Abstract
Neural oscillations have emerged as critical markers of cognitive and emotional states, offering valuable insights into psychiatric disorders. Given the potential suicide risk in patients with major depressive disorders (MDD), its underlying neurophysiological associations need to be further elucidated. This review aimed to comprehensively examine the complex relationships between neural oscillations and suicide risk in MDD. We performed a detailed analysis of electrophysiological phenomena reported in studies investigating suicide risk within depressive populations, consisting of event-related potentials (ERPs) and neuronal oscillations across theta, delta, alpha, beta, and gamma frequency bands. Notably, reduced P300 amplitude, associated with cognitive dysfunction, was observed with elevated theta and delta activity in brain regions implicated in emotional processing, correlating with heightened susceptibility to suicidal behavior. Altered alpha and beta oscillations were associated with emotional dysregulation and cognitive deficits. Importantly, gamma oscillations exhibited increased activity in individuals with suicidal tendencies, reflecting disruptions in the balance between excitatory and inhibitory neuronal circuits. Despite these findings, current research was limited by heterogeneity among study populations, small sample sizes, challenges in establishing causality, and an incomplete understanding of the biological associations underpinning these oscillations. Future research should focus on integrating multi-dimensional oscillatory features to improve individual risk prediction, employing longitudinal designs to track dynamic changes over time, and developing targeted interventions. Addressing these challenges will be critical for advancing reliable biomarkers and innovative strategies for suicide prevention in depression.
Similar content being viewed by others
Introduction
Suicide claims the lives of over 700,000 individuals worldwide annually, affecting populations across all income levels and geographic regions. Notably, approximately 90% of individuals who die by suicide have a diagnosed psychiatric disorder, with depression being the most prevalent, accounting for nearly two-thirds of these case [1,2,3]. The global burden of depressive disorders has increased significantly due to their high prevalence and substantial impact on daily functioning, which in turn elevates the risk of suicidal behavior [4,5,6]. Therefore, developing an efficient way to predict suicide risk among individuals with depression has become a critical public health goal.
Neuroimaging techniques have been extensively employed to identify potential biomarkers for suicide prediction [7,8,9]. These approaches facilitate the detection of structural and functional brain alterations associated with suicidal behavior [10,11,12]. Structural magnetic resonance imaging (sMRI) studies have consistently reported changes in key regions such as the prefrontal cortex and limbic system, including reduced gray matter volume and increased white matter hyperintensities—areas crucial for emotion regulation, decision-making, and impulse control [13,14,15,16,17]. Functional MRI (fMRI) studies have further indicated dysregulation in emotional processing, characterized by decreased activity in prefrontal regions and heightened activity within limbic circuits [18, 19]. These findings advance our understanding of the neural substrates underlying suicidal tendencies and underscore the importance of exploring brain connectivity in high-risk populations.
While sMRI and fMRI have advanced our understanding of the neuroanatomical and functional brain alterations associated with suicidal risk, these methods primarily capture static or slow-changing brain changes and are limited in reflecting the rapid neural dynamics involved in cognition and emotion regulation. To gain insight into these quick, real-time neural processes—particularly those related to impulsivity, emotional processing, and decision-making, researchers have increasingly turned to electrophysiological approaches like electroencephalography (EEG) and magnetoencephalography (MEG). These techniques provide high temporal resolution recordings of brain activity, enabling the investigation of neuronal oscillations that fluctuate on the millisecond scale [20,21,22]. Specifically, EEG offers excellent temporal resolution, allowing for the observation of rapid neuronal oscillations, which are rhythmic brain activities essential for cognition and behavior. MEG, while also providing high temporal resolution, excels in spatial resolution, enabling more precise localization of brain activity. Modifications in neural oscillations, especially in gamma and beta-gamma coupling, are connected to depressive symptoms in medication-naive individuals [23]. Disruptions in gamma oscillations are specifically pertinent to depression, and restoring these oscillations is suggested as a feasible intervention [24]. Furthermore, oscillatory activity can aid in differentiating conditions such as stress, anxiety, and depression [25,26,27]. Emerging evidence suggests that modulating oscillatory task can enhance depressive symptoms in both animals and human subjects [28]. Although the relationship between neuronal oscillations and anxiety is well established, further research is necessary to elucidate how oscillatory dynamics relate specifically to suicide risk and recovery processes.
In terms of suicide risk, both EEG and MEG can provide real-time insights into brain states [29], potentially revealing specific oscillatory patterns associated with suicidal thoughts and behaviors. Although this area of research is still developing, the potential to discover effective strategies for risk detection and intervention is promising. Understanding these oscillatory dynamics can deepen our comprehension of the neurobiological factors underlying suicidal actions, thereby enhancing our ability to predict and prevent suicide. Integrating neuroimaging and electrophysiological data can provide a more comprehensive perspective on the complex neural processes involved in suicidal behavior. However, the role of neuronal oscillations in suicide risks remains underexplored, despite their critical involvement in cognition and emotional regulation [30]. Aberrant neuronal oscillations, typically seen in mental health conditions like anxiety and schizophrenia [31], may also play a significant role in suicidal behavior. Techniques like EEG or MEG discovering atypical oscillatory activity in the brain, are essential tools for identifying these electrophysiological indicators. Current studies have introduced potential links between aberrant neuronal oscillations and suicide risk in depressive disorders. Specifically, increased theta activity in the midfrontal cortex at rest has been related to changes in cognitive control, which might exacerbate depressive symptoms and improve suicidal ideation (SI) [32,33,34,35,36]. Furthermore, reduced oscillations in the alpha and beta bands, which indicate brain interaction, and decreased posterior alpha oscillations, which predict the seriousness of anxiety, have been reported [37,38,39]. Significantly, gamma oscillations are connected to emotional facial expressions and mood-related biases in clients with major depressive disorder (MDD) [40, 41]. Previous studies also suggest that gamma activity in response to negative emotional stimuli can help distinguish between different psychiatric conditions, predict responses to antidepressant treatment, and potentially serve as an indicator of suicide risk [42,43,44,45].
Regardless of the comprehensive body of literature on neuroimaging findings associated with suicide in depression, there is still a notable gap concerning oscillatory dysfunctions specifically associated with suicide risk in clinical depression. This review aims to address this gap by examining the role of neuronal oscillations in depression and their potential connection to suicidal behavior. By completely reviewing the offered literature, identifying knowledge gaps, and proposal of electrophysiological markers, we seek to enhance understanding of the neurophysiological associations underlying suicide risk in depressive disorders.
Methods
Eligibility criteria
This narrative review aimed to explore the link between neuronal oscillations and suicide risk in depression. Studies included must focus on participants diagnosed with depression, either unipolar or bipolar, and assess suicide risk or suicidal behavior in the context of depression. Studies focusing on other psychiatric or neurological disorders were excluded unless they provide relevant comparisons to depressive disorders. The review specifically considered studies that examine neuronal oscillations or related electrophysiological characteristics, with a preference for those investigating specific oscillatory frequencies (e.g., alpha, beta, gamma) associated with depression and suicide risk. Only studies that directly related to electrophysiological findings to suicide risk, SI, suicide plan and suicidal behavior (or suicide attempt [SA]) are included. No limits were used on subjects’ demographic information. Only peer-reviewed publications in English and Chinese were included in the analysis.
Search strategy
We used a non‑systematic search strategy appropriate for this narrative review. Primary databases included PubMed/MEDLINE, Web of Science, Embase, PsycINFO, and CNKI. Searches covered records available up to August 2024. The search terms included “suicide,” “depression,” “MDD” (Major Depressive Disorder, Bipolar Disorders), “EEG” (Electroencephalography), “MEG” (Magnetoencephalography), “oscillation,” and “frequency.” These terms were combined using basic operators like this: (“suicide” OR “depression” OR “MDD” OR “suicidal”) AND (“EEG” OR “MEG”) OR (“oscillation” OR “frequency”). We carefully checked the results for relevance and duplicates to gather a complete set of literature for the study. We also did a manual search of the reference lists of the included articles and relevant reviews.
Results
A total of 27 studies were included in the current narrative review. Detailed demographics of the selected literatures (Supplementary Table 1) and the flowchart (Supplementary Fig. 1) outlining the literature selection process are provided in the supplementary materials.
Probing suicide risk through event-related potentials (ERPs) components in depression
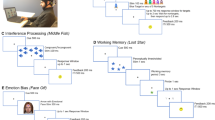
ERP studies have increasingly been utilized to investigate various aspects of suicide risk and behavior in individuals with MDD. For instance, an auditory oddball task provided insights into the potential role of P300 ERP in assessing suicide risk among patients with MDD (Fig. 1a) [46]. It was proposed that P300 habituation rates might serve as valuable predictors for suicidal tendencies. Further studies revealed differences in N200 and P300 amplitudes between suicide attempters and non-attempters during implicit emotional processing tasks [47, 48]. Using a monetary incentive delay task, evidence suggested that a blunted cue-P300 ERP might be correlated with suicidal behavior (Fig. 1b) [49]. Another investigation examined neural activity during no-go trials, which are related to inhibitory control, and found that suicide attempters with depression exhibited significantly reduced no-go P300 amplitudes. This suggests that no-go P300 may be associated with SAs in patients experiencing SI [50]. Another study employing a self-reference affect incentive task that elicited the contingent negative variation demonstrated that multimodal ERP features achieved significantly higher accuracy for classifying SI, emphasizing the importance of these EEG components in suicide prediction and prevention strategies [51]. Recent research identified electrophysiological indicators, specifically pre-treatment auditory ERPs, that predict responses to low-dose oral ketamine in individuals with depression and chronic suicidality. The predictive model indicates that serotonergic and GABAergic systems may play a significant role in determining treatment outcomes [52]. Reduced ERP amplitudes in MDD patients at moderate and high suicide risk may correlate with overall suicide risk scores [53]. In summary, these studies suggest that ERP components, particularly P300, provide important insights into the brain processes related to suicidality. Nonetheless, it is crucial to recognize that these observations are mainly correlational in nature, and definitive evidence of causality has not yet been demonstrated.
Linking low-frequency oscillations to suicidal behavior in depressive disorders
In terms of low-frequency oscillations, resting-state quantitative EEG has assessed brain markers for emotional control and depression severity. Findings showed higher theta and alpha activity in patients with MDD, suggesting a potential correlation between these frequencies and suicidality (Fig. 1c) [54]. Additionally, polysomnography study examined delta activity during sleep and found that it was reduced in MDD patients with high SI, independent of their overall depression severity. This suggests that decreased sleep-related delta activity could serve as a potential risk factor for SI [55]. The effect of accelerated intermittent theta burst stimulation (iTBS) on suicide risk was also investigated in patients with depression [56, 57], which was shown to reduce suicidal thoughts in depressive adolescents with SAs [35]. Moreover, resting-state EEG collected shortly after a SA showed a positive link between frontal theta and delta power and the duration of the suicidal process [58]. MEG studies examined the association between theta power and SI in response to emotional stimuli. It was found that variations in right-hemisphere theta power in response to negative emotional stimuli can differentiate between suicide attempters and non-attempters, underscoring the significance of theta activity in emotional processing and suicidal ideation [59]. Increased theta power in the posterior part of the default mode network (DMN) in suicide attempters was also found. This suggests a link between impairments in self-referential thinking reflected by posterior DMN theta activity and the occurrence of SAs [60].
Exploring the association between alpha and beta rhythms and suicide risk in depression
The study of alpha and beta oscillations as possible indicators of suicidality in depressive disorders has been explored more in recent literature. Frontal Alpha Asymmetry (FAA) is linked to SI in people with MDD. Findings show that depressed patients with suicidal thoughts have higher left frontal alpha power, indicating left-skewed brain activity [61]. This study suggests that SI may correlate with FAA, making it an important factor and possible indicator for understanding suicide risk in depressed individuals. In a focused study of depressed female adolescents with a history of non-suicidal self-injuries (NSSI) and SAs, clear differences in alpha power and EEG coherence were found between the NSSI and SA groups. These findings suggest that increased activity in the left hemisphere might be related to higher suicide risk [62]. Further research looked at the relationship between alpha-beta oscillations and brain function related to suicide risk. It revealed specific changes in alpha and beta power in the ventral prefrontal cortex (VPFC) and dorsal anterior cingulate cortex (dACC) in those with a history of SAs during a GO/NOGO task (Fig. 1d). This study found that alpha-beta decoupling may be related to levels of suicide risk. It indicated that alpha-beta decoupling could be considered as another electrophysiological marker for identifying suicide risk [63]. The ongoing research in this area shows the importance of alpha and beta oscillations in understanding and assessing suicidality in people with depressive disorders.
Unraveling the impact of gamma oscillations on suicidal tendencies in depression
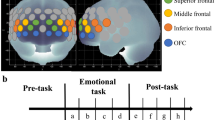
As shown in Table 1, it highlighted the correlation between gamma oscillations and suicidality in depression during both resting state and task state (Fig. 2a). Quantitative EEG analysis has found higher high-gamma power in patients with SI. This suggests that increased gamma activity may relate to suicide risk [43]. Using resting-state MEG, a study with ketamine assessed resting-state gamma power in the frontal and parietal cortices. It revealed connections between SI and gamma activity. MEG studies have also found links between gamma oscillations and SAs, proposing these oscillations as possible indicators for brain health and psychiatric conditions [59]. These findings suggest that gamma oscillations could be useful for evaluating treatment responses, especially with ketamine (Fig. 2b) [44]. A decrease in alpha-band to cortical gamma-band coupling between the right caudate and left thalamus was seen in individuals with MDD at high suicide risk. This further supports the idea that gamma oscillations are indicators of suicidality. This reduced phase amplitude coupling may show problems in brain communication that are important for emotional control [64].
a the potential correlation between resting-state and task-state gamma oscillations and suicide risk. b resting-state gamma oscillations in the fronto-parietal network are associated with suicide risk, and ketamine treatment may promote clinical improvement. c impaired gamma-band functional connectivity between the DMN, FPN, VIS, and SN is associated with the transition from suicidal ideation to behavior in depression. d heightened gamma oscillations induced by the emotional stimuli associated with suicide attempt in depression at both sensors and source space. e E/I imbalance reflected by impaired gamma oscillations contributes to elevated suicide risk in depression.
During emotional expressions recognition task, further investigation identified dysregulated gamma-band functional connectivity patterns in the SA group. Specifically, gamma connectivity within the fronto-parietal network and DMN may be crucial for understanding the progression from SI to SAs (Fig. 2c) [42]. The subsequent analyses have identified that abnormal dynamic spatial temporal features (such as fractional occupancy) of brain states in the low-gamma were related to suicide risk [65]. Notably, recent research has further revealed that that increased non-phase-locked gamma oscillations (50–70 Hz) in the occipital regions, which were induced by the emotional stimuli, could serve as potential indicators for identifying SAs in individuals with depression (Fig. 2d) [66]. Moreover, the induced visual gamma oscillations might be associated with widespread neurocognitive deficits [66]. Overall, these studies indicate that elevated gamma oscillations are associated with high suicidality in depressed patients during both resting states and emotional stimuli tasks. However, these findings are primarily based on correlational evidence, and causal relationships have yet to be established. Further research with causal evidence is necessary to fully understand their implications.
Discussion
The role of P300 in cognitive disruptions linked with suicide risk in depression
Monitoring of electrophysiological phenomena, specifically the ERPs, have emphasized the importance of the P300 component, which is an index intrinsically related to cognitive processing, attention allocation, and the alteration of working memory [67,68,69]. A decreased amplitude of the P300 component could represent disruptions in the neural pathways that oversee reward processing and attention, thereby magnifying feelings of despair and anhedonia, which are both robust predictors of SI amongst people in depression [70,71,72]. Reduced P300 amplitude may imply deficits in rapid information processing, potentially enhancing sentiments of insignificance and magnifying perceived burdensomeness. Furthermore, depressive disorders are often associated with reduced serotonergic activity, which can impact the P300 component [73,74,75]. This correlation is particularly significant considering that aberrations in serotonin are linked with impulsivity and increased suicide risks [76]. However, it is essential to note that while a reduced P300 amplitude may be associated with cognitive dysfunction, the evidence does not establish a direct causal relationship with suicidal behavior. While the P300 component shows promise as an indicator for neurocognitive vulnerabilities in individuals at risk for SI, its predictive value for suicidal behavior remains to be explored.
Low-frequency oscillations of subconscious processing and vulnerabilities in suicide risk
Low-frequency oscillations, like theta and delta bands, are usually linked to relaxation, deep sleep, and subconscious thinking [77,78,79]. Delta oscillations help with healing and regeneration, while theta oscillations relate to creativity and intuition [79,80,81]. Recent studies found higher theta power during emotional processing, especially in response to negative stimuli, in important brain areas like the amygdala and hippocampus in individuals with depression who had SAs [59]. This matches earlier research that suggests problems in theta activity may correlate with cognitive and mood issues, including depression and anxiety [82]. Additionally, there is evidence that frontal theta asymmetry could be a sign for increasing SI [54].
Delta oscillations, which are usually linked to deep sleep and cognitive control, also show higher power in those with past SAs [58]. Moreover, an interesting alpha-delta sleep pattern has been seen in patients with high SI. This pattern connects to sleep problems, especially in REM sleep, and the severity of depression [55, 83]. Resting-state MEG data also shows increased theta activity in the posterior DMN in depressed patients with SAs. This highlights the role of theta oscillations in self-reflection and self-referential thinking, which may add to suicide risk [60].
Alpha and beta oscillations as indicators for cognitive and emotional dysregulation in suicide risk
Recent studies revealed the link between alpha and beta oscillations and suicidal tendencies in depressive disorders. Different brain patterns in adolescents with depression based on their history of SAs were found [62]. Furthermore, increased left frontal alpha power correlated with depressive patients with SI compared to those without SI [61]. A phase connection has been identified between alpha/beta power and deficits in the inhibitory function, with alpha-beta decoupling observed in suicide attempters [63]. Alpha oscillations are related to cognitive load, attentional processes, and neural network synchronization. Abnormal alpha oscillations may suggest altered cognitive states or psychological dysregulation frequently seen in depressive conditions [82]. Beta oscillations are linked to analytical thought and alert states and play roles in emotion processing and regulation. Increased alpha oscillations, particularly in the left hemisphere, may be related to impaired emotion regulation, a precursor to SI and SA [84]. These oscillations not only represent cognitive and emotional states but also serve as potential signs for assessing and monitoring suicide risks in depressed patients.
Gamma oscillations as indicators of excitatory-inhibitory imbalance and their role in suicide risk in depression
Several studies have underscored the potential significance of gamma oscillations as indicators for suicide in depression. Elevated high-gamma power was correlated with dysfunction of specific brain regions in patients with SI compared to other groups [43]. This aligns with findings that emphasize gamma power as a marker for synaptic homeostasis in psychiatric disorders [44, 59]. Additionally, disrupted gamma oscillations were linked to SAs in MDD, particularly during emotional stimuli, revealing abnormal interactions among various neural networks [65].
Gamma oscillations are closely tied to the excitatory-inhibitory (E/I) balance, which is essential for information processing and neural plasticity, with gamma acting as a reflection of this balance (Fig. 2e) [85]. The abnormal gamma oscillations in depressed patients with SAs were associated with an underlying E/I imbalance within the cortical networks. Moreover, E/I imbalance may be correlated with emotional dysregulation, stress response dysfunction, and cognitive impairments, all of which can culminate in an increased risk for suicide [86]. Psychological dysregulation and abnormal anxiety feedback are characteristics of both clinical depression and suicidality, and the E/I imbalance might exacerbate these dysfunctions by impairing the brain’s ability to manage emotions and procedure stress properly [87,88,89]. Cognitive deficits, commonly associated with disrupted gamma oscillations, could further contribute to impaired decision-making and increased impulsivity, factors that are recognized to elevate suicide risk [90,91,92].
An imbalanced E/I might manifest as altered gamma oscillation patterns, therefore associating with increased suicide risk. The E/I balance is essential for the modulation of gamma power and frequency. This balance can be influenced by various external factors, such as stimulus characteristics, as well as intrinsic brain properties, including GABA levels and cortical volume [93, 94]. In the context of suicide risk, the E/I imbalance could correlate with excessive excitation or impaired inhibition, which interferes with the synchronous gamma oscillations, potentially magnifying neurocognitive dysfunctions connected to suicide risk. This concept is further supported by findings that indicate E/I imbalances in the anterior insula are associated with suicide risk, suggesting that the neural associations underlying suicidality may differ from those of depression [44]. The interplay between E/I imbalance and gamma oscillations highlights the importance of targeting inhibitory systems to deepen our understanding of the neurobiological foundations of suicidality in depression. Notably, it is important to note that these findings are primarily correlational, and causal evidence is still lacking, highlighting the need for further research to clarify these associations.
Utilizing neuronal oscillations for suicide prevention
The examination of neuronal oscillations as indicators for suicide risk in patients with MDD offers an appealing strategy for enhancing risk assessment and intervention. Stable neuronal oscillations have been identified as vital signs for distinguishing individuals at enhanced danger of suicide, giving valuable insights into their neurophysiological accounts (Fig. 3a). This identification is vital, as it permits earlier detection of at-risk people, which could lead to interventions that may alter the trajectory of their condition [95].
MEG’s exceptional combination of high spatial and temporal resolution enables precise localization of brain activity, making it effective for identifying potential indicators related to suicide risk, including specific gamma oscillation patterns. In contrast, EEG offers excellent temporal resolution and is more accessible, allowing for implementation across a broader range of clinical populations. This accessibility may facilitate wider application in clinical settings, positioning EEG as a valuable tool for ongoing research and potential interventions in suicidality. The complementary strengths of EEG and MEG underscore the importance of utilizing both methods to deepen our understanding of the neurobiological associations underlying suicidal behaviors.
By employing the normalized pipeline for analyzing neuronal oscillatory data, alongside artificial intelligence models, psychiatrists can effectively categorize clients into distinct risk categories based on these oscillatory patterns (Fig. 3b). Parameters for these models can be fine-tuned utilizing methods like cross-validation, making certain that the model attains optimal accuracy and minimizes overfitting to discriminate patients with possible high suicide risk precisely [96]. Such an approach promotes customized risk assessments, permitting targeted therapeutic interventions customized to individual needs and certain oscillatory dysfunctions.
Additionally, neuromodulation techniques, such as transcranial magnetic stimulation (TMS), neurofeedback and so forth, can be utilized to especially target neuronal oscillations, particularly in the gamma frequency band (Fig. 3c). To set criteria for these neuromodulations, professionals need to take into consideration the person’s baseline oscillatory activity, treatment frequency, and stimulation intensity [97]. This targeted modulation can boost cognitive processes, improve psychological regulation, reduce impulsivity, and promote sound decision-making [98]. It is essential to monitor patients’ responses to these interventions to adjust parameters in real-time, maximizing therapeutic efficacy. Notably, although some studies have utilized gamma neuromodulation techniques in patients with MDD to alleviate depressive symptoms [99,100,101], research specifically targeting suicide risk remains in the early stages, and robust evidence from large-scale clinical trials is currently lacking. While preliminary findings indicate potential therapeutic benefits of neuromodulation in this context, further rigorous clinical investigations are essential to establish its efficacy and safety, thereby enabling its validation as a standard clinical intervention.
Ultimately, these strategies aim to contribute to a significant reduction in suicide risk among patients. By integrating these advanced techniques into clinical practice, we can create a multifaceted treatment paradigm that not only identifies risk but actively engages with the underlying neurophysiological associations. This integrated approach positions neuronal oscillations not only as biomarkers but also as therapeutic targets for individuals at risk of suicide (Fig. 3d), fostering a more proactive and effective mental health care system.
Limitations
This review presents several challenges that warrant acknowledgment (Fig. 4a). Firstly, the encompassed studies exhibit significant heterogeneity in populations, which, given the variations in gender, races, and ages, could introduce notable variability in biomarker identification, potentially affecting the generalizability of findings. Secondly, the biological underpinnings of the observed aberrant oscillations remain ambiguously defined, even in the face of increasing multidimensional research literature. Furthermore, the complexity of suicidal behavior, being influenced by an array of social, psychological, and biological determinants, makes it challenging to pinpoint aberrant neuronal oscillations as a singular, definitive biomarker. Thirdly, the reliance of a significant portion of our cited literature on small sample sizes raises concerns about the robustness of neuroimaging findings. It underscores the urgent need for larger, more definitive studies in subsequent research.
Identifying stable oscillatory biomarkers for suicide risk assessment should account for population heterogeneity and investigate the underlying biological associations of abnormal neuronal oscillations. Integrating multimodal neuroimaging and multi-frequency oscillatory data can aid in developing individualized suicide risk assessment systems. Employing multi-strategies neuromodulation technologies to regulate neuronal oscillations to reduce suicide risk level. Collection of longitudinal oscillatory data to establish more reliable biomarkers for early identification and intervention.
Lastly, it is important to note that the cross-sectional designs of many studies included in this review preclude definitive causal conclusions. While these studies provide valuable insights into associations between variables, the lack of longitudinal data limits our ability to infer causality. Without the capacity to observe changes over time or to establish temporal precedence, we cannot ascertain whether aberrant neuronal oscillations are a cause or a consequence of suicidal behavior. This limitation highlights the need for future research utilizing longitudinal or experimental designs to clarify these relationships and strengthen causal inferences.
Future directions
Several promising directions are deserved to be explored in the future (Fig. 4b). A key area involves the precise prediction of individual-level suicide risks by integrating multi-dimensional oscillatory features. By synthesizing insights from ERP and various frequency bands such as theta, delta, alpha, beta, and gamma, we can achieve a more nuanced understanding of individualized suicide risk profile. Additionally, using multi-strategy neuromodulation techniques could effectively target specific frequency bands in patients with high suicide risk. This approach intends to improve psychological regulation and cognitive performance, eventually adding to a decrease in their suicide risk levels. By tailoring neuromodulation procedures to the unique oscillatory patterns of each patient, therapeutic outcomes can be enhanced. Moreover, to develop more steady and reputable neuronal biomarkers for identifying the progression of suicide risk, it is crucial to collect electrophysiological data from patients over a longitudinal timeframe. A longitudinal approach enables the monitoring of modifications in oscillatory patterns and their relationship with change in suicide risk, providing deeper understanding into the characteristics of suicidality. As the field progresses, these avenues will collectively advance our understanding of suicidality in depression and correlate with more effective early identification and intervention techniques.
Considerations regarding specificity and diagnose relevance
It is important to recognize that abnormalities in gamma oscillations are observed across a range of psychiatric disorders, including schizophrenia, bipolar disorder, and depression with suicidal tendencies. The shared genetic, neurobiological, and symptomatic features among these conditions complicate the interpretation of gamma oscillation data [45, 102]. The overlapping biological substrates suggest that gamma abnormalities are not inherently specific to any single disorder but may reflect common neurobiological alterations associated with various psychopathologies. Consequently, attributing gamma oscillation changes solely to suicidality within MDD remains challenging.
However, emerging research indicated heterogeneity in gamma oscillation patterns across different studies. For example, some investigations have demonstrated that gamma oscillations can effectively differentiate depression, bipolar disorder, and healthy controls under specific experimental conditions [103, 104]. Furthermore, the elicitation of gamma oscillations varies depending on the disorder and context: in schizophrenia, gamma abnormalities are often induced by sensory stimuli such as auditory or visual gratings, which reflect deficits in sensory processing [27, 105], while gamma oscillations associated with suicidal risk tend to be more closely linked to negative emotional processing [65, 66]. These differences suggest that gamma oscillation abnormalities may originate from distinct neurophysiological associations across different diseases or symptom domains.
Additionally, increased gamma activity may not necessarily serve as a direct biomarker of suicidality but could represent a compensatory or adaptive response to emotional stimuli. While gamma oscillations provide valuable insights into the neurobiological substrates underlying mental health conditions, their role as specific biomarkers for suicide risk in MDD should be interpreted with caution.
Conclusions
To conclude, the current investigations offer insights into the elaborate organizations between neural oscillations and suicidality in depression. While ERPs, theta, delta, alpha, and beta oscillations provide valuable insights into the neural dynamics associated with suicidal tendencies, gamma oscillations stand out due to a consistent body of evidence from electrophysiological studies. Their connection to critical neurotransmission systems and E/I balance suggests a nuanced neurobiological basis for suicidal behaviors in depression. These insights emphasize the importance of ongoing research to explore these neural indicators and their potential for therapeutic advancements.
References
Harwood D, Hawton K, Hope T, Jacoby R. Psychiatric disorder and personality factors associated with suicide in older people: a descriptive and case-control study. Int J Geriatr Psychiatry. 2001;16:155–65.
Hawton K, i, Comabella CC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147:17–28.
Orsolini L, Latini R, Pompili M, Serafini G, Volpe U, Vellante F, et al. Understanding the complex of suicide in depression: from research to clinics. Psychiatry investigation. 2020;17:207.
Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;33:395–405.
Obuobi-Donkor G, Nkire N, Agyapong VI. Prevalence of major depressive disorder and correlates of thoughts of death, suicidal behaviour, and death by suicide in the geriatric population—a general review of literature. Behav Sci. 2021;11:142.
Dhole AR, Petkar P, Choudhari SG, Mendhe H. Understanding the factors contributing to suicide among the geriatric population: a narrative review. Cureus. 2023;15:e46387.
Schmaal L, van Harmelen A-L, Chatzi V, Lippard ET, Toenders YJ, Averill LA, et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry. 2020;25:408–27.
Dobbertin M, Blair KS, Carollo E, Blair JR, Dominguez A, Bajaj S. Neuroimaging alterations of the suicidal brain and its relevance to practice: an updated review of MRI studies. Front Psychiatry. 2023;14:1083244.
Ballard ED, Lamontagne SJ. Neuroimaging and suicide-specific stimuli. Neuropsychopharmacol. 2024;50:308–9.
Desmyter S, Van Heeringen C, Audenaert K. Structural and functional neuroimaging studies of the suicidal brain. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:796–808.
Bani-Fatemi A, Tasmim S, Graff-Guerrero A, Gerretsen P, Strauss J, Kolla N, et al. Structural and functional alterations of the suicidal brain: an updated review of neuroimaging studies. Psychiatry Research: Neuroimaging. 2018;278:77–91.
Balcioglu YH, Kose S. Neural substrates of suicide and suicidal behaviour: from a neuroimaging perspective. Psychiatry Clin Psychopharmacol. 2018;28:314–28.
Hong S, Liu YS, Cao B, Cao J, Ai M, Chen J, et al. Identification of suicidality in adolescent major depressive disorder patients using sMRI: a machine learning approach. J Affect Disord. 2021;280:72–6.
Nawaz H, Shah I, Ali S. The amygdala connectivity with depression and suicide ideation with suicide behavior: a meta-analysis of structural MRI, resting-state fMRI and task fMRI. Prog Neuropsychopharmacol Biol Psychiatry. 2023;124:110736.
Hu J, Huang Y, Zhang X, Liao B, Hou G, Xu Z, et al. Identifying suicide attempts, ideation, and non-ideation in major depressive disorder from structural MRI data using deep learning. Asian J psychiatry. 2023;82:103511.
Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, et al. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165:740–8.
van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1:63–72.
Sublette ME, Milak MS, Galfalvy HC, Oquendo MA, Malone KM, Mann JJ. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch suicide Res. 2013;17:434–47.
Ahearn EP, Jamison KR, Steffens DC, Cassidy F, Provenzale JM, Lehman A, et al. MRI correlates of suicide attempt history in unipolar depression. Biol Psychiatry. 2001;50:266–70.
Tewarie P, Liuzzi L, O’Neill GC, Quinn AJ, Griffa A, Woolrich MW, et al. Tracking dynamic brain networks using high temporal resolution MEG measures of functional connectivity. Neuroimage. 2019;200:38–50.
da Silva FL. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–28.
Carlson TA, Grootswagers T, Robinson AK. An introduction to time-resolved decoding analysis for M/EEG. arXiv:1905.04820. [Preprint] 2019. Available from: https://arxiv.org/abs/1905.04820.
Liu X, Liu S, Li M, Su F, Chen S, Ke Y, et al. Altered gamma oscillations and beta–gamma coupling in drug-naive first-episode major depressive disorder: association with sleep and cognitive disturbance. J Affect Disord. 2022;316:99–108.
Li Q, Takeuchi Y, Wang J, Gellért L, Barcsai L, Pedraza LK, et al. Reinstating olfactory bulb-derived limbic gamma oscillations alleviates depression-like behavioral deficits in rodents. Neuron. 2023;111:2065–75.e5.
Zhang J, Emami Z, Safar K, McCunn P, Richardson JD, Rhind SG, et al. Teasing apart trauma: neural oscillations differentiate individual cases of mild traumatic brain injury from post-traumatic stress disorder even when symptoms overlap. Transl Psychiatry. 2021;11:345.
Mathalon DH, Sohal VS. Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders: it’s about time. JAMA psychiatry. 2015;72:840–4.
Hirano Y, Uhlhaas PJ. Current findings and perspectives on aberrant neural oscillations in schizophrenia. Psychiatry Clin Neurosci. 2021;75:358–68.
Okonogi T, Sasaki T. Theta-range oscillations in stress-induced mental disorders as an oscillotherapeutic target. Front Behav Neurosci. 2021;15:698753.
Cho S, van Es M, Woolrich M, Gohil C. Comparison between EEG and MEG of static and dynamic resting-state networks. Hum Brain Mapp. 2024;45:e70018.
Buzsaki G Rhythms of the Brain. 2006. https://doi.org/10.1093/acprof:oso/9780195301069.001.0001.
Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13.
Iosifescu DV, Greenwald S, Devlin P, Mischoulon D, Denninger JW, Alpert JE, et al. Frontal EEG predictors of treatment outcome in major depressive disorder. Eur Neuropsychopharmacol. 2009;19:772–7.
McLoughlin G, Gyurkovics M, Palmer J, Makeig S. Midfrontal theta activity in psychiatric illness: an index of cognitive vulnerabilities across disorders. Biol Psychiatry. 2022;91:173–82.
Wilkening J, Witteler F, Goya-Maldonado R. Suicidality and relief of depressive symptoms with intermittent theta burst stimulation in a sham-controlled randomized clinical trial. Acta Psychiatr Scand. 2022;146:540–56.
Zhao Y, He Z, Luo W, Yu Y, Chen J, Cai X, et al. Effect of intermittent theta burst stimulation on suicidal ideation and depressive symptoms in adolescent depression with suicide attempt: a randomized sham-controlled study. J Affect Disord. 2023;325:618–26.
Lee SM, Jang K-I, Chae J-H. Electroencephalographic correlates of suicidal ideation in the theta band. Clin EEG Neurosci. 2017;48:316–21.
Hanslmayr S, Staudigl T, Fellner M-C. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front Hum Neurosci. 2012;6:74.
Popov T, Miller GA, Rockstroh B, Weisz N. Modulation of α power and functional connectivity during facial affect recognition. J Neurosci. 2013;33:6018–26.
Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–35.
Dai Z, Pei C, Zhang S, Tian S, Chen Z, Zhou H, et al. Attenuated alpha–gamma coupling in emotional dual pathways with right-Amygdala predicting ineffective antidepressant response. CNS Neurosci Therapeutics. 2022;28:401–10.
Li Y, Cao D, Wei L, Tang Y, Wang J. Abnormal functional connectivity of EEG gamma band in patients with depression during emotional face processing. Clin Neurophysiol. 2015;126:2078–89.
Dai Z, Shao J, Zhou H, Chen Z, Zhang S, Wang H, et al. Disrupted fronto-parietal network and default-mode network gamma interactions distinguishing suicidal ideation and suicide attempt in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113:110475.
Arikan MK, Gunver MG, Tarhan N, Metin B. High-Gamma: A biological marker for suicide attempt in patients with depression. J Affect Disord. 2019;254:1–6.
Gilbert JR, Ballard ED, Galiano CS, Nugent AC, Zarate Jr CA. Magnetoencephalographic correlates of suicidal ideation in major depression. Biol psychiatry: Cognit Neurosci neuroimaging. 2020;5:354–63.
Fitzgerald PJ, Watson BO. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry. 2018;8:177.
Jandl M, Steyer J, Kaschka WP. Suicide risk markers in major depressive disorder: a study of electrodermal activity and event-related potentials. J Affect Disord. 2010;123:138–49.
Jiang N, Wang Y, Sun L, Song Y, Sun H An ERP study of implicit emotion processing in depressed suicide attempters. 2015 7th International Conference on Information Technology in Medicine and Education (ITME). 2015:37–40. https://doi.org/10.1109/ITME.2015.76.
Wang C, Wang Z, Cui H, Shao Y, Shi M The research progress of depression with suicide and event related potential. Chinese Journal of Behavioral Medicine and Brain Science. 2015: 565-68.
Tsypes A, Owens M, Gibb BE. Reward responsiveness in suicide attempters: An electroencephalography/event-related potential study. Biol Psychiatry: Cognit Neurosci Neuroimaging. 2021;6:99–106.
Heo IS, Kwon YJ, Lee H-Y, Lee HS, Yoon H-J, Shim S-H, et al. Electrophysiological changes related to childhood trauma in patients with major depressive disorder: an event-related potential study. Clin Psychopharmacol Neurosci. 2022;20:167.
Song W, Li H, Sun F, Wei S, Wen X, Ouyang L. Fusion of pain avoidance and the contingent negative variation induced by punitive condition predict suicide ideation in a college population. Behav Brain Res. 2023;438:114210.
Can AT, Schwenn PE, Isbel B, Beaudequin D, Bouças AP, Dutton M, et al. Electrophysiological phenotypes of suicidality predict prolonged response to oral ketamine treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2023;123:110701.
Zhou X, Lin Z, Liu J, Xiang M, Deng X, Zou Z. The relationship between event-related potential components and suicide risk in major depressive disorder. J Psychiatr Res. 2024;175:89–95.
Iosifescu D, Greenwald S, Devlin P, Perlis RH, Denninger J, Alpert JE, et al. Pretreatment frontal EEG and changes in suicidal ideation during SSRI treatment in major depressive disorder. Acta Psychiatr Scand. 2008;117:271–76.
Dolsen EA, Cheng P, Arnedt JT, Swanson L, Casement MD, Kim HS, et al. Neurophysiological correlates of suicidal ideation in major depressive disorder: hyperarousal during sleep. J Affect Disord. 2017;212:160–6.
Desmyter S, Duprat R, Baeken C, Bijttebier S, van Heeringen K. The acute effects of accelerated repetitive transcranial magnetic stimulation on suicide risk in unipolar depression: preliminary results. Psychiatria Danubina. 2014;26:48–52.
Desmyter S, Duprat R, Baeken C, Van Autreve S, Audenaert K, van Heeringen K. Accelerated intermittent theta burst stimulation for suicide risk in therapy-resistant depressed patients: a randomized, sham-controlled trial. Front Hum Neurosci. 2016;10:480.
Cáceda R, Mirmina J, Kim DJ, Rafiaa M, Carbajal JM, Akram F, et al. Low global frontal brain activity is associated with non-planned or impulsive suicide attempts. a preliminary study. J Affect Disord. 2023;326:44–8.
Gilbert JR, Gerner JL, Burton CR, Nugent AC, Zarate JrCA, Ballard ED. Magnetoencephalography biomarkers of suicide attempt history and antidepressant response to ketamine in treatment-resistant major depression. J Affect Disord. 2022;312:188–97.
Zhang S, Litvak V, Tian S, Dai Z, Tang H, Wang X, et al. Spontaneous transient states of fronto-temporal and default-mode networks altered by suicide attempt in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2022;272:1547–57.
Roh S-C, Kim JS, Kim S, Kim Y, Lee S-H. Frontal alpha asymmetry moderated by suicidal ideation in patients with major depressive disorder: a comparison with healthy individuals. Clin Psychopharmacol Neurosci. 2020;18:58.
Iznak AF, Iznak EV, Damyanovich EV, Oleichik IV. Differences of EEG frequency and spatial parameters in depressive female adolescents with suicidal attempts and non-suicidal self-injuries. Clin EEG Neurosci. 2021;52:406–13.
Dai Z, Zhou H, Zhang W, Tang H, Wang T, Chen Z, et al. Alpha-beta decoupling relevant to inhibition deficits leads to suicide attempt in major depressive disorder. J Affect Disord. 2022;314:168–75.
Chattun MR, Zhang S, Chen Y, Wang Q, Amdanee N, Tian S, et al. Caudothalamic dysfunction in drug-free suicidally depressed patients: an MEG study. Eur Arch Psychiatry Clin Neurosci. 2020;270:217–27.
Dai Z, Zhang S, Wang H, Chen Z, Zhang W, Hu X, et al. Hampered gamma oscillations induced by sad emotion underlying suicide attempt in major depressive disorder. Psychiatry Clin Neurosci. 2023;77:20–29.
Dai Z, Zhang W, Zhou H, Zhang S, Chen Z, Yao Z, et al. Gamma oscillations of visual cortex underlying emotion and cognition deficits associated with suicide attempt in major depressive disorder. Nat Ment Health. 2024;2:1–11.
Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48.
Hajcak G, Foti D. Significance?… Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology. 2020;57:e13570.
Mansor AA, Isa SM, Noor SSM. P300 and decision-making in neuromarketing. Neurosci Res Notes. 2021;4:21–6.
Beck AT, Brown GK, Steer RA, Dahlsgaard KK, Grisham JR. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life-Threatening Behav. 1999;29:1–9.
Pattan SA P300 in Depression–a Case Control Study. 2020.
Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, et al. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33:1584–602.
Rosenfeld JP, Ozsan I, Ward AC. P300 amplitude at Pz and N200/N300 latency at F3 differ between participants simulating suspect versus witness roles in a mock crime. Psychophysiology. 2017;54:640–8.
Raggi A, Serretti A, Ferri R The P300 component of the auditory event-related potential in adult psychiatric and neurologic disorders: a narrative review of clinical and experimental evidence. Int Clin Psychopharmacol. 2024;10:1097.
Smigielski L, Kometer M, Scheidegger M, Stress C, Preller KH, Koenig T, et al. P300-mediated modulations in self–other processing under psychedelic psilocybin are related to connectedness and changed meaning: a window into the self–other overlap. Hum Brain Mapp. 2020;41:4982–96.
Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–9.
Lie MEK, Falk-Petersen CB, Piilgaard L, Griem-Krey N, Wellendorph P, Kornum BR. GABAA receptor β1-subunit knock-out mice show increased delta power in NREM sleep and decreased theta power in REM sleep. Eur J Neurosci. 2021;54:4445–55.
Li Y, Hou S, Li F, Long S, Yang Y, Li Y, et al. Preoperative recovery sleep ameliorates postoperative cognitive dysfunction aggravated by sleep fragmentation in aged mice by enhancing Eeg Delta-wave activity and Lfp Theta oscillation in hippocampal Ca1. Brain Res Bull. 2024;211:110945.
Li Y, Li Q, Zou X, Zhong Z, Ouyang Q, Zeng Q, et al. Effects of CPAP treatment on electroencephalographic activity in patients with obstructive sleep apnea syndrome during deep sleep: Preliminary findings of a cross-sectional study. Chron Respir Dis. 2023;20:14799731231215094.
Zhang Y, Lahmann I, Baum K, Shimojo H, Mourikis P, Wolf J, et al. Oscillations of Delta-like1 regulate the balance between differentiation and maintenance of muscle stem cells. Nat Commun. 2021;12:1318.
Cassidy JM, Wodeyar A, Wu J, Kaur K, Masuda AK, Srinivasan R, et al. Low-frequency oscillations are a biomarker of injury and recovery after stroke. Stroke. 2020;51:1442–50.
Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–95.
Riemann D. Voderholzer U. Primary insomnia: a risk factor to develop depression?. J Affect Disord. 2003;76:255–9.
Mackey S, Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 2014;40:2777–96.
Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25.
Marchisella F, Creutzberg KC, Begni V, Sanson A, Wearick-Silva LE, Tractenberg SG, et al. Exposure to prenatal stress is associated with an excitatory/inhibitory imbalance in rat prefrontal cortex and amygdala and an increased risk for emotional dysregulation. Front Cell Dev Biol. 2021;9:653384.
Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51.
Wang H-L, Sun Y-X, Liu X, Wang H, Ma Y-N, Su Y-A, et al. Adolescent stress increases depression-like behaviors and alters the excitatory-inhibitory balance in aged mice. Chin Med J. 2019;132:1689–99.
Han K, Lee M, Lim H-K, Jang MW, Kwon J, Lee CJ, et al. Excitation-inhibition imbalance leads to alteration of neuronal coherence and neurovascular coupling under acute stress. J Neurosci. 2020;40:9148–62.
Anticevic A, Schleifer C, T. Cho Y. Emotional and cognitive dysregulation in schizophrenia and depression: understanding common and distinct behavioral and neural mechanisms. Dialogues Clin Neurosci. 2015;17:421–34.
Kühnel A, Widmann A, Colic L, Herrmann L, Demenescu L, Leutritz A, et al. Impaired cognitive self-awareness mediates the association between alexithymia and excitation/inhibition balance in the pgACC. Psychol Med. 2020;50:1727–35.
Bi D, Wen L, Wu Z, Shen Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of alzheimer’s disease. Alzheimers Dement. 2020;16:1312–29.
Ray S, Maunsell JH. Do gamma oscillations play a role in cerebral cortex?. Trends Cogn Sci. 2015;19:78–85.
Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—a review of multimodal imaging studies. Neurosci Biobehav Rev. 2014;47:36–52.
Morrison AP, French P, Stewart SL, Birchwood M, Fowler D, Gumley AI, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233.
Koppe G, Meyer-Lindenberg A, Durstewitz D. Deep learning for small and big data in psychiatry. Neuropsychopharmacology. 2021;46:176–90.
Antal A, Luber B, Brem A-K, Bikson M, Brunoni AR, Kadosh RC, et al. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract. 2022;7:146–65.
Ouellet J, McGirr A, Van den Eynde F, Jollant F, Lepage M, Berlim MT. Enhancing decision-making and cognitive impulse control with transcranial direct current stimulation (tDCS) applied over the orbitofrontal cortex (OFC): a randomized and sham-controlled exploratory study. J Psychiatr Res. 2015;69:27–34.
Pathak Y, Salami O, Baillet S, Li Z, Butson CR. Longitudinal changes in depressive circuitry in response to neuromodulation therapy. Front Neural Circuits. 2016;10:50.
Sun Y, Giacobbe P, Tang CW, Barr MS, Rajji T, Kennedy SH, et al. Deep brain stimulation modulates gamma oscillations and theta–gamma coupling in treatment resistant depression. Brain Stimul. 2015;8:1033–42.
Frank AC, Scangos KW, Larson PS, Norbu T, Lee AT, Lee AM. Identification of a personalized intracranial biomarker of depression and response to DBS therapy. BraStimulation: Basic, Translational, Clin Res Neuromodulation. 2021;14:1002–04.
Tao Y, Zhao R, Yang B, Han J, Li Y. Dissecting the shared genetic landscape of anxiety, depression, and schizophrenia. J Transl Med. 2024;22:373.
Isomura S, Onitsuka T, Tsuchimoto R, Nakamura I, Hirano S, Oda Y, et al. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J Affect Disord. 2016;190:800–6.
Tao P, Dai Z, Shao J, Tang H, Zhang S, Yao Z, et al. Gamma band VMPFC-PreCG. L connection variation after the onset of negative emotional stimuli can predict mania in depressive patients. J Psychiatr Res. 2023;158:165–71.
Tada M, Kirihara K, Koshiyama D, Nagai T, Fujiouka M, Usui K, et al. Alterations of auditory-evoked gamma oscillations are more pronounced than alterations of spontaneous power of gamma oscillation in early stages of schizophrenia. Transl Psychiatry. 2023;13:218.
Acknowledgements
We also would like to express our gratitude to AJE and YIMORE for its invaluable assistance in polishing this manuscript. Its advanced language processing capabilities helped enhance the clarity and coherence of our writing, significantly contributing to the overall quality of the paper. This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (24KJB310001).
Author information
Authors and Affiliations
Contributions
Zhongpeng Dai conceptualized and designed the structure of the review, contributed to the interpretation of the literature, and drafted the manuscript. Jia Miao performed literature searches, organized key findings, and contributed to the manuscript revisions. Hongliang Zhou critically reviewed the manuscript for intellectual content, and contributed to the refinement of the manuscript. Huan Wang supervised the project, provided fundings, and contributed to the final editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, Z., Jia, M., Zhou, H. et al. Uncovering oscillatory dysregulation associated with suicide risk in major depressive disorder: a narrative review. Transl Psychiatry 16, 25 (2026). https://doi.org/10.1038/s41398-025-03800-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03800-x