Abstract
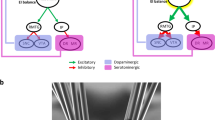
The habenula is a small epithalamic structure composed of two distinct subregions, the medial (MHb) and lateral (LHb) habenula. It serves as a critical hub for integrating fronto-limbic and brainstem signals to regulate motivation, mood, and reward processing. Therefore, it is unsurprising that dysfunction of the habenula has been implicated in several mood disorders including major depressive disorder (MDD), a debilitating mood disorder marked by low mood and feelings of hopelessness. This review synthesizes recent advances in understanding the habenula’s neurocircuitry, molecular landscape, and role in MDD pathophysiology, while evaluating its potential as a therapeutic target. Specifically, emerging evidence highlights subregion-specific pathology. Indeed, in MDD and in animal models of depression, the MHb has been shown to exhibit marked downregulation of calcium-dependent activator protein for secretion 2 (CAPS2) and deficits in nicotinic acetylcholine receptor-mediated signaling. While in the LHb, dysregulated expression profiles of inward-rectifying potassium channel Kir4.1, the β isoform of calcium/calmodulin-dependent protein kinase II (CaMKIIβ), protein phosphatase 2 A (PP2A), and small nucleolar RNA SNORA69 have been found in animal models of depression and MDD postmortem studies. Structural imaging and postmortem neurohistological studies in MDD patients have further revealed habenular volume changes, reduced neuronal cell counts, diminished cell area, and abnormal functional connectivity. As research unravels the habenula’s complexities, its potential in treating mood disorders grows increasingly salient, offering new avenues for intervention in mental health.
Similar content being viewed by others
References
Herrera ML, Rubio NG, Quintanilla JP, Manuel Huerta V, Osorio-Forero A, Cárdenas, et al. Effects of electrical stimulation of the habenula on the modulation of emotional responses in Wistar rats. Acta Colombiana de Psicología. 2018;21:224–35.
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–71.
Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5.
Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–52.
Snell RS Clinical neuroanatomy. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. p. 542.
Díaz E, Bravo D, Rojas X, Concha ML. Morphologic and immunohistochemical organization of the human habenular complex. J Comp Neurol. 2011;519:3727–47.
Kim Jwon, Naidich TP, Ely BA, Yacoub E, De Martino F, Fowkes ME, et al. Human habenula segmentation using myelin content. NeuroImage. 2016;130:145–56.
Roman E, Weininger J, Lim B, Roman M, Barry D, Tierney P, et al. Untangling the dorsal diencephalic conduction system: a review of structure and function of the stria medullaris, habenula and fasciculus retroflexus. Brain Struct Funct. 2020;225:1437–58.
Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13.
Andres KH, Düring MV, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–50.
Fore S, Palumbo F, Pelgrims R, Yaksi E. Information processing in the vertebrate habenula. Semin Cell Dev Biol. 2018;78:130–9.
He N, Sethi SK, Zhang C, Li Y, Chen Y, Sun B, et al. Visualizing the lateral habenula using susceptibility weighted imaging and quantitative susceptibility mapping. Magnetic Reson Imaging. 2020;65:55–61.
Müller UJ, Ahrens M, Vasilevska V, Dobrowolny H, Schiltz K, Schlaaff K, et al. Reduced habenular volumes and neuron numbers in male heroin addicts: A post-mortem study. Eur Arch Psychiatry Clin Neurosci. 2021;271:835–45.
Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–66.
Artymyshyn R, Murray M. Substance P in the interpeduncular nucleus of the rat: normal distribution and the effects of deafferentation. J Comp Neurol. 1985;231:78–90.
Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Øktedalen O, et al. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. an immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–70.
Benarroch EE. Habenula: recently recognized functions and potential clinical relevance. Neurology. 2015;85:992–1000.
Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc B: Biol Sci. 2008;364:1005–20.
Morley BJ. The interpeduncular nucleus. Int Rev Neurobiol. 1986;28:157–82.
Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–73.
Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47.
Mehlman ML, Marcroft JL, Taube JS. Anatomical projections to the dorsal tegmental nucleus and abducens nucleus arise from separate cell populations in the nucleus prepositus hypoglossi, but overlapping cell populations in the medial vestibular nucleus. J Comp Neurol. 2021;529:2706–26.
Lima LB, Bueno D, Leite F, Souza S, Gonçalves L, Furigo IC, et al. Afferent and efferent connections of the interpeduncular nucleus with special reference to circuits involving the habenula and raphe nuclei. J Comp Neurol. 2017;525:2411–42.
Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–76.
Quina LA, Walker A, Morton G, Han V, Turner EE. GAD2 expression defines a class of excitatory lateral habenula neurons in mice that project to the raphe and pontine tegmentum. eNeuro. 2020;7:ENEURO.0527–19.
Wagner F, Bernard R, Derst C, French L, Veh RW. Microarray analysis of transcripts with elevated expressions in the rat medial or lateral habenula suggest fast GABAergic excitation in the medial habenula and habenular involvement in the regulation of feeding and energy balance. Brain Struct Funct. 2016;221:4663–89.
Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93.
Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. a horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–46.
Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–30.
Hikosaka O. Habenula. Scholarpedia. 2007;2:2703.
Brown PL, Shepard PD. Functional evidence for a direct excitatory projection from the lateral habenula to the ventral tegmental area in the rat. J Neurophysiol. 2016;116:1161–74.
Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJY, Stephenson-Jones M, et al. The lateral habenula circuitry: reward processing and cognitive control. J Neurosci. 2016;36:11482–8.
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a major GABAergic afferent to midbrain dopamine neurons, selectively encodes aversive stimuli and promotes behavioral inhibition. Neuron. 2009;61:786–800.
Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–9.
Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–30.
Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–72.
Park MR. Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull. 1987;19:581–6.
Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr Bull. 2006;32:417–21.
Sonkusare S, Ding Q, Zhang Y, Wang L, Gong H, Mandali A, et al. Power signatures of habenular neuronal signals in patients with bipolar or unipolar depressive disorders correlate with their disease severity. Transl Psychiatry. 2022;12:1–9.
Wang RY, Aghajanian GK. Physiological Evidence for Habenula as Major Link Between Forebrain and Midbrain Raphe. Science. 1977;197:89–91.
Bains N, Abdijadid S Major Depressive Disorder. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. http://www.ncbi.nlm.nih.gov/books/NBK559078/.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edition. Arlington, VA: American Psychiatric Association; 2013. p. 947.
Cowen PJ. Serotonin and depression: pathophysiological mechanism or marketing myth?. Trends Pharmacol Sci. 2008;29:433–6.
Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69:4–7.
Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. AJP. 1965;122:509–22.
Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118.
Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: multivariate granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–72.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacol. 2010;35:192–216.
Wang Q, Timberlake MA, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109.
Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1:9.
Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–47.
Petković A, Chaudhury D. Encore: Behavioural animal models of stress, depression and mood disorders. Front Behav Neurosci. 2022;16:931964.
Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Sig Transduct Target Ther. 2024;9:1–32.
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–16.
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64.
Codeluppi SA, Xu M, Bansal Y, Lepack AE, Duric V, Chow M, et al. Prefrontal cortex astroglia modulate anhedonia-like behavior. Mol Psychiatry. 2023;28:4632–41.
Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–55.
Miller WR, Seligman ME, Kurlander HM. Learned helplessness, depression, and anxiety. J Nerv Ment Dis. 1975;161:347–57.
Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9.
Planchez B, Surget A, Belzung C. Animal models of major depression: Drawbacks and challenges. J Neural Transm. 2019;126:1383–408.
Tian X, Russo SJ, Li L. Behavioral animal models and neural-circuit framework of depressive disorder. Neurosci Bull. 2025;41:272–88.
Yoo H, Yang SH, Kim JY, Yang E, Park HS, Lee SJ, et al. Down-regulation of habenular calcium-dependent secretion activator 2 induces despair-like behavior. Sci Rep. 2021;11:3700.
Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, et al. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci. 2011;108:373–8.
Cisternas FA, Vincent JB, Scherer SW, Ray PN. Cloning and characterization of human CADPS and CADPS2, new members of the Ca2+-dependent activator for secretion protein family. Genomics. 2003;81:279–91.
Speidel D, Varoqueaux F, Enk C, Nojiri M, Grishanin RN, Martin TFJ, et al. A Family of Ca2+-Dependent Activator Proteins for Secretion. J Biol Chem. 2003;278:52802–9.
Han S, Yang SH, Kim JY, Mo S, Yang E, Song KM, et al. Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior. Sci Rep. 2017;7:900.
Frahm S, Antolin-Fontes B, Görlich A, Zander JF, Ahnert-Hilger G, Ibañez-Tallon I. An essential role of acetylcholine-glutamate synergy at habenular synapses in nicotine dependence. Nelson SB, editor elife. 2015;4:e11396.
Ohno Y, Kinboshi M, Shimizu S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int J Mol Sci. 2018;19:3313.
Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554:323–7.
Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606.
Hoffman L, Farley MM, Waxham MN. Calcium-calmodulin-dependent protein kinase II isoforms differentially impact the dynamics and structure of the actin cytoskeleton. Biochemistry. 2013;52:1198–207.
Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–20.
Zalcman G, Federman N, Romano A. CaMKII Isoforms in learning and memory: localization and function. Front Mol Neurosci. 2018;11:445.
Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Hervé D, et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–61.
Shaye H, Stauch B, Gati C, Cherezov V. Molecular mechanisms of metabotropic GABAB receptor function. Sci Adv. 2021;7:eabg3362.
Seo JS, Zhong P, Liu A, Yan Z, Greengard P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol Psychiatry. 2018;23:1113–9.
Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14:673–80.
Zhang Z, Zhang W, Fang Y, Wang N, Liu G, Zou N, et al. A potentiation of REM sleep-active neurons in the lateral habenula may be responsible for the sleep disturbance in depression. Curr Biol. 2024;34:3287–300.e6.
Zheng Z, Liu Y, Mu R, Guo X, Feng Y, Guo C, et al. A small population of stress-responsive neurons in the hypothalamus-habenula circuit mediates development of depression-like behavior in mice. Neuron. 2024;112:3924–39.e5.
Li X, Liu X, Liu J, Zhou F, Li Y, Zhao Y, et al. Neuronal TCF7L2 in lateral habenula is involved in stress-induced depression. Int J Mol Sci. 2024;25:12404.
Guo F, Wang Y. TCF7l2, a nuclear marker that labels premyelinating oligodendrocytes and promotes oligodendroglial lineage progression. Glia. 2023;71:143–54.
Koga Y, Kajitani N, Miyako K, Takizawa H, Boku S, Takebayashi M. TCF7L2: A potential key regulator of antidepressant effects on hippocampal astrocytes in depression model mice. J Psychiatr Res. 2024;170:375–86.
Liu D, Nguyen TTL, Gao H, Huang H, Kim DC, Sharp B, et al. TCF7L2 lncRNA: a link between bipolar disorder and body mass index through glucocorticoid signaling. Mol Psychiatry. 2021;26:7454–64.
Lin R, Mitsuhashi H, Fiori LM, Denniston R, Ibrahim EC, Belzung C, et al. SNORA69 is up-regulated in the lateral habenula of individuals with major depressive disorder. Sci Rep. 2024;14:8258.
Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809.
Borchardt EK, Martinez NM, Gilbert WV. Regulation and Function of RNA Pseudouridylation in Human Cells. Annu Rev Genet. 2020;54:309–36.
Fiori LM, Kos A, Lin R, Théroux JF, Lopez JP, Kühne C, et al. miR-323a regulates ERBB4 and is involved in depression. Mol Psychiatry. 2021;26:4191–204.
Bean JC, Lin TW, Sathyamurthy A, Liu F, Yin DM, Xiong WC, et al. Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain. J Neurosci. 2014;34:13549–66.
Bruce LL, Kornblum HI, Seroogy KB. Comparison of thalamic populations in mammals and birds: expression of ErbB4 mRNA. Brain Res Bull. 2002;57:455–61.
Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159:494–503.
Lawson RP, Drevets WC, Roiser JP. Defining the habenula in human neuroimaging studies. NeuroImage. 2013;64:722–7.
Lawson RP, Nord CL, Seymour B, Thomas DL, Dayan P, Pilling S, et al. Disrupted habenula function in major depression. Mol Psychiatry. 2017;22:202–8.
Liu WH, Valton V, Wang LZ, Zhu YH, Roiser JP. Association between habenula dysfunction and motivational symptoms in unmedicated major depressive disorder. Soc Cognit Affect Neurosci. 2017;12:1520–33.
Schmidt FM, Schindler S, Adamidis M, Strauß M, Tränkner A, Trampel R, et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur Arch Psychiatry Clin Neurosci. 2016;267:107–15.
Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain EE, Neumeister A, et al. Habenula volume in bipolar disorder and major depressive disorder: a high resolution MRI Study. Biol Psychiatry. 2011;69:336–43.
Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychological Med. 2010;40:557–67.
Ambrosi E, Arciniegas DB, Curtis KN, Patriquin MA, Spalletta G, Sani G, et al. Resting-State functional connectivity of the habenula in mood disorder patients with and without suicide-related behaviors. J Neuropsychiatry Clin Neurosci. 2019;31:49–56.
Jung JY, Cho SE, Kim N, Kang CK, Kang SG. Decreased resting-state functional connectivity of the habenula-cerebellar in a major depressive disorder. Front Psychiatry. 2022;13:925823.
Qiao D, Zhang A, Sun N, Yang C, Li J, Zhao T, et al. Altered static and dynamic functional connectivity of habenula associated with suicidal ideation in first-episode, drug-naïve patients with major depressive disorder. Front Psychiatry. 2020;11:608197.
Wu Y, Chu Z, Chen X, Zhu Y, Xu X, Shen Z. Functional connectivity between the habenula and posterior default mode network contributes to the response of the duloxetine effect in major depressive disorder. NeuroReport. 2024;35:380.
Wu Z, Wang C, Ma Z, Pang M, Wu Y, Zhang N, et al. Abnormal functional connectivity of habenula in untreated patients with first-episode major depressive disorder. Psychiatry Res. 2020;285:112837.
Yang L, Jin C, Qi S, Teng Y, Li C, Yao Y, et al. Alterations of functional connectivity of the lateral habenula in subclinical depression and major depressive disorder. BMC Psychiatry. 2022;22:588.
Zhu Z, Wang S, Lee TMC, Zhang R. Habenula functional connectivity variability increases with disease severity in individuals with major depression. J Affect Disord. 2023;333:216–24.
Fujita I. The inferior temporal cortex: architecture, computation, and representation. J Neurocytol. 2002;31:359–71.
Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI Evidence for a double gradient of information convergence in the temporal lobes. J Cognit Neurosci. 2012;24:1766–78.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Luan S, Zhang L, Wang R, Zhao H, Liu C. A resting-state study of volumetric and functional connectivity of the habenular nucleus in treatment-resistant depression patients. Brain Behav. 2019;9:e01229.
Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–39.
Ichikawa N, Siegle GJ, Jones NP, Kamishima K, Thompson WK, Gross JJ, et al. Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2011;11:354–71.
Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci. 2002;22:9651–5.
Rudolph S, Badura A, Lutzu S, Pathak SS, Thieme A, Verpeut JL, et al. Cognitive-Affective functions of the cerebellum. J Neurosci. 2023;43:7554–64.
Siuda K, Chrobak AA, Starowicz-Filip A, Tereszko A, Dudek D. Emotional disorders in patients with cerebellar damage-case studies. Psychiatr Pol. 2014;48:289–97.
Gao J, Li Y, Wei Q, Li X, Wang K, Tian Y, et al. Habenula and left angular gyrus circuit contributes to response of electroconvulsive therapy in major depressive disorder. Brain Imaging Behav. 2021;15:2246–53.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83.
Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–40.
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–9.
Li W, Xie K, Ngetich RK, Zhang J, Jin Z, Li L. Inferior frontal gyrus-based resting-state functional connectivity and medium dispositional use of reappraisal strategy. Front Neurosci. 2021;15:681859.
Schaum M, Pinzuti E, Sebastian A, Lieb K, Fries P, Mobascher A, et al. Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans. Elife. 2021;10:e61679.
Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 Enhances depolarization-induced GABA release. Neuron. 2007;54:599–610.
Mannekote Thippaiah S, Pradhan B, Voyiaziakis E, Shetty R, Iyengar S, Olson C, et al. Possible role of parvalbumin interneurons in meditation and psychiatric illness. JNP. 2022;34:113–23.
Webster JF, Vroman R, Balueva K, Wulff P, Sakata S, Wozny C. Disentangling neuronal inhibition and inhibitory pathways in the lateral habenula. Sci Rep. 2020;10:8490.
Luo B, Liu Z, Lin D, Chen W, Ren D, Yu Z, et al. ErbB4 promotes inhibitory synapse formation by cell adhesion, independent of its kinase activity. Transl Psychiatry. 2021;11:1–11.
Sun Y, Ikrar T, Davis MF, Gong N, Zheng X, Luo ZD, et al. Neuregulin-1 (NRG1)/ErbB4 signaling regulates visual cortical plasticity. Neuron. 2016;92:160–73.
Belzeaux R, Formisano-Tréziny C, Loundou A, Boyer L, Gabert J, Samuelian JC, et al. Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J Psychiatr Res. 2010;44:1205–13.
Abdelaziz HA, Abdelbaki TN, Dean YE, Assem S. Is neuregulin-1 (NRG-1) a potential blood biomarker linking depression to obesity? a case-control study. BMC Psychiatry. 2023;23:670.
Mei L, Nave KA. Neuregulin-ERBB signaling in nervous system development and neuropsychiatric diseases. Neuron. 2014;83:27–49.
Wang N, Zhang GF, Liu XY, Sun HL, Wang XM, Qiu LL, et al. Downregulation of Neuregulin 1-ErbB4 signaling in parvalbumin interneurons in the rat brain may contribute to the antidepressant properties of ketamine. J Mol Neurosci. 2014;54:211–8.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: a randomized control study. J Affect Disord. 2018;225:709–14.
Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacol. 2024;49:41–50.
Matveychuk D, Thomas RK, Swainson J, Khullar A, MacKay MA, Baker GB, et al. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol. 2020;10:2045125320916657.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22.
Xu J, Guo C, Liu Y, Wu G, Ke D, Wang Q, et al. Nedd4l downregulation of NRG1 in the mPFC induces depression-like behaviour in CSDS mice. Transl Psychiatry. 2020;10:1–15.
Jewett BE, Thapa B Physiology, NMDA Receptor. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. http://www.ncbi.nlm.nih.gov/books/NBK519495/.
Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA Receptors: Molecular and Functional Distinction of Subtypes. Science. 1992;256:1217–21.
Rădulescu I, Drăgoi AM, Trifu SC, Cristea MB. Neuroplasticity and depression: Rewiring the brain’s networks through pharmacological therapy (Review). Exp Ther Med. 2021;22:1131.
Wang S, Bian L, Yin Y, Guo J. Targeting NMDA receptors in emotional disorders: their role in neuroprotection. Brain Sci. 2022;12:1329.
Zhu JM, Li KX, Cao SX, Chen XJ, Shen CJ, Zhang Y, et al. Increased NRG1-ErbB4 signaling in human symptomatic epilepsy. Sci Rep. 2017;7:141.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581.
Shi X, Zhang Q, Li J, Liu X, Zhang Y, Huang M, et al. Disrupting phosphorylation of Tyr-1070 at GluN2B selectively produces resilience to depression-like behaviors. Cell Rep. 2021;36:109612.
Abraham ME, Ong V, Gendreau J, Brown NJ, Choi EH, Shlobin NA, et al. Investigating deep brain stimulation of the habenula: a review of clinical studies. Neuromodulation. 2023;26:292–301.
Korlatowicz A, Pabian P, Solich J, Kolasa M, Latocha K, Dziedzicka-Wasylewska M, et al. Habenula as a possible target for treatment-resistant depression phenotype in wistar kyoto rats. Mol Neurobiol. 2023;60:643–54.
Sartorius, Kiening A, Kirsch KL, Gall P, von CC, Haberkorn U, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–11.
Wang Z, Jiang C, Guan L, Zhao L, Fan T, Wang J, et al. Deep brain stimulation of habenula reduces depressive symptoms and modulates brain activities in treatment-resistant depression. Nat Ment Health. 2024;2:1045–52.
Zhang Y, Ma L, Zhang X, Yue L, Wang J, Zheng J, et al. Deep brain stimulation in the lateral habenula reverses local neuronal hyperactivity and ameliorates depression-like behaviors in rats. Neurobiol Dis. 2023;180:106069.
Germann J, Mameli M, Elias GJB, Loh A, Taha A, Gouveia FV, et al. Deep brain stimulation of the habenula: systematic review of the literature and clinical trial registries. Front Psychiatry. 2021;12:730931.
Elias GJB, Boutet A, Joel SE, Germann J, Gwun D, Neudorfer C, et al. Probabilistic mapping of deep brain stimulation: insights from 15 years of therapy. Ann Neurol. 2021;89:426–43.
Davis LL, Frazier E, Husain MM, Warden D, Trivedi M, Fava M, et al. Substance use disorder comorbidity in major depressive disorder: a confirmatory analysis of the STAR*D cohort. Am J Addictions. 2006;15:278–85.
Hunt GE, Malhi GS, Lai HMX, Cleary M. Prevalence of comorbid substance use in major depressive disorder in community and clinical settings, 1990-2019: systematic review and meta-analysis. J Affect Disord. 2020;266:288–304.
Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Dependence. 1995;39:197–206.
Hashemzadeh I, Marquez-Arrico JE, Hashemzadeh K, Navarro JF, Adan A. Circadian functioning and quality of life in substance use disorder patients with and without comorbid major depressive disorder. Front Psychiatry. 2021;12:750500.
Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–7.
Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, et al. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci. 2014;34:9789–802.
Tandon S, Keefe KA, Taha SA. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion. J Physiol. 2017;595:1393–412.
Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–9.
Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12.
Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J Neurosci. 2012;32:12641–6.
Zuo W, Xiao C, Gao M, Hopf FW, Krnjević K, McIntosh JM, et al. Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms. Sci Rep. 2016;6:32937.
Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601.
Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–8.
Boulos LJ, Darcq E, Kieffer BL. Translating the habenula—from rodents to humans. Biol Psychiatry. 2017;81:296–305.
Cameron S, Weston-Green K, Newell KA. The disappointment centre of the brain gets exciting: a systematic review of habenula dysfunction in depression. Transl Psychiatry. 2024;14:1–27.
Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21:277–95.
Chen C, Wang M, Yu T, Feng W, Xu Y, Ning Y, et al. Habenular functional connections are associated with depression state and modulated by ketamine. J Affect Disord. 2024;345:177–85.
Acknowledgements
We would like to thank all the members of the McGill Group for Suicide Studies (MGSS) for their valuable input and support in this work. This project was supported by grants from the Canadian Institutes of Health Research (CIHR) and the National Institutes of Health (NIH). Financial support was also provided by Health Canada through the Canada Brain Research Fund, an innovative partnership between the Government of Canada (through Health Canada) and Brain Canada. Additional funding was contributed by the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains for Healthy Lives initiative, as well as by the Fonds de recherche du Québec - Santé (FRQS) through the Quebec Network on Suicide, Mood Disorders, and Related Disorders.
Author information
Authors and Affiliations
Contributions
Feiteng Lin led the literature search, defined the structural framework, and wrote the original draft and subsequent revisions. Kayleigh Casmey provided critical feedback and editing on the draft. Sierra Codeluppi-Arrowsmith contributed to the literature search, suggested key references, and provided revisions to the drafts. Gustavo Turecki provided senior oversight, contributed to the structural framework and conceptual scope of the review, and performed the final critical revision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, F., Casmey, K., Codeluppi-Arrowsmith, S.A. et al. The Habenula’s role in major depressive disorder: recent insights from preclinical and human studies. Transl Psychiatry (2026). https://doi.org/10.1038/s41398-026-03867-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-026-03867-0