Abstract
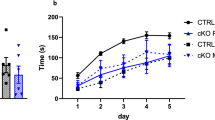
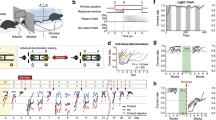
Social valence is the directional emotional significance affiliated with social experiences. Maladaptive social information processing has been linked to mood disorder susceptibility, which is more prevalent in women. To determine whether there are sex differences in social valence processing, we employed behavioral tasks that associated conspecific identity recognition with either positive or negative valence, as well as tasks in which valence information originated from social targets. Male mice demonstrated identity recognition regardless of social valence. While male and female mice performed similarly in the positive social valence task, female mice did not show identity recognition following the negative social valence task. In vivo calcium imaging of the dorsal CA1 further revealed sex differences in negative social valence processing with reduced hippocampal representation of social information in female mice. Finally, enhancing dorsal CA1 neuronal activity by ampakine rescued identity recognition in female mice. These results suggest that sex differences in social valence processing may contribute to the heightened vulnerability to social stress-related mood disorders in women.
Similar content being viewed by others
Data availability
All MATLAB codes are available at https://github.com/tpwonglab/sexe-diff-social-memory. Raw data of all figures can be found in main text and supplementary materials. MATLAB data from calcium imaging will be shared on Zenodo after the publication of the paper.
Materials availability
All MATLAB codes are available at https://github.com/tpwonglab/sexe-diff-social-memory. Raw data of all figures can be found in main text and supplementary materials. MATLAB data from calcium imaging will be shared on Zenodo after the publication of the paper.
References
Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. Am Sociol Rev. 1995;60:104–25.
Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Curr Directions Psychological Sci. 2005;14:64–8.
Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89.
Hammen C. Interpersonal stress and depression in women. J Affect Disord. 2003;74:49–57.
Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–27.
Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National comorbidity survey. i: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96.
Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56.
Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92.
Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J Neurosci. 2005;25:11239–47.
Chiang MC, Huang AJY, Wintzer ME, Ohshima T, McHugh TJ. A role for CA3 in social recognition memory. Behav Brain Res. 2018;354:22–30.
Pena RR, Pereira-Caixeta AR, Moraes MF, Pereira GS. Anisomycin administered in the olfactory bulb and dorsal hippocampus impaired social recognition memory consolidation in different time-points. Brain Res Bull. 2014;109:151–7.
Kong E, Lee KH, Do J, Kim P, Lee D. Dynamic and stable hippocampal representations of social identity and reward expectation support associative social memory in male mice. Nat Commun. 2023;14:2597.
Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–41.
Danjo T, Toyoizumi T, Fujisawa S. Spatial representations of self and other in the hippocampus. Science. 2018;359:213–8.
Omer DB, Maimon SR, Las L, Ulanovsky N. Social place-cells in the bat hippocampus. Science. 2018;359:218–24.
Zhang TR, Larosa A, Di Raddo ME, Wong V, Wong AS, Wong TP. Negative memory engrams in the hippocampus enhance the susceptibility to chronic social defeat stress. J Neurosci. 2019;39:7576–90.
Kim J, Lei Y, Lu XY, Kim CS. Glucocorticoid-glucocorticoid receptor-HCN1 channels reduce neuronal excitability in dorsal hippocampal CA1 neurons. Mol Psychiatry. 2022;27:4035–49.
Colon LM, Poulos AM. Contextual processing elicits sex differences in dorsal hippocampus activation following footshock and context fear retrieval. Behav Brain Res. 2020;393:112771.
Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology. 2017;42:397–407.
Larosa A, Xu QW, Mitrikeski NM, Wong TP. Behavioral tasks for examining identity recognition in mice. J Vis Exp. 2025;7:e67547.
Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, et al. Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry. 2019;86:657–68.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302.
Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009; Appendix 4: Appendix 4I. https://doi.org/10.1002/0471142301.nsa04is48.
Resendez SL, Jennings JH, Ung RL, Namboodiri VM, Zhou ZC, Otis JM, et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc. 2016;11:566–97.
Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21:1281–9.
Pnevmatikakis EA, Giovannucci A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J Neurosci Methods. 2017;291:83–94.
Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife. 2018;7:e28728.
Larosa A, Zhang TR, Wong AS, Fung CYH, Long XLYJ, Singh P, et al. Diminished social memory and hippocampal correlates of social interactions in chronic social defeat stress susceptibility. Biol Psychiatry Glob Open Sci. 2025;5:100455.
Kummer KK, Hofhansel L, Barwitz CM, Schardl A, Prast JM, Salti A, et al. Differences in social interaction- vs. cocaine reward in mouse vs. rat. Front Behav Neurosci. 2014;8:363.
Toth I, Neumann ID, Slattery DA. Social fear conditioning: a novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology. 2012;37:1433–43.
Jacobs SA, Tsien JZ. genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PLoS One. 2012;7:e36387.
Kondo Y, Hayashi H. Neural and hormonal basis of opposite-sex preference by chemosensory signals. Int J Mol Sci. 2021;22:8311.
Chari T, Griswold S, Andrews NA, Fagiolini M. The stage of the estrus cycle is critical for interpretation of female mouse social interaction behavior. Front Behav Neurosci. 2020;14:113.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Leroy F, Brann DH, Meira T, Siegelbaum SA. Input-timing-dependent plasticity in the hippocampal CA2 region and its potential role in social memory. Neuron. 2017;95:1089–102.e1085.
Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27:12025–32.
Larosa A, Wong TP. The hippocampus in stress susceptibility and resilience: reviewing molecular and functional markers. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110601.
Liang B, Zhang L, Barbera G, Fang W, Zhang J, Chen X, et al. Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron. 2018;100:700–14.e709.
Asok A, Hijazi J, Harvey LR, Kosmidis S, Kandel ER, Rayman JB. Sex differences in remote contextual fear generalization in mice. Front Behav Neurosci. 2019;13:56.
Zhu E, Mathew D, Jee HJ, Sun M, Liu W, Zhang Q, et al. AMPAkines have site-specific analgesic effects in the cortex. Mol Pain. 2024;20:17448069231214677.
Goode LK, Fusilier AR, Remiszewski N, Reeves JM, Abiraman K, Defenderfer M, et al. Examination of diurnal variation and sex differences in hippocampal neurophysiology and spatial memory. eNeuro. 2022;9:ENEURO.0124–22.2022.
Trott JM, Krasne FB, Fanselow MS. Sex differences in contextual fear learning and generalization: a behavioral and computational analysis of hippocampal functioning. Learn Mem. 2022;29:283–96.
Poggi G, Bergamini G, Dulinskas R, Madur L, Greter A, Ineichen C, et al. Engagement of basal amygdala-nucleus accumbens glutamate neurons in the processing of rewarding or aversive social stimuli. Eur J Neurosci. 2024;59:996–1015.
Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, et al. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One. 2011;6:e17405.
Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep. 2017;7:12838.
Ayash S, Schmitt U, Muller MB. Chronic social defeat-induced social avoidance as a proxy of stress resilience in mice involves conditioned learning. J Psychiatr Res. 2020;120:64–71.
Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92.
Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am J Psychiatry. 2017;174:125–34.
Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13.
Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, et al. Autobiographical memory specificity and emotional disorder. Psychol Bull. 2007;133:122–48.
Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calandreau L, et al. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–3.
Lesuis SL, Brosens N, Immerzeel N, van der Loo RJ, Mitric M, Bielefeld P, et al. Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biol Psychiatry. 2021;90:494–504.
Engeland WC, Massman L, Miller L, Leng S, Pignatti E, Pantano L, et al. Sex differences in adrenal bmal1 deletion-induced augmentation of glucocorticoid responses to stress and ACTH in Mice. Endocrinology. 2019;160:2215–29.
Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300.
Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–85.
Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–40.
Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA. 2005;102:14063–8.
Sorensen AT, Cooper YA, Baratta MV, Weng FJ, Zhang Y, Ramamoorthi K, et al. A robust activity marking system for exploring active neuronal ensembles. Elife. 2016;5:e13918.
Hassan SI, Bigler S, Siegelbaum SA. Social odor discrimination and its enhancement by associative learning in the hippocampal CA2 region. Neuron. 2023;111:2232–46.e2235.
Rao RP, von Heimendahl M, Bahr V, Brecht M. Neuronal Responses to Conspecifics in the Ventral CA1. Cell Rep. 2019;27:3460–72.e3463.
Herlitz A, Loven J. Sex differences and the own-gender bias in face recognition: a meta-analytic review. Vis Cogn. 2013;21:1306–36.
Lewin C, Herlitz A. Sex differences in face recognition-women’s faces make the difference. Brain Cogn. 2002;50:121–8.
Yuan Y, Xu Y, Zhang W, Guan L. Sex differences in the effects of threats on self-face recognition in social and natural scenes. Curr Psychol. 2022;41:4158–70.
Kraines MA, Kelberer LJA, Wells TT. Sex differences in attention to disgust facial expressions. Cogn Emot. 2017;31:1692–7.
McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–55.
Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage. 2005;28:618–26.
Chang J, Yu R. Hippocampal connectivity in the aftermath of acute social stress. Neurobiol Stress. 2019;11:100195.
O’Neil EB, Newsome RN, Li IH, Thavabalasingam S, Ito R, Lee AC. Examining the role of the human hippocampus in approach-avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J Neurosci. 2015;35:15039–49.
Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–93.
Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA. 2002;99:10789–94.
Acknowledgements
We would like to thank the UCLA Miniscope team and Daniel Aharoni’s lab for developing and sharing the miniscope technology. The present study used the services of the Molecular and Cellular Microscopy Platform in the Douglas Research Centre. Melina Jaramillo Garcia helped set up the imaging verification. We also thank Dr. Rosemary Bagot for the helpful discussions and advice.
Funding
Natural Sciences and Engineering Research Council of Canada RGPIN-2021-03739 (TPW). Canadian Institutes of Health Research PJ8 179866 and PJT 183587 (TPW). Fonds de recherche du Québec – Nature et technologies 326838 (TPW).
Author information
Authors and Affiliations
Contributions
Conceptualization: AL, QWX, MPB, TPW. Methodology: AL, QWX, ASW, JQL, MY, BWW. Investigation: AL, QWX. Funding acquisition: TPW. Project administration: TPW. Supervision: TPW. Writing—original draft: AL, TPW. Writing—review & editing: AL, TPW.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Larosa, A., Xu, Q.W., Yaghoubi, M. et al. Social valence dictates sex differences in identity recognition. Transl Psychiatry (2026). https://doi.org/10.1038/s41398-026-03854-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-026-03854-5