that mimic the developing human brain. Crucially, these organoids recapitulate the species-specific aspects of human brain development, such as the formation of an outer subventricular zone (oSVZ), which is underdeveloped in rodent models. This allows for a more accurate investigation of early neurodevelopmental processes that are difficult to model in non-human animals. These models are increasingly employed because they can replicate the complex architecture of the human brain and model the abnormalities associated with ASD-linked genetic factors. For example, brain organoids harboring CNTNAP2 mutations demonstrated abnormalities in embryonic cortical development. Specifically, these organoids exhibited an increased number of neural progenitor cells, leading to larger organoid sizes. This finding mirrors the brain enlargement observed in clinical patients carrying CNTNAP2 mutations [80]. However, a significant limitation of current in vitro models is that iPSC-derived cells, organoids, and assembloids largely reflect fetal developmental stages and often fail to recapitulate the mature physiological characteristics observed in postnatal tissues. Therefore, developing advanced protocols to promote further maturation and model postnatal developmental trajectories is a critical next step for the field.
Currently, the majority of ASD studies using hiPSC-derived organoid models primarily focus on cerebral organoids that simulate cortical development and functions [66, 81, 82]. Although the cortex is crucial for processing and storing sensory information, a cortex-centric view overlooks the essential earlier stages of the somatosensory system, including sensory neurons, DRG, and the thalamus, which receive and relay incoming signals. Consequently, this narrow perspective inevitably leads to an incomplete and potentially misleading picture of the pathophysiology underlying sensory dysfunction in ASD. From this perspective, to overcome the limitations of single-region models, researchers have begun developing assembloids. This innovative approach involves integrating multiple, distinct region-specific organoids to recapitulate the complex neural circuits implicated in ASD. As previously mentioned, the imbalance between excitatory and inhibitory neuronal activity is proposed to be a major driver of ASD pathophysiology. In this regard, traditional cerebral organoid models primarily generating excitatory neurons have limitations in modeling E/I networks. However, recent advancements have enabled the development of ganglionic eminence organoids, where inhibitory neurons are generated and migrate to cortical areas [83, 84]. These organoids can now be fused with cerebral organoids to create cortical-ganglionic assembloids, allowing for the construction of functional E/I networks. Additionally, methods for producing thalamic organoids, specifically those mimicking the ventral posterior lateral thalamic nuclei capable of generating inhibitory neurons in the thalamic reticular nucleus, have further expanded the scope of these models [85]. By integrating these techniques, researchers can now create brain organoids that recapitulate balanced E/I networks, providing a robust platform for testing the E/I imbalance hypothesis in ASD. Recent organoid studies have revealed how ASD-associated genetic variants can disrupt the development, maturation, and reciprocal regulation of excitatory and inhibitory neurons, potentially leading to aberrations in the E/I balance of the somatosensory system. Building on this, the developing assembloid models will allow us to directly test whether E/I imbalances are induced at the network level using electrophysiological recordings and calcium/voltage imaging. This will provide an avenue to investigate underlying mechanisms and explore potential rescue strategies. Ultimately, knowledge gained from these in vitro systems can be translated to in vivo animal models to verify whether correcting these circuit-level dysfunctions can ameliorate abnormal social behaviors.
An advanced assembloid model was recently developed to integrate multiple brain regions. For example, cortical spheroids, spinal spheroids, and skeletal muscle spheroids were generated independently from hiPSCs and then physically connected to form a motor system assembloid [86]. Similarly, sensory system assembloids were created by connecting sensory neurons, the spinal cord, thalamus, and cortex [87]. The functional integrity of these assembloid models was validated through calcium imaging and electrophysiological analyses, demonstrating descending pathways from the CNS to the PNS in the motor system assembloid model and ascending pathways from the sensory system to the CNS in the sensory system assembloid.
While multi-regional assembloids offer exciting new avenues for ASD research, significant challenges concerning their reproducibility and reliability must be addressed. A primary concern is the considerable variability in model properties. As demonstrated by Lancaster and colleagues, organoids intended to model the same brain region can exhibit different cellular compositions and developmental stages depending on the differentiation protocol used [88]. Furthermore, significant variations can arise from the specific iPSC line employed, and even between different batches of the same line. Consequently, there is a critical need to standardize protocols to generate organoids with compositions that more faithfully recapitulate their in vivo counterparts. Efforts must also focus on minimizing process-induced variability and developing robust metrics for quality control to validate the maturity and composition of each organoid and assembloid prior to functional analysis. A further limitation lies in how these assembloids model neural connectivity. Current methods typically rely on fusing organoids via direct physical contact. This approach, however, does not fully replicate the in vivo environment, where distinct brain regions are connected by long-range axonal projections. Recent research has demonstrated that connecting two cerebral organoids via a bundle of reciprocally extended axons, rather than through direct contact, promotes the development of more mature neural networks. Furthermore, during their trajectory, these developing axons are influenced by a complex milieu of various neuronal and non-neuronal cells, which are absent in current assembloid models. Although this simplified system allows for the direct assessment of how the constructed circuit contributes to the acquisition and processing of sensory information, its physiological relevance remains a key question. Therefore, findings from assembloid models must be critically validated against data from more complex systems, such as in vivo non-human models or clinical data analyses.
Beyond these structural challenges, a major functional limitation is that assembloids cannot receive direct sensory inputs, making it difficult to model the experience-dependent plasticity central to sensory development. To circumvent this, researchers are exploring methods to mimic sensory signaling, such as using optogenetics to activate specific neural populations with light or applying chemical agonists to stimulate sensory pathways. However, a significant hurdle remains in designing these artificial stimuli with physiologically relevant resolution, duration, and intensity. It is also essential to validate whether these inputs can genuinely induce long-term, plasticity-like changes in the network. This challenge is amplified when considering that ASD often involves deficits in sensory discrimination, not just detection. As current assembloid systems lack the resolution to model these nuanced perceptual deficits, future work should move beyond assessing the propagation of single inputs to investigate how these models integrate and discriminate between multiple sensory signals. The aforementioned techniques, such as patterned optogenetic or chemical stimulation, provide a promising avenue to begin exploring these more sophisticated network functions, bridging the gap to real-world sensory impairments in ASD (Table 2).
Conclusions
Further advancements are necessary to accurately mimic and validate E/I networks at every stage of the somatosensory system. As discussed, future research will greatly benefit from integrating assembloid models with the aforementioned advanced techniques, such as optogenetics and calcium/voltage imaging, to systematically explore the effects of E/I imbalance on network function. Beyond these in vitro analyses, a critical next step is to bridge the gap to in vivo validation. Further advancements are necessary to accurately mimic and validate E/I networks at every stage of the somatosensory system. As discussed, future research will greatly benefit from integrating assembloid models with the aforementioned advanced techniques, such as optogenetics and calcium/voltage imaging, to systematically explore the effects of E/I imbalance on network function. Beyond these in vitro analyses, a critical next step is to bridge the gap to in vivo validation. To this end, xenotransplantation approaches, where human brain organoids are implanted into the brains of animal models, are emerging as a powerful strategy [89, 90]. This method offers the unique advantage of allowing human-derived neural circuits to mature and be tested within a living network system. However, significant species-specific differences between the human implant and the host (e.g., a rodent) can prevent the circuits from becoming fully functional or may cause them to operate in an altered, constrained manner. Despite this, by applying these innovative methods in parallel, researchers aim to elucidate the causality between sensory integration deficits and ASD pathogenesis, deepen our understanding of the core pathological mechanisms, and ultimately pave the way for developing effective therapeutic interventions (Fig. 3).
Region-specific organoids, including sensory nerve, spinal cord, thalamus, ganglionic eminence and cortex, are fused to model the human somatosensory system. Neuronal activity and connectivity are assessed using multi-electrode array(MEA) recording and calcium or voltage imaging. human somatosensory system with distinct organoids. Created with BioRender.com.
References
Nelson EL, Danforth C, Needham AW. Infant reaching and grasping: Frameworks for testing developmental cascades. Infant Behav Dev. 2025;80:102104.
Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–36.
Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79.
Kuhl PK. Early language acquisition: cracking the speech code. Nat Rev Neurosci. 2004;5:831–43.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th edition) 2013; 31, 50–58.
World Health Organization. International classification of diseases for mortality and morbidity statistics (11th revision) 2018; 416-33.
Robertson CE, Baron-Cohen S. Sensory perception in autism. Nat Rev Neurosci. 2017;18:671–84.
Kilroy E, Aziz-Zadeh L, Cermak S. Ayres theories of autism and sensory integration revisited: what contemporary neuroscience has to say. Brain Sci. 2019;9:68.
Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, et al. Evidence for diminished multisensory integration in autism spectrum disorders. J Autism Dev Disord. 2014;44:3161–7.
Balasco L, Provenzano G, Bozzi Y. Sensory abnormalities in autism spectrum disorders: a focus on the tactile domain, from genetic mouse models to the clinic. Front Psychiatry. 2020;10:1016.
Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. 2007;61:190–200.
Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69:48–54.
Schaffler MD, Middleton LJ, Abdus-Saboor I. Mechanisms of tactile sensory phenotypes in autism: current understanding and future directions for research. Curr Psychiatry Rep. 2019;21:134.
Cascio CJ. Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord. 2010;2:62–69.
Hertenstein MJ, Verkamp JM, Kerestes AM, Holmes RM. The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet, Soc, Gen Psychol Monogr. 2006;132:5–94.
Main M, Stadtman J. Infant response to rejection of physical contact by the mother. J Am Acad Child Psychiatry. 1981;20:292–307.
Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159:727–37.
Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–32.
Huber E, Jiang F, Fine I. Responses in area hMT+ reflect tuning for both auditory frequency and motion after blindness early in life. Proc Natl Acad Sci. 2019;116:10081–6.
Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605.
Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Investig. 2016;126:1983–97.
Lane SJ, Mailloux Z, Schoen S, Bundy A, May-Benson TA, Parham LD, et al. Neural foundations of ayres sensory integration®. Brain Sci. 2019;9:153.
Oh S, Jang J-S, Jeon A-R, Kim G, Kwon M, Cho B, et al. Effectiveness of sensory integration therapy in children, focusing on Korean children: A systematic review and meta-analysis. World J Clin Cases. 2024;12:1260–71.
Yamanishi Y, Orita Y, Nagayoshi M, Nishimura R, Shinjyo T, Masuda K, et al. Examining the effectiveness of ayres sensory integration® intervention for children with developmental coordination disorder in improving motor coordination and daily activity function: a randomized controlled Trial. Cureus. 2025;17:e76971.
Parham LD, Cohn ES, Spitzer S, Koomar JA, Miller LJ, Burke JP, et al. Fidelity in sensory integration intervention research. Am J Occup Ther. 2007;61:216–27.
Kashefimehr B, Kayihan H, Huri M. The effect of sensory integration therapy on occupational performance in children with autism. OTJR: Occup, Particip Heal. 2018;38:75–83.
Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Res Dev Disabil. 2006;27:70–84.
Watling R, Deitz J, Kanny EM, McLaughlin JF. Current practice of occupational therapy for children with autism. Am J Occup Ther. 1999;53:498–505.
Hunt J, Hooydonk E, van, Faller P, Mailloux Z, Schaaf R. Manualization of occupational therapy using ayres sensory integration® for autism. OTJR: Occup, Particip Heal. 2017;37:141–8.
Iwanaga R, Honda S, Nakane H, Tanaka K, Toeda H, Tanaka G. Pilot study: efficacy of sensory integration therapy for Japanese children with high-functioning autism spectrum disorder. Occup Ther Int. 2014;21:4–11.
Hojo S, Mizukoshi A, Azuma K, Okumura J, Mizuki M, Miyata M. New criteria for multiple chemical sensitivity based on the Quick Environmental Exposure and Sensitivity Inventory developed in response to rapid changes in ongoing chemical exposures among Japanese. PLoS ONE. 2019;14:e0215144.
Hojo S, Kumano H, Yoshino H, Kakuta K, Ishikawa S. Application of quick environment exposure sensitivity inventory (QEESI©) for Japanese population: study of reliability and validity of the questionnaire. Toxicol Ind Heal. 2003;19:41–49.
Raditha C, Handryastuti S, Pusponegoro HD, Mangunatmadja I. Positive behavioral effect of sensory integration intervention in young children with autism spectrum disorder. Pediatr Res. 2023;93:1667–71.
Camarata S, Miller LJ, Wallace MT. Evaluating sensory integration/sensory processing treatment: issues and analysis. Front Integr Neurosci. 2020;14:556660.
Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–39.
Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–53.
Hao J, Bonnet C, Amsalem M, Ruel J, Delmas P. Transduction and encoding sensory information by skin mechanoreceptors. Pflügers Arch - Eur J Physiol. 2015;467:109–19.
Roudaut Y, Lonigro A, Coste B, Hao J, Delmas P, Crest M. Touch sense. Channels. 2012;6:234–45.
Tortora, G, and Derrickson, BH Principles of anatomy & physiology (14th edition). WILEY 2016; 547-59.
Bear, MF, Connors, BW, and Paradiso, MA Neuroscience: Exploring the brain (4th edition). Wolters Kluwer 2015. 416-33.
Alloway KD. Neuroscience. Dale Purves, George J. Augustine, David Fitzpatrick, Lawrence C. Katz, Anthony-Samuel LaMantia, James O. McNamara, S. Mark Williams. Q Rev Biol 2001; 76:526–526.
Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A-S, McNamara JO, Williams SM. Neuroscience (3th edition). Sinauer Associates 2004. 203–206.
Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, et al. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 2015;13:1246–57.
Chen X, Tang S-J. Neural circuitry polarization in the spinal dorsal horn (SDH): a novel form of dysregulated circuitry plasticity during pain pathogenesis. Cells. 2024;13:398.
Lee E, Lee J, Kim E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81:838–47.
Culotta L, Penzes P. Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD. Mol Autism. 2020;11:32.
Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577:392–8.
Kania A. Sensational developments in somatosensory development?. Curr Opin Neurobiol. 2021;66:212–23.
Reed-Geaghan EG, Wright MC, See LA, Adelman PC, Lee KH, Koerber HR, et al. Merkel cell-driven BDNF signaling specifies SAI neuron molecular and electrophysiological phenotypes. J Neurosci. 2016;36:4362–76.
Díaz-Balzac CA, Rahman M, Lázaro-Peña MI, Martin Hernandez LA, Salzberg Y, Aguirre-Chen C, et al. Muscle- and skin-derived cues jointly orchestrate patterning of somatosensory dendrites. Curr Biol. 2016;26:2379–87.
Marmigère F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–27.
Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64.
Onishi K, Tian R, Feng B, Liu Y, Wang J, Li Y, et al. LRRK2 mediates axon development by regulating Frizzled3 phosphorylation and growth cone–growth cone communication. Proc Natl Acad Sci. 2020;117:18037–48.
Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc Natl Acad Sci. 2014;111:E3005–E3014.
Roome RB, Bourojeni FB, Mona B, Rastegar-Pouyani S, Blain R, Dumouchel A, et al. Phox2a defines a developmental origin of the anterolateral system in mice and humans. Cell Rep. 2020;33:108425.
Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Lüscher C, et al. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511:471–4.
Matsui A, Tran M, Yoshida AC, Kikuchi SS, Mami U, Ogawa M, et al. BTBD3 controls dendrite orientation toward active axons in mammalian neocortex. Science. 2013;342:1114–8.
Colonnese MT, Phillips MA. Thalamocortical function in developing sensory circuits. Curr Opin Neurobiol. 2018;52:72–79.
Moreno-Juan V, Filipchuk A, Antón-Bolaños N, Mezzera C, Gezelius H, Andrés B, et al. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat Commun. 2017;8:14172.
Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG, Nowakowski TJ. Development and arealization of the cerebral cortex. Neuron. 2019;103:980–1004.
Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9:eaag2882 https://doi.org/10.1126/scitranslmed.aag2882.
Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600.
Wolff JJ, Swanson MR, Elison JT, Gerig G, Pruett JR, Styner MA, et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism. 2017;8:8.
Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Prim. 2020;6:5.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44.
Paulsen B, Velasco S, Kedaigle AJ, Pigoni M, Quadrato G, Deo AJ, et al. Autism genes converge on asynchronous development of shared neuron classes. Nature. 2022;602:268–73.
Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, et al. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med. 2017;9:eaah6733 https://doi.org/10.1126/scitranslmed.aah6733.
Chen Q, Deister CA, Gao X, Guo B, Lynn-Jones T, Chen N, et al. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat Neurosci. 2020;23:520–32.
Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASDs. Cell. 2016;166:299–313.
Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell. 2019;178:867–86.e24.
Seah C, Breen MS, Rusielewicz T, Bader HN, Xu C, Hunter CJ, et al. Modeling gene × environment interactions in PTSD using human neurons reveals diagnosis-specific glucocorticoid-induced gene expression. Nat Neurosci. 2022;25:1434–45.
Hansen DV, Lui JH, Parker PRL, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–61.
Rostock C, Schrenk-Siemens K, Pohle J, Siemens J. Human vs. mouse nociceptors – similarities and differences. Neuroscience. 2018;387:13–27.
Grunwald L-M, Stock R, Haag K, Buckenmaier S, Eberle M-C, Wildgruber D, et al. Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism. Transl Psychiatry. 2019;9:179.
Russo FB, Brito A, Freitas AMde, Castanha A, Freitas BCde, Beltrão-Braga PCB. The use of iPSC technology for modeling Autism Spectrum Disorders. Neurobiol Dis. 2019;130:104483.
Adams JW, Vinokur A, de Souza JS, Austria C, Guerra BS, et al. Loss of GTF2I promotes neuronal apoptosis and synaptic reduction in human cellular models of neurodevelopment. Cell Rep. 2024;43:113867.
Wu W, Yao H, Negraes PD, Wang J, Trujillo CA, de Souza JS, et al. Neuronal hyperexcitability and ion channel dysfunction in CDKL5-deficiency patient iPSC-derived cortical organoids. Neurobiol Dis. 2022;174:105882.
Mok RSF, Zhang W, Sheikh TI, Pradeepan K, Fernandes IR, DeJong LC, et al. Wide spectrum of neuronal and network phenotypes in human stem cell-derived excitatory neurons with Rett syndrome-associated MECP2 mutations. Transl Psychiatry. 2022;12:450.
Jourdon A, Wu F, Mariani J, Capauto D, Norton S, Tomasini L, et al. Modeling idiopathic autism in forebrain organoids reveals an imbalance of excitatory cortical neuron subtypes during early neurogenesis. Nat Neurosci. 2023;26:1505–15.
Jong, de JO, Llapashtica C, Genestine M, Strauss K, Provenzano F, et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder. Nat Commun. 2021;12:4087.
Li C, Fleck JS, Martins-Costa C, Burkard TR, Themann J, Stuempflen M, et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature. 2023;621:373–80.
Rabeling A, Goolam M. Cerebral organoids as an in vitro model to study autism spectrum disorders. Gene Ther. 2023;30:659–69.
Samarasinghe RA, Miranda OA, Buth JE, Mitchell S, Ferando I, Watanabe M, et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat Neurosci. 2021;24:1488–1500.
He Z, Dony L, Fleck JS, Szałata A, Li KX, Slišković I, et al. An integrated transcriptomic cell atlas of human neural organoids. Nature. 2024;635:690–8.
Kiral FR, Cakir B, Tanaka Y, Kim J, Yang WS, Wehbe F, et al. Generation of ventralized human thalamic organoids with thalamic reticular nucleus. Cell Stem Cell. 2023;30:677–88.e5.
Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, et al. Generation of functional human 3D cortico-motor assembloids. Cell. 2020;183:1913–29.e26.
Kim J, Imaizumi K, Jurjuț O, Kelley KW, Wang D, Thete MV, et al. Human assembloid model of the ascending neural sensory pathway. Nature. 2025;642:1–11.
Chiaradia I, Imaz-Rosshandler I, Nilges BS, Boulanger J, Pellegrini L, Das R, et al. Tissue morphology influences the temporal program of human brain organoid development. Cell Stem Cell. 2023;30:1351–67.e10.
Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610:319–26.
Chiola S, Napan KL, Wang Y, Lazarenko RM, Armstrong CJ, Cui J, et al. Defective AMPA-mediated synaptic transmission and morphology in human neurons with hemizygous SHANK3 deletion engrafted in mouse prefrontal cortex. Mol Psychiatry. 2021;26:4670–86.
Mao W, Watanabe T, Cho S, Frost JL, Truong T, Zhao X, et al. Shank1 regulates excitatory synaptic transmission in mouse hippocampal parvalbumin-expressing inhibitory interneurons. Eur J Neurosci. 2015;41:1025–35.
Qin Y, Du Y, Chen L, Liu Y, Xu W, Liu Y, et al. A recurrent SHANK1 mutation implicated in autism spectrum disorder causes autistic-like core behaviors in mice via downregulation of mGluR1-IP3R1-calcium signaling. Mol Psychiatry. 2022;27:2985–98.
Won H, Lee H-R, Gee HY, Mah W, Kim J-I, Lee J, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5.
Grabrucker S, Pagano J, Schweizer J, Urrutia-Ruiz C, Schön M, Thome K, et al. Activation of the medial preoptic area (MPOA) ameliorates loss of maternal behavior in a Shank2 mouse model for autism. EMBO J. 2021;40:EMBJ2019104267.
Speed HE, Kouser M, Xuan Z, Reimers JM, Ochoa CF, Gupta N, et al. Autism-associated insertion mutation (InsG) of Shank3 exon 21 causes impaired synaptic transmission and behavioral deficits. J Neurosci. 2015;35:9648–65.
Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, et al. Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Rep. 2015;11:1400–13.
Kim S, Kim Y-E, Song I, Ujihara Y, Kim N, Jiang Y-H, et al. Neural circuit pathology driven by Shank3 mutation disrupts social behaviors. Cell Rep. 2022;39:110906.
Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–29.
Lv D, Liu A, Yi Z, Mu M, Wu M, Li X, et al. Neuroligin 1 regulates autistic-like repetitive behavior through modulating the activity of striatal D2 receptor-expressing medium spiny neurons. Adv Sci. 2025;12:2410728.
Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–32.
Lai ESK, Nakayama H, Miyazaki T, Nakazawa T, Tabuchi K, Hashimoto K, et al. An autism-associated neuroligin-3 mutation affects developmental synapse elimination in the cerebellum. Front Neural Circuits. 2021;15:676891.
Ozkan ED, Creson TK, Kramár EA, Rojas C, Seese RR, Babyan AH, et al. Reduced cognition in Syngap1 mutants is caused by isolated damage within developing forebrain excitatory neurons. Neuron. 2014;82:1317–33.
Haetzel LM, Iafrati J, Cording KR, Farhan M, Noveir SD, Rumbaugh G, et al. Haploinsufficiency of Syngap1 in striatal indirect pathway neurons alters motor and goal-directed behaviors in mice. J Neurosci. 2024;44:e1264232024.
Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–90.
Pizzamiglio L, Capitano F, Rusina E, Fossati G, Menna E, Léna I, et al. Neurodevelopmental defects in Dravet syndrome Scn1a +/− mice: Targeting GABA-switch rescues behavioral dysfunctions but not seizures and mortality. Neurobiol Dis. 2025;207:106853.
Jurgensen S, Castillo PE. Selective dysregulation of hippocampal inhibition in the mouse lacking autism candidate gene CNTNAP2. J Neurosci. 2015;35:14681–7.
Gandhi T, Canepa CR, Adeyelu TT, Adeniyi PA, Lee CC. Neuroanatomical alterations in the CNTNAP2 mouse model of autism spectrum disorder. Brain Sci. 2023;13:891.
Jang S-S, Takahashi F, Huguenard JR. Reticular thalamic hyperexcitability drives autism spectrum disorder behaviors in the Cntnap2 model of autism. Sci Adv. 2025;11:eadw4682.
Karayannis T, Au E, Patel JC, Kruglikov I, Markx S, Delorme R, et al. Cntnap4 differentially contributes to GABAergic and dopaminergic synaptic transmission. Nature. 2014;511:236–40.
Zhang W, Huang J, Gao F, You Q, Ding L, Gong J, et al. Lactobacillus reuteri normalizes altered fear memory in male Cntnap4 knockout mice. EBioMedicine. 2022;86:104323.
Huang T-N, Chuang H-C, Chou W-H, Chen C-Y, Wang H-F, Chou S-J, et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–7.
Hsu T-T, Huang T-N, Wang C-Y, Hsueh Y-P. Deep brain stimulation of the Tbr1-deficient mouse model of autism spectrum disorder at the basolateral amygdala alters amygdalar connectivity, whole-brain synchronization, and social behaviors. PLoS Biol. 2024;22:e3002646.
Bacon C, Schneider M, Magueresse CL, Froehlich H, Sticht C, Gluch C, et al. Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol Psychiatry. 2015;20:632–9.
Li X, Hao S, Zou S, Tu X, Kong W, Jiang T, et al. Cortex-restricted deletion of Foxp1 impairs barrel formation and induces aberrant tactile responses in a mouse model of autism. Mol Autism. 2023;14:34.
Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–7.
Hooshmandi M, Truong VT, Fields E, Thomas RE, Wong C, Sharma V, et al. 4E-BP2-dependent translation in cerebellar Purkinje cells controls spatial memory but not autism-like behaviors. Cell Rep. 2021;35:109036.
Ash RT, Palagina G, Fernandez-Leon JA, Park J, Seilheimer R, Lee S, et al. Increased reliability of visually-evoked activity in area V1 of the MECP2-duplication mouse model of autism. J Neurosci. 2022;42:6469–82.
Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68.
Monday HR, Nieto AM, Yohannes SA, Luxu S, Wong KW, Bolio FE et al. Physiological and molecular impairment of PV circuit homeostasis in mouse models of autism. bioRxiv 2025; 2025.01.08.632056.
Vogt D, Cho KKA, Lee AT, Sohal VS, Rubenstein JLR. The parvalbumin/somatostatin ratio is increased in pten mutant mice and by human PTEN ASD alleles. Cell Rep. 2015;11:944–56.
Shin S, Santi A, Huang S. Conditional pten knockout in parvalbumin- or somatostatin-positive neurons sufficiently leads to autism-related behavioral phenotypes. Mol Brain. 2021;14:24.
Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162:375–90.
Wang Y, Chiola S, Yang G, Russell C, Armstrong CJ, Wu Y, et al. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. Nat Commun. 2022;13:5688.
Villa CE, Cheroni C, Dotter CP, López-Tóbon A, Oliveira B, Sacco R, et al. CHD8 haploinsufficiency links autism to transient alterations in excitatory and inhibitory trajectories. Cell Rep. 2022;39:110615.
Courchesne E, Taluja V, Nazari S, Aamodt CM, Pierce K, Duan K, et al. Embryonic origin of two ASD subtypes of social symptom severity: the larger the brain cortical organoid size, the more severe the social symptoms. Mol Autism. 2024;15:22.
Papes, Camargo F, Souza AP, de JS, Carvalho VMA, Szeto RA, et al. Transcription Factor 4 loss-of-function is associated with deficits in progenitor proliferation and cortical neuron content. Nat Commun. 2022;13:2387.
Meng X, Yao D, Imaizumi K, Chen X, Kelley KW, Reis N, et al. Assembloid CRISPR screens reveal impact of disease genes in human neurodevelopment. Nature. 2023;622:359–66.
Miura Y, Kim J, Jurjuț O, Kelley KW, Yang X, Chen X et al. Assembloid model to study loop circuits of the human nervous system. bioRxiv 2024; 2024.10.13.617729.
Tasnim A, Alkislar I, Hakim R, Turecek J, Abdelaziz A, Orefice LL, et al. The developmental timing of spinal touch processing alterations predicts behavioral changes in genetic mouse models of autism spectrum disorders. Nat Neurosci. 2024;27:484–96.
Wood ET, Cummings KK, Jung J, Patterson G, Okada N, Guo J, et al. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl Psychiatry. 2021;11:39.
Wagner L, Banchik M, Okada NJ, McDonald N, Jeste SS, Bookheimer SY, et al. Associations between thalamocortical functional connectivity and sensory over-responsivity in infants at high likelihood for ASD. Cereb Cortex. 2023;33:8075–86.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76.
Ogawa T, Yamada S, Fukushi S, Imai Y, Kawada J, Ikeda K, et al. Formation and Long-Term Culture of hiPSC-Derived Sensory Nerve Organoids Using Microfluidic Devices. Bioengineering. 2024;11:794.
Inglis GAS. Charting the development of human dorsal root ganglia. Nat Neurosci. 2025;28:217–217.
Lee J-H, Shin H, Shaker MR, Kim HJ, Park S-H, Kim JH, et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat Biomed Eng. 2022;6:435–48.
Seo K, Cho S, Shin H, Shin A, Lee J, Kim JH, et al. Symmetry breaking of human pluripotent stem cells (hPSCs) in micropattern generates a polarized spinal cord-like organoid (pSCO) with dorsoventral organization. Adv Sci. 2023;10:2301787.
Fitzgerald MQ, Chu T, Puppo F, Blanch R, Chillón M, Subramaniam S, et al. Generation of ‘semi-guided’ cortical organoids with complex neural oscillations. Nat Protoc. 2024;19:2712–38.
Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim K-Y, Sun P, et al. hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 2019;24:487–97.e7.
Patton MH, Thomas KT, Bayazitov IT, Newman KD, Kurtz NB, Robinson CG, et al. Synaptic plasticity in human thalamocortical assembloids. Cell Rep. 2024;43:114503.
Xiang Y, Cakir B, Park I-H. Generation of regionally specified human brain organoids resembling thalamus development. STAR Protoc. 2020;1:100001.
Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 2019;25:558–69.e7.
Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–8.
Nevalainen P, Lauronen L, Pihko E. Development of human somatosensory cortical functions – what have we learned from magnetoencephalography: a review. Front Hum Neurosci. 2014;8:158.
Wang L, Ma L, Yang J, Wu J. Human somatosensory processing and artificial somatosensation. Cyborg Bionic Syst. 2021;2021:1–11.
Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: boulder committee revisited. Nat Rev Neurosci. 2008;9:110–22.
Lu T, Wang M, Zhou W, Ni Q, Yue Y, Wang W, et al. Decoding transcriptional identity in developing human sensory neurons and organoid modeling. Cell. 2024;187:7374–93.e28.
Acknowledgements
This work was supported by the National Research Foundation (NRF) grants funded by the Ministry of Science and ICT (MSIT), South Korea (grant number: RS-2023-00266171 to YO, YJK, and JS, and RS-2025-00554061 to JS). All schematics were created using BioRender with a full license to publish.
Author information
Authors and Affiliations
Contributions
Taeyeon Kim, Juwon Lee, and Jinsoo Seo conceived the idea of this review. Taeyeon Kim, Juwon Lee, Jiye Lee, Hyeokjin Jo, and Jinsoo Seo wrote the manuscript. Yohan Oh and Yong Jun Kim provided scientific insights and reviewed the manuscript. Taeyeon Kim and Juwon Lee worked on the figures and tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, T., Lee, J., Lee, J. et al. Sensory abnormalities in autism spectrum disorder and their in vitro modeling. Transl Psychiatry 15, 534 (2025). https://doi.org/10.1038/s41398-025-03778-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03778-6