Abstract
Background
There is no current consensus on the presence of viral infections in acute apical abscesses; therefore, this protocol for a systematic review and meta-analysis is designed to detail the procedures required to investigate the prevalence of viral infections in acute apical abscesses, a common dental condition characterized by pus accumulation due to bacterial infection. Viral infections in oral tissues have been linked to systemic health risks, including chronic inflammation and oncogenesis, which further emphasize the importance of understanding their role in acute apical abscesses.
Methods/design
We adopted a systematic review and meta-analysis protocol design followed by PRISMA guidelines. A priori protocol was registered in PROSPERO with registry number: CRD42023468287. Inclusion criteria will be established according to the PICO framework; hence, we will include original articles with no restriction on publication date or population group. The selective screening of information will be conducted by peers, starting with titles, abstracts, and keywords, and finally reviewing the full text. The risk of bias will be assessed using the ROBINS tool, and the certainty of the evidence will be evaluated following the GRADE guidelines. We will perform a random-effects meta-analysis, utilizing the Freeman-Tukey double arcsine transformation, to estimate the pooled prevalence of viral infections in acute apical abscesses, assess heterogeneity using the Q-test and I² statistic, evaluate potential publication bias with funnel plots and Egger’s test, and conduct sensitivity analyses to ensure robust results.
Discussion
At present, no consensus exists regarding the prevalence of viral infections in acute apical abscesses that could inform clinical dental practice. Moreover, the existing body of knowledge on this subject is notably limited. This approach is intended to provide data that will facilitate the improvement of clinical practice and serve as a methodological framework for studying various pathologies. By elucidating the prevalence of viral infections, the findings of this study could enhance diagnostic accuracy and inform more targeted and effective treatment strategies, ultimately improving patient outcomes.
Similar content being viewed by others
Introduction
The oral cavity is known to be the second most diverse microbial environment, encompassing approximately 700 species, including bacteria, fungi, viruses, and protozoa. This environment is notably intricate, with microorganisms colonizing tooth surfaces, soft oral mucosal tissues, and, as recent studies have emphasized, dental abscesses. These microbes play a vital role in both oral and systemic health [1,2,3].
Acute apical abscesses are a common issue in both dentistry and oral medicine, marked by the accumulation of pus at the apex of a tooth due to bacterial infection of the pulp tissue [4]. While these abscesses are extensively studied, there is growing interest in the role of viruses within them. Viruses, often neglected in discussions about dental infections, could significantly affect the development and severity of acute apical abscesses. They may act as reservoirs for viral infections that could go unnoticed by patients and healthcare providers alike [5, 6].
Research has identified the presence of various viruses, including Human Papillomavirus (HPV), Epstein-Barr Virus (EBV), and Cytomegalovirus (CMV), in potentially malignant lesions of the oral cavity and periodontium [7,8,9], as well as Human Herpesvirus (HHV) in Hodgkin’s lymphoma [10]. However, the prevalence of these viruses in acute apical abscesses—a frequent problem in dental settings—varies. Gaining a comprehensive understanding of the different viruses present in acute apical abscesses is essential to mitigate potential risks, as some of these viruses are associated with a high carcinogenic potential in the oral cavity.
Therefore, this manuscript aims to outline the steps necessary to conduct a systematic review and meta-analysis. This approach is intended to provide data that will facilitate the improvement of clinical practice and serve as a methodological framework for studying various pathologies.
Methods study design
We adopted a systematic review and meta-analysis design followed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The study protocol was registered on the International Prospective Register of Systematic Reviews (registry number: CRD42023468287).
Study selection
We will conduct electronic searches across PubMed, Google Scholar, Cochrane Library, and Elsevier databases to identify studies focused on the presence of viral infections in acute apical abscesses. The search strategy is detailed in Table 1.
Relevant publications will be searched from the inception of the databases up to June, 2025, with no restrictions on date or language. The titles and abstracts of the retrieved documents will be independently screened by two authors (G.A.S.R. and M.A.C.D.) to evaluate their relevance to our research objective. Following this, the authors will review the full text of all potentially relevant studies, applying the eligibility criteria to select studies for qualitative data synthesis. Any discrepancies between the authors reviewing the full texts will be resolved through consensus between C.P.S. and M.A.C.M.
Eligibility criteria
The eligibility criteria for the studies will be developed based on the components of the PECOS statement (population, exposure, comparators, outcome, and study design) [12], addressing the research question: “What is the prevalence of viruses in acute apical abscesses in humans?” (Table 2). We will include all original studies that investigate the presence of any type of virus in acute apical abscesses. Reviews, conference papers, and comments will be excluded.
Periodontal abscesses will also be excluded to maintain a focused scope, as their etiology and clinical management differ significantly from acute apical abscesses. The emphasis on original studies ensures the inclusion of primary data, minimizing
biases associated with secondary analyses and providing a robust foundation for systematic review and meta-analysis.
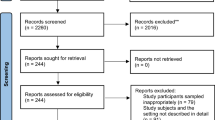
The process of searching and selecting studies to be included in this review will be summarized in a flow diagram based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Fig. 1).
Data extraction and quality assessment
Data from the eligible studies will be extracted independently by two authors (M.A.G.R. and J.A.H.M.) using a pre-piloted standard form. Information to be extracted will include: first author, year of publication, country where the study was conducted; sample size and sex of participants; age of participants; prevalence outcomes with virus subtypes (if reported); conclusions, findings, participants’ HIV status, and physical status according to the ASA Classification System, oral hygiene status, and risky sexual practices. When data of interest is not reported, authors will be contacted via email.
To ensure inter-rater reliability during the data extraction process, two authors (M.A.G.R. and J.A.H.M.) will extract data independently using a standardized form. Discrepancies between the extracted data will be resolved through discussion and, if necessary, consultation with a third author (G.C.V.). Cohen’s kappa statistics will be calculated to quantify the level of agreement between the reviewers, with a kappa value above 0.75 considered indicative of excellent agreement.
Risk of bias in each study will be evaluated using the Risk Of Bias In Non-randomized Studies—of Exposures (ROBINS-E) tool [13]. ROBINS-E tool assesses seven domains of bias: (1) confounding, (2) selection of participants into the study, (3) classification of exposures, (4) departures from intended exposures, (5) missing data, (6) measurement of outcomes, and (7) selection of the reported result.
The information from the included articles and the evaluation of their quality will be used to develop the qualitative synthesis of the data.
The certainty of the evidence will be assessed using the GRADE framework (Grading of Recommendations Assessment, Development, and Evaluation) [14], considering the study design, risk of bias, imprecision, inconsistency, indirectness, and publication bias to rate the level of evidence. The two aforementioned processes will be conducted by M.A.C.M. and supervised by G.C.V and M.A.C.D.
Statistical analysis
If sufficient quantitative data are available, we will estimate the pooled prevalence of viral infections in acute apical absences, and its 95% confidence interval based on the data reported in the studies will be included in this review. The pooled prevalence will be estimated by random-effects meta-analysis to account for heterogeneity across studies. Due to the potential low frequency of some viral infections, we will use the Freeman-Tukey double arcsine transformation in the meta-analysis to stabilize the prevalence variations reported in each primary study, allowing for a robust estimate of the pooled prevalence [15]. As a secondary analysis, we will conduct independent meta-analyses to determine the pooled prevalence of viral infections in acute apical abscesses according to the type of virus.
The Freeman-Tukey double arcsine transformation was selected for its ability to stabilize variances when dealing with prevalence rates that are near 0% or 100%, which can otherwise lead to misleading results in meta-analyses. This approach ensures that the pooled prevalence estimates are robust and not disproportionately influenced by studies reporting extreme values.
Statistical heterogeneity among the selected studies will be assessed using the Q-test (with p > 0.10 indicating the presence of heterogeneity) and the I² statistic (range 0–100%) [16]. According to Cochrane’s criteria, an I² < 30% indicates low heterogeneity, an I² between 30 and 60% indicates moderate heterogeneity, and an I² ≥ 75% indicates considerable heterogeneity. We will perform subgroup meta-analyses and meta-regression analyses in the presence of moderate or greater heterogeneity to identify potential sources of heterogeneity, such as geographic region, ASA Physical Status Classification, patient age, underlying medical conditions, poor oral hygiene, weak immune system, and risky sexual practices. These subgroup analyses aim to reveal unique trends or risk factors associated with viral infections in acute apical abscesses, which may provide insights into specific patient populations or clinical scenarios that warrant tailored interventions.
Potential publication bias will be assessed through visual inspection of funnel plots to detect asymmetric patterns and will be complemented with Egger’s test (with a p-value < 0.10 indicating publication bias) [17]. As a sensitivity analysis, we will explore the effect of individual studies on the pooled prevalence of viral infections in acute apical abscesses by excluding one study at a time and re-estimating the pooled prevalence. All statistical analyses will be conducted using STATA software. This analysis will be conducted by J.A.H.M. and supervised by C.P.S.
Conclusion
We anticipate identifying a significant prevalence of viral infections within acute apical abscesses, with variations in the prevalence rates depending on the specific viral subtypes involved. We expect the pooled prevalence estimates to reveal noteworthy heterogeneity across different geographic regions, patient demographics, and clinical conditions. The analysis may uncover correlations between viral presence and specific risk factors, including poor oral hygiene, immunocompromised status, and risky sexual behaviors. In addition, we predict that the systematic review and meta-analysis will underscore the necessity for incorporating viral diagnostics in the routine evaluation of acute apical abscesses, given the potential implications for both oral and systemic health. Ultimately, the findings are expected to contribute valuable insights into the role of viruses in dental infections, potentially influencing clinical practices and future research directions.
Data availability
The data generated when this systematic review and meta-analysis is completed will be available in an online repository (Mendeley Data).
Change history
22 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41405-025-00334-8
References
Baker JL, Bor B, Agnello M, Shi W, He X. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol. 2017;25:362–74. https://doi.org/10.1016/J.TIM.2016.12.012
Betz SJ. HPV-related papillary lesions of the oral mucosa: a review. Head Neck Pathol. 2019;13:80–90. https://doi.org/10.1007/s12105-019-01003-7
Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122–8. https://doi.org/10.4103/JOMFP.JOMFP_304_18
Siqueira JF, Rôças IN. Present status and future directions: microbiology of endodontic infections. Int Endod J. 2022;55:512–30. https://doi.org/10.1111/IEJ.13677
Petti S, Lodi G. The controversial natural history of oral herpes simplex virus type 1 infection. Oral Dis. 2019;25:1850–65. https://doi.org/10.1111/ODI.13234
Santosh AR, Muddana K. Viral infections of oral cavity. J Fam Med Prim Care. 2020;9:36. https://doi.org/10.4103/JFMPC.JFMPC_807_19
Rahman R, Gopinath D, Buajeeb W, Poomsawat S, Johnson NW. Potential role of Epstein-Barr virus in oral potentially malignant disorders and oral squamous cell carcinoma: a scoping review. Viruses. 2022;14. https://doi.org/10.3390/V14040801
Alramadhan SA, Fitzpatrick SG, Bhattacharyya I, Islam MN, Cohen DM. Changing trends in Benign Human Papillomavirus (HPV) related epithelial neoplasms of the oral cavity: 1995–2015. Head Neck Pathol. 2022;16:738–45. https://doi.org/10.1007/s12105-022-01426-9
de la Cour CD, Sperling CD, Belmonte F, Syrjänen S, Kjaer SK. Human papillomavirus prevalence in oral potentially malignant disorders: systematic review and meta-analysis. Oral Dis. 2021;27:431–8. https://doi.org/10.1111/ODI.13322
Wells MJ, Jacobson S, Levine PH. An evaluation of HHV-6 as an etiologic agent in Hodgkin lymphoma and brain cancer using IARC criteria for oncogenicity. Infect Agent Cancer. 2019;14. https://doi.org/10.1186/S13027-019-0248-3
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10. https://doi.org/10.1186/S13643-021-01626-4
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7. https://doi.org/10.1186/1472-6947-7-16
Risk of bias tools - ROBINS-E tool. Accessed February 11, 2024. https://www.riskofbias.info/welcome/robins-e-tool
GRADE Handbook | Cochrane Training. Accessed February 11, 2024. https://training.cochrane.org/resource/grade-handbook
Chen Y, Chen D, Wang Y, Han Y. Using Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Aesthetic Plast Surg. 2023;47:83–4. https://doi.org/10.1007/s00266-022-02977-6
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186
Harbord RM, Egger M, Sterne JAC. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. https://doi.org/10.1002/sim.2380
Acknowledgements
The authors would like to thank Jefatura de Investigación Pregrado of the Facultad de Odontología of the Universidad Autónoma de Nuevo León, and the División de Investigación of the Hospital Juárez de México for their support on this research.
Funding
This research was self-funded and did not receive any financial support.
Author information
Authors and Affiliations
Contributions
G.C.-V.: conceptualization, methodology, writing—original draft. G.A.S.-R.: formal analysis, investigation, data curation. M.A.G.-R.: supervision, writing—review & editing, project administration. J.Á.H.-M.: data curation, resources, visualization. M.A.C.-M.: methodology, validation, writing—review & editing. C.S.-P.: investigation, funding acquisition, resources. A.A.C.-D.: writing—original draft & funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hernández-Mariano, J.Á., Sánchez-Ramírez, G.A., Cano-Verdugo, G. et al. Protocol for a systematic review and meta-analysis of the prevalence of viral infections in acute apical abscesses. BDJ Open 11, 34 (2025). https://doi.org/10.1038/s41405-025-00320-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41405-025-00320-0