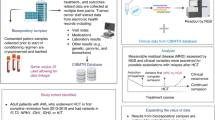
Abstract
Systematic reviews apply rigorous methodologies to address a pre-specified, clearly formulated clinical research question. The conclusion that results is often cited to more robustly inform decision-making by clinicians, third-party payers and managed care organizations about the clinical question of interest. While systematic reviews provide a rigorous standard, they may be unfeasible when the task is to create general disease-focused guidelines comprised of multiple clinical practice questions versus a single major clinical practice question. Collaborating transplantation and cellular therapy societal committees also recognize that the quantity and or quality of reference sources may be insufficient for a meaningful systematic review. As the conduct of systematic reviews has evolved over time in terms of grading systems, reporting requirements and use of technology, here we provide current guidance in methodologies, resources for reviewers, and approaches to overcome challenges in conducting systematic reviews in transplantation and cellular therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
03 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41409-021-01316-8
References
Jones R, Horowitz M, Wall D, Wingard J, Wolff S, McCarthy P, et al. Evidence-based reviews and the role of blood and marrow transplantation in the treatment of selected disease: an ASBMT policy statement. American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2000;6:523.
Jones R, Horowitz M, Wall D, Wingard J, Wolff S, McCarthy P, et al. ASBMT policy statement regarding the methodology of evidence-based reviews in evaluating the role of blood and marrow transplantation in the treatment of selected diseases. American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2000;6:524–5.
Schmidt LM, Gotzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pr. 2005;54:334–8.
Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–80.
Petticrew M. Systematic reviews from astronomy to zoology: myths and misconceptions. BMJ. 2001;322:98–101.
Garritty C, Stevens A, Hamel C, Golfam M, Hutton B, Wolfe D. Knowledge synthesis in evidence-based medicine. Semin Nucl Med. 2019;49:136–44.
Sargeant JM, O’Connor AM. Introduction to systematic reviews in animal agriculture and veterinary medicine. Zoonoses Public Health. 2014;61(Suppl 1):3–9.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108.
Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic press; 1985.
OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. UK: Oxford Centre for Evidence-Based Medicine Oxford; 2011.
Shipp MA, Abeloff MD, Antman KH, Carroll G, Hagenbeek A, Loeffler M, et al. International Consensus Conference on High-Dose Therapy with Hematopoietic Stem Cell Transplantation in Aggressive Non-Hodgkin’s Lymphomas: report of the jury. J Clin Oncol. 1999;17:423–9.
Chalmers TC, Berrier J, Sacks HS, Levin H, Reitman D, Nagalingam R. Meta-analysis of clinical trials as a scientific discipline. II: replicate variability and comparison of studies that agree and disagree. Stat Med. 1987;6:733–44.
Jones R, Nieto Y, Rizzo JD, Wall D, Wingard JR, McCarthy P Jr, et al. The evolution of the evidence-based review: evaluating the science enhances the art of medicine–statement of the Steering Committee for Evidence-Based Reviews of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2005;11:819–22.
Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–6.
Hahn T, Wolff SN, Czuczman M, Fisher RI, Lazarus HM, McCarthy PL, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of diffuse large cell B-cell non-Hodgkin’s lymphoma: an evidence-based review. Biol Blood Marrow Transpl. 2001;7:308–31.
Hahn T, Wingard JR, Anderson KC, Bensinger WI, Berenson JR, Brozeit G, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: an evidence-based review. Biol Blood Marrow Transpl. 2003;9:4–37.
Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transpl. 2005;11:823–61.
Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: an evidence-based review. Biol Blood Marrow Transpl. 2006;12:1–30.
Oliansky DM, Rizzo JD, Aplan PD, Arceci RJ, Leone L, Ravindranath Y, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transpl. 2007;13:1–25.
Oliansky DM, Appelbaum F, Cassileth PA, keating A, Kerr J, Nieto Y, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: an evidence-based review. Biol Blood Marrow Transpl. 2008;14:137–80.
Oliansky DM, Antin JH, Bennett JM, Deeg HF, Engelhardt C, Heptinstall KV, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transpl. 2009;15:137–72.
Jones RB, Nieto Y, Wall D, Wingard JR, Rizzo JD, Mccarthy PL Jr, et al. Methodology for updating published evidence-based reviews evaluating the role of blood and marrow transplantation in the treatment of selected diseases: a policy statement by the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2009;15:761–2.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8:iii–iv.1–72
Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009:CD001055.
University of York NHS Centre for Reviews Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York, UK: Centre for Reviews and Dissemination, University of York; 2009.
Graham ID, Tetroe J, Gagnon M. Lost in translation: just lost or beginning to find our way? Ann Emerg Med. 2009;54:313–4. discussion 4
IOM (Institute of Medicine). In: Eden J, Levit L, Berg A, Morton S, editors. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The Nationaal Academies Press; 2011.
Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Chapter 2: determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, LiT, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 6.1 (updated September 2020). Cochrane; 2020. Available from http://www.training.cochrane.org/handbook.
Oliansky DM, Larson RA, Weisdorf D, Dillon H, Ratko TA, Wall D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukemia: update of the 2006 evidence-based review. Biol Blood Marrow Transpl. 2012;18:18–36. Available from http://www.training.cochrane.org/handbook.
Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transpl. 2012;18:505–22.
Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, et al. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2015;21:1155–66.
Oliansky DM, Czuczman M, Fisher RI, Irwin FD, Lazarus HM, Omel J, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transpl. 2011;17:20–47.
DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the American Society of Transplantation and Cellular Therapy. Biol Blood Marrow Transpl. 2019;25:2113–3123.
Dholaria B, Savani BN, Hamilton BK, Oran B, Lui HD, Tallman MS et al. Hematopoietic cell transplantation in the treatment of newly disgnosed adult acute myeloid leukemia: an evidence-based review from the American Society of Transplantation and Cellular Therapy. Biol Blood Marrow Transpl. 2020; e-pub ahead of print 20 Sep 2020; https://doi.org/10.1016/j.bbmt.2020.09.020.
Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncery TR, Langston A, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001.
Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–54.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Morton S, Berg A, Levit L, Eden J. Standards for Initiating a Systematic Review. Finding what works in health care: standards for systematic reviews. Washington, DC: National Academies Press; 2011.
McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how that will be grouped for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 6.1 (updated September 2020). Cochrane; 2020. http://www.training.cochrane.org/handbook.
Moher D, Shamseer, Clarke M, Dhersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRIMSA-P) 2015 statement. Syst Rev. 2015;4:1.
PROSPERO: International prospective register of systematic reviews. (Accessed 13 Aug, 2020 https://www.crd.york.ac.uk/prospero/.)
Cochrane Library. (Accessed 13 Aug, 2020 https://www.cochranelibrary.com/.
F1000 Research. (Accessed 13 Aug, 2020 https://f1000research.com/
Joanna Briggs Institute. (Accessed 13 Aug, 2020 https://joannabriggs.org/.
OSF registries. https://osf.io/registries.
Zenodo (Accessed 13 Aug, 2020 https://zenodo.org/.
Male-specific late effects after hematopoietic cell transplantation. 2020. (Accessed 13 Aug 2020 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020147640.
Wu YP, Aylward BS, Roberts MC, Evans SC. Searching the scientific literature: implications for quantitative and qualitative reviews. Clin Psychol Rev. 2012;32:553–7.
Morton S, Berg A, Levit L, Eden J. Standards for Finding and Assessing Individual Studies. Finding what works in health care: standards for systematic reviews. Washington, DC: National Academies Press; 2011.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62:944–52.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Joannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Altman DG. Measuring agreement. In: Altman DG, editors. Practical Statistics for Medical Research. London, UK: CRC press; 1991.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Guyatt GH, Oxman AD, Schűnemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Alonso-Coello P, Schűnemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schűnemann HJ, GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8.
Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NH, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019;366:I44898.
Rashidi A, Meybodi MA, Cao W, Chu H, Warkick ED, Devine S, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation in myelodysplastic syndromes: systematic review and meta-analysis. Biol Blood Marrow Transpl. 2020;26:e138–e41.
Sterne JA, Hernan MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Aulakh S, Reljic T, Yassine F, Ayala E, Chavez JC, Chanan-Khan A et al. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther 2020; epub ahead of print 15 May 2020; https://doi.org/10.1016/j.hemonc.2020.05.002.
Parrondo RD, Reljic T, Iqbal M, Ayala E, Tun HW, Kharfan-Dabaja MA et al. Efficacy of autologous and allogeneic hematopoietic cell transplantation in Waldenstrӧm Macroglobulinemia: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2020; epub ahead of print 2 Jun 2020; https://doi.org/10.1016/j.clml.2020.05.021.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Prod ESRC Methods Program Version 1. 2006;1(April):b92.
Bushman BJ, Wang MC. Vote-counting procedures in meta-analysis. In: Cooper HHL, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis, 2nd edn. New York, NY, US: Russell Sage Foundation; 2009. p. 207–20.
Cwikel J, Behar L, Rabson-Hare J. A comparison of a vote count and a meta-analysis review of intervention research with adult cancer patients. Res Soc Work Pract. 2000;10:139–58.
Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: SAGE publications, Inc; 2001.
Verbeek J, Ruotsalainen J, Hoving JL. Synthesizing study results in a systematic review. Scand J Work Environ Health. 2012;38:282–90.
Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: an overview with suggestions for practice. Res Synth Methods. 2010;1:66–80.
Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med. 2002;21:1503–11.
Lehrnbecher T, Fisher BT, Phillips B, Alexander S, Ammann RA, Beauchemin M, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis. 2020;71:226–36.
Berkman ND, Lohr KN, Ansari MT, Balk EM, Kane R, McDonagh M, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015;68:1312–24.
Development and Update of the NCCN Guidelines. (Accessed 13 Aug, 2020 https://www.nccn.org/professionals/development.aspx.
USPHS/IDSA Prevention of Opportunistic Infections Working Group. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA). MMWR Recomm Rep. 1999;48:1–59.61–6.
Page M, McKenzie J, Bossuty P, Boutron I, Hoffmann T, Mulrow C et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. (Accessed 18 Oct, 2020) https://osf.io/preprints/metaarxiv/v7gm2.
Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
APA Publications Communications Board Working Group on Journal Article Reporting Standards. Reporting standards for research in psychology: why do we need them? What might they be? Am Psychol. 2008;63:839–51.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–30.
Khera N. From evidence to clinical practice in blood and marrow transplantation. Blood Rev. 2015;29:351–7.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–73.
Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transpl. 2019;25:2305–21.
Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical utilization of chimeric antigen receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transpl. 2019;54:1868–80.
Bubalo J, Carpenter PA, Majhail N, Perales MA, Marks DI, Shaughnessy P, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transpl. 2014;20:600–16.
McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–62.
Montoto S, Corradini P, Dreyling M, Ghielmini M, Kimby E, López-Guillelrmo A, et al. Indications for hematopoietic stem cell transplantation in patients with follicular lymphoma: a consensus project of the EBMT-Lymphoma Working Party. Haematologica. 2013;98:1014–21.
Krӧger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–33.
Kharfan-Dabaja MA, Kumar A, Hamadani M, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2016;22:2117–25.
Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, Reimer P, Gisselbrecht C, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: aninternational collaborative effort on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2017;23:1826–38.
Kanate AS, Kumar A, Dreger P, Dreyling M, Le Gouill S, Corradini P, et al. Maintenance therapies for Hodgkin and non-Hodgkin lymphomas after autologous transplantation: a consensus project of ASBMT, CIBMTR, and the Lymphoma Working Party of EBMT. JAMA Oncol. 2019;5:715–22.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazii F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167.
Acknowledgements
We thank Keith A. Laycock, PhD, ELS, for scientific editing and Matthew J. Page, PhD for critical review of the paper.
Funding
The National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health supported this publication under Award Number K23HL150232 (SMB). AS is the recipient of an American Society of Scholar Award. The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the NHLBI and the National Institute of Allergy and Infectious Diseases (NIAID); R21HL140314 and U01HL128568 from the NHLBI; HHSH250201700006C, SC1MC31881-01-00 and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612 and BARDA. The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, HRSA or any other agency of the United States Government.
Author information
Authors and Affiliations
Contributions
AS wrote the first draft of the paper. SB and LJB critically reviewed and wrote several sections. AS and SB contributed equally to the paper. All authors provided input, reviewed the final paper, and agreed with its content.
Corresponding author
Ethics declarations
Conflict of interest
AS is a paid consultant for Spotlight Therapeutics and CRISPR Therapeutics/Vertex Pharmaceuticals and Novartis. Between 2017 and 2020, Hélène Schoemans has participated in advisory boards for Incyte (2018) and Janssen & Novartis (2020). She has received speaker’s fees from Jazz Pharmaceuticals (2017); Novartis & Incyte (2018) and Incyte, Jazz Pharmaceuticals & Takeda (2019). She has received travel grants from EBMT (2017); EBMT, Celgene & Abbvie (2018); EBMT & Incyte (2019) and EBMT & Gilead (2020). Research funding was also received from Novartis for an investigator-initiated study and consultancy (2020). None of the other authors has a financial disclosure to make.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is co-published in the journals Bone Marrow Transplantation and Transplantation and Cellular Therapy https://doi.org/10.1038/s41409-020-01199-1 or https://doi.org/10.1016/j.jtct.2020.12.002
The original online version of this article was revised: the Funding information was incorrect.
Rights and permissions
About this article
Cite this article
Sharma, A., Badawy, S.M., Suelzer, E.M. et al. Systematic reviews in hematopoietic cell transplantation and cellular therapy: considerations and guidance from the American Society for Transplantation and Cellular Therapy, European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research late effects and quality of life working committee. Bone Marrow Transplant 56, 786–797 (2021). https://doi.org/10.1038/s41409-020-01199-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-020-01199-1