Abstract
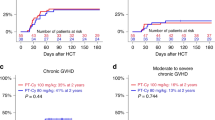
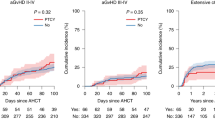
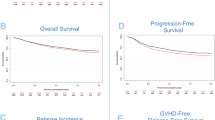
PT-CY use in T cell-replete haploidentical HCT has significantly improved outcomes. However, hyperhydration with MESNA in CY administration poses a challenge, in patients with cardiac/ renal problems. PT-CY also increases VOD risk with prior exposure to hepatotoxic drugs. Katsanis et al. in a phase Ia trial in patients undergoing HCT for hematological malignancies showed that partially replacing PT-CY with PT-BEN had comparable outcomes to conventional PT-CY. We conducted an ambispective study in 54 patients [haplo (39), MSD(14), and MUD(1)] with nonmalignant hematological disorders and hematological malignancies in pediatric and adult patients undergoing HCT (MAC/RIC) from February 2019 to May 2024. GvHD prophylaxis comprised of PT-CY/BEN (PT-CY 50 mg/kg Day +3; PT-BEN 90 mg/m2 Day +4) in a prospective arm (n = 21) and PT-CY/CY (50 mg/kg on Days +3, +4; comparator arm) in ambispective (prospective 12; retrospective 21) arm. In both groups, immunosuppression with CNI and MMF was also given. PT-CY/BEN was comparable to PT-CY/CY in terms of safety, efficacy, and GVHD prevention. In the PT-CY/BEN group, there was earlier neutrophil (0.008) and platelet (0.0057) engraftment with significantly lower BK viremia. Incidence of bacterial infection, TRM, EFS, and OS were comparable in both groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
McCurdy SR, Kasamon YL, Kanakry CG, Bolaños-Meade J, Tsai HL, Showel MM, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica 2017;102:391–400.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Posttransplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWPEBMT. J Hematol Oncol. 2018;11:40.
George B, Kulkarni U, Lionel S, Devasia AJ, Aboobacker FN, Lakshmi KM, et al. Haploidentical transplantation is feasible and associated with reasonable outcomes despite major infective complications-A single center experience from India. Transpl Cell Ther 2022;28:45.e1–45.e8.
Copelan OR, Sanikommu SR, Trivedi JS, Butler C, Ai J, Ragon BK, et al. Higher incidence of hemorrhagic cystitis following haploidentical related donor transplantation compared with matched related donor transplantation. Biol Blood Marrow Transpl. 2019;25:785–90.
Al Malki MM, Dadwal S, Yang D, Mokhtari S, Cao T, Gendzekhadze K, et al. High Incidence of CMV reactivation after haploidentical donor hematopoietic cell transplantation using high-dose post-transplant cyclophosphamide, and its impact on transplant outcomes. Blood 2017;130:4494. https://doi.org/10.1182/blood.V130.Suppl_1.4494.4494.
Stokes J, Molina MS, Hoffman EA, Simpson RJ, Katsanis E. Immunomodulatory effects of bendamustine in hematopoietic cell transplantation. Cancers. 2021;13:1702. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8038415/.
Stokes J, Hoffman EA, Zeng Y, Larmonier N, Katsanis E. Post-transplant bendamustine reduces GvHD while preserving GvL in experimental haploidentical bone marrow transplantation. Br J Haematol. 2016;174:102–16.
Katsanis E, Maher K, Roe DJ, Simpson RJ. Progressive substitution of posttransplant cyclophosphamide with bendamustine: A phase I study in haploidentical bone marrow transplantation. EJHaem. 2020;1:286–92.
Gómez-Centurión I, Gallardo Morillo AI, Pérez Martínez A, Cabrero Calvo M, Chinea A, González L, et al. Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease after Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation with PostTransplantation Cyclophosphamide: A Study on Behalf of the Spanish Hematopoietic Stem Cell Transplantation and Cellular Therapy Group (GETH). Transpl Cell Ther. 2024;30:914.e1-914.e8. Available from: https://www.sciencedirect.com/science/article/pii/S2666636724004408.
Kumar R, Kapoor R, Das SR, Yanamandra U, Pramanik S, Sharma S, et al. Cyclophosphamide Followed By Intravenous Busulfan (Cy-Bu) As Myeloablative Conditioning: Impact on Venoocclusive Disease and Transplant Outcomes, Real World Experience. Blood 2017;130:3226. https://doi.org/10.1182/blood.V130.Suppl_1.3226.3226.
Katsanis E, Stea B, Kovacs K, Truscott L, Husnain M, Khurana S, et al. Feasibility and efficacy of partially replacing post-transplantation cyclophosphamide with bendamustine in pediatric and young adult patients undergoing Haploidentical Bone Marrow Transplantation. Transpl Cell Ther 2022;28:390.e1–390.e10.
Moiseev I, Bondarenko S, Morozova E, Vlasova Y, Dotsenko A, Epifanovskaya O, et al. Graft-versus-host disease prophylaxis with post-transplantation bendamustine in patients with refractory acute leukemia: a dose-ranging study. Transpl Cell Ther. 2021;27:601.e1–601.e7.
Definition of EFS - NCI Dictionary of Cancer Terms - NCI [Internet]. 2011 [cited 2025 Jan 23]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/efs
Sureda A, Corbacioglu S, Greco R, Kröger N, Carreras E, editors. The EBMT Handbook: Hematopoietic cell transplantation and cellular therapies [Internet]. Cham: Springer International Publishing; 2024 [cited 2024 Dec 11]. Available from: https://link.springer.com/10.1007/978-3-031-44080-9.
Kitko CL, Pidala J, Schoemans HM, Lawitschka A, Flowers ME, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transpl Cell Ther 2021;27:545–57.
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIHCIBMTR Task Force position statement on standardized terminology & guidance for graft versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15.
Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the Detection and Treatment of Donor-specific Anti-HLA Antibodies (DSA) in Haploidentical Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2018;53:521–34.
Ibrahim U, Keyzner A Daratumumab for donor-specific anti-HLA antibody desensitization in a case of HLA-mismatched allogeneic stem cell transplantation. Hematol Transfus Cell Ther. 2022; Available from: https://www.sciencedirect.com/science/article/pii/S2531137922000086.
Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood 1995;85:2742–6.
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17.
Author information
Authors and Affiliations
Contributions
VN conceptualized, designed the study, and primarily reviewed the manuscript. MK was involved with manuscript writing, patient management, data collection, entry, and analysis. SP was responsible for statistical analysis. VS, SB, SK, JP, NM, DS, GS, CP and were involved with patient management and final manuscript review. UY reviewed the final manuscript and helped in the statistical analysis. Vivek N, AW provided dermatology consultations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, V., Kathrotiya, M., Shirure, V. et al. Feasibility and efficacy of partial replacement of post transplantation cyclophosphamide with bendamustine on day +4 for graft versus host disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 60, 994–1001 (2025). https://doi.org/10.1038/s41409-025-02581-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02581-7