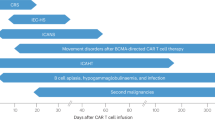
Abstract
CAR T-cell therapies have revolutionized the treatment of hematologic malignancies; however, alongside the well-known complications of cytokine release syndrome (CRS), neurotoxicity, immune effector cell–associated hematotoxicity (ICAHT), and infections, other non-classical toxicities are emerging. This review, developed by the EBMT Practice Harmonization and Guidelines Committee, addresses the management of other critical post-CAR T-cell complications including hemophagocytic lymphohistiocytosis (HLH), graft-versus-host disease (GvHD), thrombotic microangiopathy (TMA), coagulation disorders and secondary malignancies. These complications, though less frequent, present a significant challenge, often contributing to morbidity and mortality with no standardized management protocols. This review provides best practice recommendations for early identification, risk mitigation, and therapeutic interventions, supported by limited but emerging clinical evidence. Comprehensive expert guidance is essential for optimal management of these under-explored toxicities following CAR T-cell therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle C, Brudno J, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Hayden PJ, Roddie C, Bader P, Basak G, Bonig H, Chabannon C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33:259–75.
Yakoub-Agha IR, Greco R, Onida F, de la Camara R, Ciceri F, Cobagioglu S, et al. Practice harmonization workshops of EBMT: an expert-based approach to generate practical and contemporary guidelines within the arena of hematopoietic cell transplantation and cellular therapy. Bone Marrow Transpl. 2023;58:696–700.
Janka GE, Aricò M. Clinical features, diagnosis and therapy of familial haemophagocytic lymphohistiocytosis. Acta Paediatr. 2021;110:2723–8.
Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–81.
Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–16.
Sandler RD, Tattersall RS, Schoemans H, Greco R, Badoglio M, Labopin M, et al. Diagnosis and management of secondary HLH/MAS following HSCT and CAR-T cell therapy in adults; a review of the literature and a survey of practice within EBMT centres on behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP). Front Immunol. 2020;11:524.
Rejeski K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142:865–77.
Lichtenstein DA, Schischlik F, Shao L, Steinberg S, Yates B, Wang H, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138:2469–84.
Hines MR, Knight TE, McNerney KO, Leick M, Jain T, Ahmed S, et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl Cell Ther. 2023;29:438.e1–e16.
Löfstedt A, Jädersten M, Meeths M, Henter JI. Malignancy-associated hemophagocytic lymphohistiocytosis in Sweden: incidence, clinical characteristics, and survival. Blood. 2024;143:233–42.
Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang HW, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138:2469–84.
McNerney KO, Si Lim SJ, Ishikawa K, Dreyzin A, Vatsayan A, Chen JJ, et al. HLH-like toxicities predict poor survival after the use of tisagenlecleucel in children and young adults with B-ALL. Blood Adv. 2023;7:2758–71.
Hines MR, Keenan C, Maron Alfaro G, Cheng C, Zhou Y, Sharma A, et al. Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol. 2021;194:701–7.
Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters P, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38:1938–50.
Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016;174:203–17.
Tsuji T, Hirano T, Yamasaki H, Tsuji M, Tsuda H. A high sIL-2R/ferritin ratio is a useful marker for the diagnosis of lymphoma-associated hemophagocytic syndrome. Ann Hematol. 2014;93:821–6.
Böhm S, Wustrau K, Pachlopnik Schmid J, Prader S, Ahlmann M, Yacobovich J, et al. Survival in primary hemophagocytic lymphohistiocytosis, 2016 to 2021: etoposide is better than its reputation. Blood. 2024;143:872–81.
Scala JJ, Eckrich MJ, Lipak K, Yates B, Yuan C, Wang HW, et al. Treatment strategies for progressive immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome: case series. Haematologica. 2024;109:3439–45.
Peterlin P, Garnier A, Le Bourgeois A, Jullien M, Seguin A, Eveillard M, et al. Dramatic recovery after etoposide phosphate infusion for hemophagocytic lymphohistiocytosis/macrophage activation syndrome following treatment with tisagenlecleucel in a young patient with relapsed acute lymphoblastic leukemia: a case report. Acta Haematol. 2022;145:537–41.
Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–22.
Wang J, Wang Y, Wu L, Wang X, Jin Z, Gao Z, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105:e210–e212.
Ortí G, Peczynski C, Boreland W, O’Reilly M, von Bonin M, Balduzzi A, et al. Graft-versus-host disease after anti-CD19 chimeric antigen receptor T-cell therapy following allogeneic hematopoietic cell transplantation: a transplant complications and paediatric diseases working parties joint EBMT study. Leukemia. 2025;39:431–7.
Lu W, Lyu H, Xiao X, Bai X, Zhang M, Wang J, et al. Prophylactic donor-derived CD19 CAR-T cell infusion for preventing relapse in high-risk B-ALL after allogeneic hematopoietic stem cell transplantation. Leukemia. 2024;38:1419–22.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim S, Rose J, Telford W, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39.
Benjamin R, Graham C, Yallop D, Jozwik A, Mirci-Danicar O, Lucchini G, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396:1885–94.
Del Bufalo F, Becilli M, Rosignoli C, De Angelis B, Algeri M, Hanssens L, et al. Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL. Blood. 2023;142:146–57.
Anwer F, Shaukat AA, Zahid U, Husnain M, McBride A, Persky D, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9:123–30.
Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–9.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte R, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159.
Minson A, Hamad N, Cheah CY, Tam C, Blombery P, Westerman D, et al. CAR T cells and time-limited ibrutinib as treatment for relapsed/refractory mantle cell lymphoma: the phase 2 TARMAC study. Blood. 2024;143:673–84.
Kenderian S, Ruella M, Shestova O, Kim M, Klichinsky M, Chen F, et al. Ruxolitinib Prevents Cytokine Release Syndrome after Car T-Cell Therapy Without Impairing the Anti-Tumor Effect in a Xenograft Model. Biology of Blood and Marrow Transplantation. 2017;23:S19–S20.
Han H, Wang L, Ding Y, Neuber B, Hückelhoven-Krauss A, Lin M, et al. Extracorporeal photopheresis as a promising strategy for the treatment of graft-versus-host disease after CAR T-cell therapy. Blood Adv. 2024;8:2675–90.
Gavriilaki E, Sakellari I, Gavriilaki M, Anagnostopoulos A. A new era in endothelial injury syndromes: toxicity of CAR-T cells and the role of immunity. Int J Mol Sci. 2020;21:3886.
Luft T, Dreger P, Radujkovic A. Endothelial cell dysfunction: a key determinant for the outcome of allogeneic stem cell transplantation. Bone Marrow Transpl. 2021;56:2326–35.
Wu MS, Koirala A. Thrombotic microangiopathy following chimeric antigen receptor T-cell therapy. Clin Nephrol Case Stud. 2023;11:17–21.
Taneja A, Jain T. CAR-T-OPENIA: Chimeric antigen receptor T-cell therapy-associated cytopenias. EJHaem. 2021;3:32–38.
Schoettler ML, Carreras E, Cho B, Dandoy C, Ho V, Jodele S, et al. Harmonizing definitions for diagnostic criteria and prognostic assessment of transplantation-associated thrombotic microangiopathy: a report on behalf of the European Society for Blood and Marrow Transplantation, American Society for Transplantation and Cellular Therapy, Asia-Pacific Blood and Marrow Transplantation Group, and Center for International Blood and Marrow Transplant Research. Transpl Cell Ther. 2023;29:151–63.
Bindal P, Patell R, Chiasakul T, Lauw M, Ko A, Wang TF, et al. A meta-analysis to assess the risk of bleeding and thrombosis following chimeric antigen receptor T-cell therapy: communication from the ISTH SSC Subcommittee on Hemostasis and Malignancy. J Thromb Haemost. 2024;22:2071–80.
Wang Y, Qi K, Cheng H, Cao J, Shi M, Qiao J, et al. Coagulation disorders after chimeric antigen receptor T cell therapy: analysis of 100 patients with relapsed and refractory hematologic malignancies. Biol Blood Marrow Transpl. 2020;26:865–75.
Dong R, Wang Y, Lin Y, Sun X, Xing C, Zhang Y, et al. The correlation factors and prognostic significance of coagulation disorders after chimeric antigen receptor T cell therapy in hematological malignancies: a cohort study. Ann Transl Med. 2022;10:975.
Johnsrud A, Craig J, Baird J, Spiegel J, Muffly L, Zehnder J, et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5:4465–75.
Schorr C, Forindez J, Espinoza-Gutarra M, Mehta R, Grover N, Perna F. Thrombotic Events are unusual toxicities of chimeric antigen receptor T-cell therapies. Int J Mol Sci. 2023;24:8349.
Farge D, Frere C, Connors JM, Khorana AA, Kakkar A, Ay C, et al. International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23:e334–e347.
Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–74.
Verdun N, Marks P. Secondary cancers after chimeric antigen receptor T-cell therapy. N Engl J Med. 2024;390:584–6.
Elsallab M, Ellithi M, Lunning MA, D´Angelo C, Ma J, Perales MA, et al. Second primary malignancies after commercial CAR T-cell therapy: analysis of the FDA Adverse Events Reporting System. Blood. 2024;143:2099–105.
Hamilton MP, Sugio T, Noordenbos T, Shi S, Bulterys P, Liu CL, et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N Engl J Med. 2024;390:2047–60.
Ghilardi G, Fraietta JA, Gerson JN, Van Deerlin V, Morrissetti DJ, et al. T cell lymphoma and secondary primary malignancy risk after commercial CAR T cell therapy. Nat Med. 2024;30:984–9.
Cordas Dos Santos DM, Tix T, Shouval R, Gafter-Gvili A, Alberge JB, Cliff ERS, et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat Med. 2024;30:2667–78.
Tix T, Alhomoud M, Shouval R, Scheffer Cliff E, Perales MA, Cordas Dos Santos D, et al. Second primary malignancies after CAR T-cell therapy: A systematic review and meta-analysis of 5,517 lymphoma and myeloma patients. Clin Cancer Res. 2024;30:4690–700.
Kobbe G, Brüggemann M, Baermann BN, Wiegard M, Trautmann H, Yousefian S, et al. Aggressive lymphoma after CD19 CAR T-cell therapy. N Engl J Med. 2024;391:1217–26.
Jadlowsky JK, Hexner EO, Marshall A, Grupp SA, Frey NV, Riley JL, et al. Long-term safety of lentiviral or gammaretroviral gene-modified T cell therapies. Nat Med. 2025. https://doi.org/10.1038/s41591-024-03478-6. Online ahead of print.
Dulery R, Guiraud V, Choquet S, Thieblemont C, Bachy E, Barete S, et al. T cell malignancies after CAR T-cell therapy in the DESCAR-T registry. Nat Med. 2025. https://doi.org/10.1038/s41591-024-03458-w. Online ahead of print.
Ozdemirli M, Loughney TM, Deniz E, Chahine J, Albitar M, Pittaluga S, et al. Indolent CD4+ CAR T-cell lymphoma after cilta-cel CAR T-cell therapy. N Engl J Med. 2024;390:2074–82.
Author information
Authors and Affiliations
Contributions
GO: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. GD: participated in the meeting, wrote the original draft, revised the manuscript and approved the final version. CG: conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. ZP: conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. AA: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. FB: participated in the meeting, wrote the original draft, revised the manuscript and approved the final version. MD: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. JH: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. CR: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. OS: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. WVJ: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. RV: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. MA: participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. AR: developed the methodology, conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. FO: developed the methodology, conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. ISO: developed the methodology, conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. IYA: developed the methodology, conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version. OP: conceptualized the subject for this workshop, participated in the meeting, wrote the original draft, revised the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
GO has received consulting fees from BMS, Incyte, Norvartis, Pfizer, and Sanofi; travel support from BMS, Incyte, Norvartis, and Sanofi; institutional research grant from Incyte; and honoraria from BMS, Incyte, Jazz, Norvartis, Pfizer, and Sanofi. GD received honoraria from Kite/Gilead, BMS. JIH: consultant for Sobi. FdB received support from the Italian Ministry of Health (project code: GR-2021-12372614). N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, Cellectis, and BMS, and serves in advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Sanofi, Takeda, Roche, Novartis, Bayer, Adaptive, Merck, Kite Pharma, Pfizer, AbbVie, and Servier, all paid to institution. RV: has received consulting fees from Takeda, Novartis, Kite/ Gilead, and Seagen; received honoraria from Takeda, Janseen, and Kite/Gilead, and travel grants from Takeda and Kite/Gilead. IYA received honoraria from Kite/Gilead, BMS, Novartis, Janssen. OP has received honoraria or travel support from Alexion, Gilead, Jazz, MSD, Neovii, Novartis, Pfizer, and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Apogepha, Alexion, Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Orca Bio, Priothera, Sanofi, Shionogi, and SOBI.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortí, G., Dachy, G., Graham, C.E. et al. Less frequent complications following CAR T-cell therapies: hemophagocytic lymphohistiocytosis, graft-versus-host disease, thrombotic microangiopathy, coagulation disorders and secondary malignancies: best practice recommendations from the EBMT Practice Harmonization and Guidelines Committee. Bone Marrow Transplant 60, 751–758 (2025). https://doi.org/10.1038/s41409-025-02567-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02567-5
This article is cited by
-
Optimizing CAR T cell therapy for solid tumours: a clinical perspective
Nature Reviews Clinical Oncology (2025)