Abstract
Extranodal (EN) diffuse large B-cell lymphoma (DLBCL) has been historically associated with inferior survival outcomes compared to nodal DLBCL. However, outcomes of patients with EN DLBCL following chimeric antigen receptor T-cell (CAR-T) therapy are not well established. In this multi-center retrospective cohort study, we evaluated the outcomes of patients with EN DLBCL who underwent CAR-T in the relapsed/refractory (R/R) setting. The primary objective was overall survival (OS), while secondary objectives included progression-free survival (PFS), response rates, and toxicity rates. A total of 218 patients were included in the analysis. The most common sites of EN involvement were skin/soft tissue (25%), bone (22%), and lung (17%). Overall response rate (ORR) and complete response rate (CRR) at first post-treatment evaluation were 62% (n = 127) and 40% (n = 82), respectively. Median follow-up was 3.5 years. Median PFS and OS were 4.0 months (95% CI = 3.1–7.2) and 25.7 months (95% CI = 16.1–51.6), respectively. Cytokine release syndrome (CRS) of any grade occurred in 73% (n = 159) of patients, and 6% (n = 12) had grade ≥ 3 CRS. Immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade occurred in 37% (n = 81) of patients, and 19% (n = 41) developed grade ≥ 3 ICANS. In the multivariable analysis, factors that were independently prognostic of inferior OS were 3 or more lines of therapy prior to CAR-T, bulky disease at the time of CAR-T, hepatobiliary, and pancreas involvement, while refractory disease to the most recent therapy prior to CAR-T was associated with inferior PFS. Future studies should further evaluate outcomes of CAR-T in patients with specific EN sites of involvement that appear to be associated with inferior survival such as the liver and pancreas.
Similar content being viewed by others
Introduction
Extranodal (EN) lymphoma is defined by involvement of sites outside of the lymph nodes, spleen, thymus and oropharyngeal lymphoid tissue [1]. Up to 50% of patients with diffuse large B-cell lymphoma (DLBCL) have EN involvement at diagnosis [2, 3]. The most common sites of EN disease in DLBCL are the gastrointestinal tract, bone, bone marrow, and skin/soft tissue [3, 4]. EN DLBCL is distinct from nodal DLBCL in its molecular pathogenesis, clinical presentation, and prognosis [5, 6]. In addition, EN lymphoma is a highly heterogeneous disease, and biologic features and prognosis vary significantly based on the specific site of EN involvement [3, 6,7,8]. Low prevalence of each EN site has resulted in scarce data to inform best practices and optimal management in these patients [6].
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape of relapsed or refractory (R/R) DLBCL with approval in second line for primary refractory and early relapse as well as in the third line [9,10,11,12,13,14]. Prior studies evaluating the outcomes of EN DLBCL receiving CAR-T have been limited in study size and have shown conflicting findings on the impact of EN disease at time of CAR-T on outcomes [15, 16]. We therefore sought to evaluate outcomes and toxicities in patients with EN DLBCL undergoing CAR-T in the R/R setting using a large, multi-institutional database.
Patients and methods
Study Design
This is a multicenter retrospective cohort study of patients with EN R/R large B-cell lymphoma (LBCL) receiving CAR T-cell therapy from 8 US academic centers between January 1, 2016, and March 1, 2024. To be eligible, R/R LBCL patients must have received anti-CD19 CAR T-cell therapy. Patients with active EN involvement (either primary or secondary EN disease) at the time of apheresis were included. Patients with primary central nervous system (CNS) lymphoma were excluded, while those with secondary CNS lymphoma were excluded from the main (primary) analysis but were included in the secondary analysis. Patients who had EN disease at diagnosis but not at the time of CAR-T (n = 25) were excluded from the primary analysis and were only included in the exploratory analysis.
Study objectives and definitions
The primary objective was to evaluate the overall survival (OS) of patients with EN R/R LBCL undergoing CAR-T. Secondary objectives included progression-free survival (PFS), response rates (overall response rate [ORR] and complete response rate [CRR]) to CAR-T, and CAR-T toxicities including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), prolonged (>28 days post CAR-T infusion) need for transfusion of packed red blood cells (PRBCs) or platelets, and prolonged (>28 days post CAR-T infusion) need for granulocyte stimulating agents.
In this study, we retrospectively identified patients with LBCL and included patients with any subtype of DLBCL and high-grade B-cell lymphoma (HGBCL), as well as transformed indolent lymphoma histologies. Patients with disease that arises from an EN site are deemed to have primary EN lymphoma, while patients with disease arising from a nodal site and spreading to an EN site have secondary EN disease. OS was defined as the time from CAR-T infusion to death from any cause, and PFS was calculated from CAR-T infusion to radiologic or symptomatic disease progression, death from any cause, with censoring for those alive at the time of last follow up. ORR was defined as the proportion of patients achieving either a partial or complete response to CAR T-cell therapy, as determined by institutional radiologic response criteria on the first post-treatment positron emission tomography/computed tomography (PET/CT). CRR was defined as the proportion of patients showing no evidence of disease on the first post-treatment PET/CT, based on institutional radiologic response criteria.
Ethics approval and consent to participate
The study was approved by the institutional review board at Northwestern University and at all participating sites and was conducted in compliance with the Declaration of Helsinki. As this was a retrospective study, informed consent was waived.
Statistical analysis
Descriptive statistics were used to describe the demographic and disease characteristics. Median and range were used to summarize continuous variables, whereas categorical variables were described using frequency and percentages. PFS and OS were estimated using the Kaplan-Meier method. The Cox proportional hazards models were used to evaluate hazard ratios associated with the risk of progression or death. The univariable analysis for both OS and PFS was conducted using the same set of variables, which included all EN sites and other clinically important variables. Variables found to be significant in the univariable analysis were included in the multivariable analysis. Exploratory analyses were performed for other variables outside of those predetermined for univariable and multivariable models. Analyses were performed using R version 4.3.2, and all the estimates were reported with 95% confidence interval (CI).
Results
Patient characteristics
A total of 218 patients were included in the analysis (see Consort diagram, Figure S1). Baseline characteristics of the cohort are outlined in Table 1. The median age at diagnosis was 62 years (range: 20–90), with 64% (n = 140) males. At the time of CAR-T, 74% (n = 161) of the patients had DLBCL, 19% (n = 41) had high-grade B-cell lymphoma (HGBCL), and 7% (n = 16) had transformed indolent lymphoma. The median follow up time was 3.50 years (range: 0.08–6.99).
Sites of EN involvement
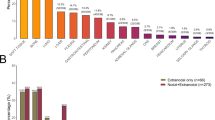
At the time of CAR-T, 62% (n = 134) of patients had 1 EN site involved, and 38% (n = 83) of patients had presence of two or more EN sites. The most common site involved was skin/soft tissue in 25% (n = 55) of patients, followed by bone (22%, n = 48) and lung (17%, n = 38) involvement. Other common sites involved included the gastrointestinal (GI) tract, bone marrow and liver. Distribution of EN sites involved at the time of CAR-T is illustrated in Fig. 1. Outcomes by site of EN involvement are outlined in Table S1.
Permission granted by Wiley to reproduce figure from: https://doi.org/10.1002/ajh.25493.
Response rates and Toxicities
ORR and CRR at first post-treatment evaluation were 62% (n = 127) and 40% (n = 82), respectively. Response rates are detailed in Table S2.
CRS of any grade occurred in 73% (n = 159) of patients, and 6% (n = 12) had grade ≥ 3 CRS. The median duration of CRS was 4 days (range: 1–57). ICANS of any grade occurred in 37% (n = 81) of patients, and 19% (n = 41) developed grade ≥ 3 ICANS. The median duration of ICANS was 5 days (range: 1–188).
Prolonged need for PRBC or platelet transfusion occurred in 19% (n = 39) and 20% (n = 42) of the patients, respectively. Prolonged need for granulocyte colony-stimulating factor (G-CSF) occurred in 36% (n = 75) of patients. A summary of CAR-T toxicities is outlined in Table 2.
Progression-free survival
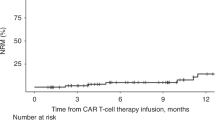
The median PFS was 4.0 months (95% CI = 3.1–7.2). The 1-, 3-, and 5-year PFS estimates were 38% (95% CI = 32–45), 33% (95% CI = 27–40), and 30% (95% CI = 24–38), respectively (Fig. 2A).
In the univariable analysis, factors associated with significantly inferior PFS included age-adjusted IPI ≥ 2, bulky disease at the time of CAR-T, defined as a tumor measuring ≥ 7 centimeters (cm), refractory disease to the most recent therapy prior to CAR-T, use of bridging therapy prior to CAR-T, and EN disease involving the liver/biliary tract (Table S3). In the multivariable analysis, refractory disease to the most recent therapy prior to CAR-T remained associated with significantly inferior PFS, with a hazard ratio (HR) of 2.55 (95% CI = 1.36–4.79, p < 0.01, Table S3).
Overall survival
The median OS was 25.7 months (95% CI = 16.1–51.6). The 1-, 3-, and 5-year OS estimates were 63% (95% CI = 56–70), 45% (95% CI = 38–53), and 41% (95% CI = 35–50), respectively (Fig. 2B).
In the univariable analysis, factors associated with significantly inferior OS included ≥ 3 lines of therapy prior to CAR-T, age-adjusted IPI ≥ 2, bulky disease at the time of CAR-T (≥7 cm), refractory disease to the most recent therapy prior to CAR-T, use of bridging therapy prior to CAR-T, EN involvement of the liver/biliary tract, and pancreas (Table 3). After controlling for these factors in the multivariable analysis, patients receiving ≥ 3 lines of therapy prior to CAR-T (HR = 2.20, 95% CI = 1.28–3.78, p < 0.01), bulky disease at the time of CAR-T (HR = 2.56, 95% CI = 1.40–4.66, p = 0.01), liver/biliary tract involvement (HR = 2.05, 95% CI = 1.01–4.15, p = 0.05) and pancreas involvement (HR = 6.2, 95% CI = 2.20–17.46, p < 0.01) remained independently prognostic of inferior OS (Table 3).
Outcomes for patients with EN disease including secondary CNS involvement who underwent CAR-T
As secondary CNS involvement accounts for the highest risk EN disease, we performed secondary analysis of patients with EN involvement including those with secondary CNS lymphoma (n = 236) who underwent CAR-T.
CRS of any grade occurred in 74% (n = 175) of patients and 9% (n = 15) had grade ≥ 3 CRS. ICANS of any grade occurred in 38% (n = 90) of patients, and 19% (n = 45) developed grade ≥ 3 ICANS. The rates of CRS and ICANS were similar to that seen in patients with EN disease without secondary CNS involvement. A summary of CAR-T toxicities for patients with EN disease who underwent CAR-T based on secondary CNS involvement are outlined in Table S4. The response rates (ORR and CRR) were comparable for patients with EN disease who underwent CAR-T regardless of the secondary CNS involvement (see Table S5).
The median PFS was 4.0 months (95% CI = 3.1–7.2). The 1-, 3-, and 5-year PFS estimates were 38% (95% CI = 32–44), 32% (95% CI = 26–39), and 29% (95% CI = 23-36), respectively (Figure S2A). The median OS was 23.8 months (95% CI = 16.0–37.9). The 1-, 3-, and 5-year OS estimates were 62% (95% CI = 56–68), 44% (95% CI = 38–52), and 41% (95% CI = 34–49), respectively (Figure S2B). These survival estimates were comparable to patients with EN disease who underwent CAR-T without secondary CNS involvement.
Exploratory analysis
We considered whether patients who had EN disease at initial diagnosis but no longer had EN disease at the time of apheresis would have different outcomes from patients who had active EN disease at apheresis (Table S6). An exploratory analysis comparing these patient groups showed no differences in toxicity (Table S7) or efficacy outcomes (Table S8 and Figures S3).
Discussion
In this multi-institutional retrospective cohort study, we evaluated the outcomes of patients with R/R LBCL receiving CAR T-cell therapy and made several important observations. First, the ORR and CRR were 62% and 40%, respectively. Second, the rates of grade ≥3 CRS and ICANS were 6% and 19%, respectively. Third, refractory disease to the most recent therapy prior to CAR-T was associated with significantly inferior PFS in the multivariable analysis. Lastly, we found that 3 or more lines of therapy prior to CAR-T, bulky disease at the time of CAR-T, hepatobiliary, and pancreas involvement were independently associated with significantly inferior OS.
Previous real-world studies evaluating outcomes associated with CAR-T in R/R LBCL found ORR and CRR ranging between 60-80% and 30-65%, respectively [17,18,19,20], which is comparable to the response rates noted in our study. Similarly, the median PFS following CAR-T in our study was in line with the published data (3-9 months) [17,18,19,20], however, the median OS post-CAR-T was slightly longer in our study (25.7 months) relative to the prior studies (12–22 months) [17,18,19,20]. There was a higher proportion of patients in our study that had received 1–2 lines of therapy prior to CAR-T versus 3 or more prior lines of therapy, which may account for this difference in OS. Of note, most of these real-world studies did not provide information regarding the proportion of patients with EN LBCL versus nodal LBCL in their cohort.
In prior studies, the most common toxicities post CAR-T included CRS, ICANS, and prolonged cytopenias [17,18,19,20]. Rate of CRS typically ranged between 55-85%, with 5-10% developing grade ≥ 3 CRS. Rate of ICANS ranged between 30–55%, with 5–25% developing grade ≥ 3 ICANS. Prolonged cytopenia (most commonly neutropenia and thrombocytopenia) occurred in 10–25% of patients [17,18,19,20]. In line with these studies, we found a comparable rate of all-grade and grade ≥ 3 CRS (73% and 6%, respectively), all-grade and grade ≥ 3 ICANS (37% and 19%, respectively) and prolonged need for blood count support (18% for PRBC transfusions, 20% for platelet transfusions, 36% for G-CSF) in our study.
A few retrospective studies have compared the outcomes of EN DLBCL and nodal DLBCL following CAR-T. One retrospective study of 37 patients with R/R DLBCL treated with CAR-T (n = 19 patients with EN DLBCL, n = 18 patients with nodal DLBCL) showed that one-year PFS and OS were inferior in patients with EN DLBCL compared to nodal DLBCL (PFS = 83.3% vs. 42.1%, p = 0.008; OS = 94.4% vs. 63.2%, p = 0.020) [15]. This study had a small sample size and limited median follow up of 13 months. A separate study evaluated 126 patients receiving CAR-T for R/R DLBCL in patients with EN DLBCL (n = 72) versus nodal DLBCL (n = 52) and showed similar median PFS (10.8 vs. 14.1 months, p = 0.126) and OS (15.4 vs. 18.4 months, p = 0.10) between the two groups [16]. A third retrospective study of 47 patients who underwent CAR-T for R/R LBCL (n = 25 patients with EN DLBCL, n = 22 patients with nodal DLBCL) showed no statistically significant difference in OS between patients with versus without EN disease at the time of CAR-T [21]. A fourth recently published study of 516 patients with R/R LBCL (n = 177 patients with only nodal disease, n = 66 patients with only EN disease, n = 273 patients with both nodal and EN disease) showed no outcome difference between patients with nodal versus EN disease, although patients with both nodal and EN involvement had shorter PFS and OS [22]. Our results support the findings of the latter studies suggesting similar outcomes to CAR-T in patients with EN R/R LBCL compared to nodal LBCL, as the PFS, OS, and toxicity rates in our cohort were comparable to those traditionally described in the literature for R/R LBCL.
We identified several risk factors in patients with EN R/R LBCL undergoing CAR-T that were independently prognostic of inferior OS, including three or more lines of therapy prior to CAR-T and bulky disease at the time of CAR-T. The site of EN involvement is also important, as evidenced by our findings that involvement of the liver/biliary tract and pancreas at the time of CAR-T portended poor OS. Findings from a recently published study detailed above (n = 516) also indicated poorer outcomes (shorter PFS) in patients with liver and pancreas involvement [22]. We postulate that different sites of involvement may be associated with distinct tumor microenvironments, with some features resulting in additional mechanisms of resistance to CAR-T.
Of note, given the poor prognosis associated with CAR-T for patients with secondary CNS involvement [4, 23,24,25], we performed additional analysis evaluating the outcomes of patients with EN disease with secondary CNS involvement who underwent CAR-T. The toxicity and efficacy outcomes in this cohort were found to be comparable in those without secondary CNS involvement. One potential reason for this might be related to the small sample size of the patients with secondary CNS lymphoma who underwent CAR-T (n = 18) in the current study.
Our study is limited by the inherent limitations of the retrospective study design. The molecular and clinical characteristics of primary versus secondary disease are distinct from each other [6], however, due to sample size limitations we did not have sufficient statistical power to evaluate the differences in outcomes between primary and secondary EN LBCL. Given the relatively small sample size of each individual EN site, we were not able to further analyze trends regarding patient/disease characteristics within these subgroups. We could not analyze the outcomes based on nodal and extranodal versus extranodal only given the way data was collected. Lastly, the study was not designed to compare the outcomes of those with and without EN disease at CAR-T and the results of the exploratory analysis should be interpreted as such.
Conclusions
In this large, multi-institutional study evaluating the outcomes of patients with EN R/R LBCL undergoing CAR-T, the response rates, survival outcomes and the rate of high-grade CRS and ICANS were comparable to the real-world outcomes of CAR-T in R/R LBCL. Future studies should further evaluate outcomes of CAR-T in patients with specific EN sites of involvement that appear to be associated with inferior survival such as the liver and pancreas.
Data availability
Data is available upon request to the corresponding author as permitted by the IRB.
References
Krol ADG, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14:131–9.
Suresh Babu MC, Sreevalli A. Extranodal diffuse large B-cell lymphoma: Real-world experience from an Indian tertiary cancer center. Cancer Res Stat Treat. 2022;5:119–121.
Nair SC, Gogia A, Arora S, Kumar L, Sharma A, Biswas A, et al. Clinicopathologic features and outcomes of diffuse large B-cell lymphoma with extranodal involvement: a retrospective analysis. Cancer Res Stat Treat. 2022;5:67–74.
Ollila TA, Olszewski AJ. Extranodal diffuse large B cell lymphoma: molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. 2018;19:38.
Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation-a population-based study of 1575 cases. Br J Haematol. 2004;124:151–9.
Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v91–v102.
Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol. 2014;89:310–4.
El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D, et al. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: A Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol. 2015;90:1041–6.
Epperla N, Kumar A, Abutalib SA, Awan FT, Chen Y-B, Gopal AK, et al. ASTCT Clinical Practice Recommendations for Transplantation and Cellular Therapies in Diffuse Large B Cell Lymphoma. Transplant Cell Ther, Off Publ Am Soc Transplant Cell Ther. 2023;29:548–55.
Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl J Med. 2022;386:640–54.
Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141:1675–84.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl J Med. 2019;380:45–56.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England J Med. 377:2531–44.
Song Z, Xu L, Tang G, Gao L, Wang L, Ni X, et al. Prognostic differences of refractory/relapsed nodal and extranodal diffuse large B-cell lymphoma in the chimeric antigen receptor T cell therapy era. Clin Chim Acta. 2022;532:72–8.
Beyar KO, Perry C, Grisariu-Greenzaid S, Yehudai-Ofir D, Luttwak E, Avni B, et al. Response rates of extra-nodal diffuse large B cell lymphoma to anti-CD19-CAR T cells: A real word retrospective multicenter study. Eur J Haematol. 2023;111:63–71.
Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transpl Cell Ther. 2022;28:581.e1–e8.
Crombie JL, Nastoupil LJ, Andreadis C, Isufi I, Hunter B, Winter A, et al. Multicenter, Real-World Study in Patients with R/R Large B-Cell Lymphoma (LBCL) Who Received Lisocabtagene Maraleucel (liso-cel) in the United States (US). Blood. 2023;142:104.
Jacobson CA, Munoz J, Sun F, Kanters S, Limbrick-Oldfield EH, Spooner C, et al. Real-world outcomes with chimeric antigen receptor T cell therapies in large B cell lymphoma: a systematic review and meta-analysis. Transpl Cell Ther. 2024;30:77.e1–e15.
Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021;10:3214–23.
Voltin CA, Gödel P, Beckmann L, Heger JM, Kobe C, Kutsch N, et al. Outcome prediction in patients with large B-cell lymphoma undergoing chimeric antigen receptor T-cell therapy. Hemasphere. 2023;7:e817.
Iacoboni G, Rejeski K, Navarro V, van Meerten T, Rampotas A, Martín-López A, et al. Site-specific analysis of extranodal involvement in large B-cell lymphoma reveals distinct efficacy with chimeric antigen receptor T-cell therapy. Leukemia. 2025.
El-Galaly TC, Cheah CY, Bendtsen MD, Nowakowski GS, Kansara R, Savage KJ, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer. 2018;93:57–68.
Epperla N, Feng L, Shah NN, Fitzgerald L, Shah H, Stephens DM, et al. Outcomes of patients with secondary central nervous system lymphoma following CAR T-cell therapy: a multicenter cohort study. J Hematol Oncol. 2023;16:111.
Epperla N, Hashmi H, Ahn KW, Allbee-Johnson M, Chen AI, Wirk B, et al. Outcomes of patients with secondary central nervous system lymphoma treated with chimeric antigen receptor T-cell therapy: A CIBMTR analysis. Br J Haematol. 2024;205:1202–7.
Author information
Authors and Affiliations
Contributions
FS was involved in the study design, collection and assembly of data, data interpretation, and writing the first draft. SB was involved in data analysis and data interpretation and provided critical and insightful comments. PGD was involved in the collection of data, data interpretation and provided critical and insightful comments. MJ was involved in the collection of data, data interpretation and provided critical and insightful comments. KA was involved in the collection of data, data interpretation and provided critical and insightful comments. AEK was involved in the collection of data, data interpretation and provided critical and insightful comments. AG was involved in the collection of data, data interpretation and provided critical and insightful comments. YKT was involved in the collection of data, data interpretation and provided critical and insightful comments. BM was involved in the collection of data, data interpretation and provided critical and insightful comments. KA was involved in the collection of data, data interpretation and provided critical and insightful comments. AH was involved in the collection of data, data interpretation and provided critical and insightful comments. JPA was involved in the collection of data, data interpretation and provided critical and insightful comments. OA was involved in the collection of data, data interpretation and provided critical and insightful comments. KP was involved in the collection of data, data interpretation and provided critical and insightful comments. AT was involved in the collection of data, data interpretation and provided critical and insightful comments. ISL was involved in the collection of data, data interpretation and provided critical and insightful comments. LF was involved in the collection of data, data interpretation and provided critical and insightful comments. TAO was involved in the collection of data, data interpretation and provided critical and insightful comments. MJM was involved in the collection of data, data interpretation and provided critical and insightful comments. JK was involved in the collection of data, data interpretation and provided critical and insightful comments. RK was involved in the collection of data and data interpretation and provided critical and insightful comments. NE was involved in the conception and study design, collection and assembly of data, data interpretation, and critically edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
NE: Research Funding (institution) from Beigene, ADC Therapeutics, Lilly, Incyte, and Ipsen. Advisory board for Genentech, CRISPR Therapeutics and Ipsen. RK: Advisory Board: BMS, Genentech/Roche, Abbvie, GenMab; Speakers Bureau: BeiGene, AstraZeneca, BMS. JPA: Research support: ADC Therapeutics, Genmab, AbbVie, and BeiGene. Consultancy: ADC Therapeutics, Genentech, AbbVie, Lilly, Novartis, and Regeneron. TAO: Ad board for ADC therapeutics, Honoraria and research support for ONO pharmaceuticals.
Third party material
Permission granted by Wiley to reproduce Fig. 1 from: https://doi.org/10.1002/ajh.25493.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
St-Pierre, F., Bhatta, S., Doukas, P.G. et al. Impact of extranodal involvement at CAR T-cell therapy on outcomes in patients with relapsed or refractory large B-cell lymphoma—Results from a multicenter cohort study. Blood Cancer J. 15, 110 (2025). https://doi.org/10.1038/s41408-025-01318-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01318-5
This article is cited by
-
Anatomical patterns at first confirmed relapse or refractory disease predict outcomes in diffuse large B-cell lymphoma
International Journal of Hematology (2025)