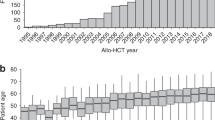
Abstract
We assessed whether the incidence and outcomes of chronic Graft-versus-Host Disease (cGvHD) after allogeneic hematopoietic stem cell transplantation (alloHSCT) have changed over 30 years. We studied 102,275 adults with hematological malignancies receiving a first alloHSCT from identical siblings or unrelated donors. We compared 3 decades: (I) 1990–1999 vs. (II) 2000–2009 vs. (III) 2010–2019. Over time, patients were older at transplantation, received more PBSC, unrelated donor transplants, reduced intensity conditioning, in vivo T-cell depletion and an ATG prophylaxis, and less TBI. cGvHD incidence at 48 months was 37.3% [36.2–38.4] in I decade vs. 44.9% [44.3–45.5] in II decade vs. 39.1% [38.7–39.5] in III decade, and incidence of extensive cGvHD at 48 months was 18.1% [17.3–19] vs. 22.2% [21.7–22.6] vs. 19.2% [18.9–19.5] over decades. In multivariate analysis, more cGvHD developed in II than in I decade (HR 1.14, 95% CI 1.09–1.21), but no difference was found between III and I decade (HR 1.01, 95% CI 0.96–1.06). Among patients with cGvHD, NRM at 48 months decreased over decades (21.3% [19.8–22.8] vs. 21% [20.3–21.7] vs. 19.7% [19.1–20.2], p < 0.001). Our data show unchanged cGvHD incidences over time and a high NRM in patients after cGvHD diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data analyzed in this study were provided and approved by the Transplant Complications Working Party (TCWP) of the EBMT. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request after approval of the scientific board of the TCWP of EBMT.
References
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. Pathology Working Group report. Biol Blood Marrow Transpl. 2006;12:31–47.
Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft versus-host disease: III. Biomarker Working Group report. Biol Blood Marrow Transpl. 2006;12:126–37.
Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Response Criteria Working Group. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transpl. 2006;12:252–66.
Couriel D, Carpenter PA, Cutler C, Bolańos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl. 2006;12:375–96.
Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, et al. Design of Clinical Trials Working Group. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transpl. 2006;12:491–505.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transpl. 2015;21:389–401.
Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transpl. 2015;21:589–603.
Paczesny S, Hakim FT, Pidala J, Cooke K, Lathrop J, Griffith LM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transpl. 2015;21:780–92.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transpl. 2015;21:984–99.
Carpenter PA, Kitko CL, Elad S, Flowers MED, Gea-Banacloche JC, Halter JP, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl. 2015;21:1167–87.
Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol Blood Marrow Transpl. 2015;21:1343–59.
Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transpl. 2014;49:168–73.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159.
Malard F, Mohty M. Updates in chronic graft-versus-host disease management. Am J Hematol. 2023;98:1637–44.
Lee SJ, Zeiser R. FDA-approved therapies for chronic GVHD. Blood. 2025;145:795–800.
Wolff D, Cutler C, Lee SJ, Pusic I, Bittencourt H, White J, et al. Axatilimab in Recurrent or Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2024;391:1002–14.
Williams KM, Inamoto Y, Im A, Hamilton B, Koreth J, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2020 Etiology and Prevention Working Group Report. Transpl Cell Ther. 2021;27:452–66.
Kitko CL, Pidala J, Schoemans HM, Lawitschka A, Flowers ME, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transpl Cell Ther. 2021;27:545–57.
Pidala J, Kitko C, Lee SJ, Carpenter P, Cuvelier GDE, Holtan S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIb. The 2020 Preemptive Therapy Working Group Report. Transpl Cell Ther. 2021;27:632–41.
DeFilipp Z, Couriel DR, Lazaryan A, Bhatt VR, Buxbaum NP, Alousi AM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2020 Treatment of Chronic GVHD Report. Transpl Cell Ther. 2021;27:729–37.
Wolff D, Radojcic V, Lafyatis R, Cinar R, Rosenstein RK, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transpl Cell Ther. 2021;27:817–35.
Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transpl. 2015;21:266–74.
Bachier CR, Aggarwal SK, Hennegan K, Milgroom A, Francis K, Dehipawala S, et al. Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis. Transpl Cell Ther. 2021;27:504.e1–504.e6.
Im A, Rashidi A, Wang T, Hemmer M, MacMillan ML, Pidala J, et al. Risk Factors for Graft-versus-Host Disease in Haploidentical Hematopoietic Cell Transplantation Using Post-Transplant Cyclophosphamide. Biol Blood Marrow Transpl. 2020;26:1459–68.
Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation—Results from a Single-Center Observational Study. Biol Blood Marrow Transpl. 2016;22:1781–91.
Kanakry CG, Bolaños-Meade J, Kasamon YL, Zahurak M, Durakovic N, Furlong T, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129:1389–93.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–68.
Langer, Lelas R, Rittenschober A, Piekarska M, Sadowska-Klasa A, Sabol I, et al. Retrospective analysis of the incidence and outcome of late acute and chronic graft-versus-host disease—an analysis from transplant centers across Europe. Front Transpl. 2024;3:1332181.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Othman J, Greenwood M, Moore J, Larsen S, Watson AM, Arthur C, et al. Unrelated donor transplant recipients given thymoglobuline have superior GRFS when compared to matched related donor recipients undergoing transplantation without ATG. Biol Blood Marrow Transpl. 2020;26:1868–75.
Remberger M, Tjønnfjord GE, Abrahamsen IW, Ali M, Myhre AE, Gedde-Dahl T, et al. Superior graft-versus-host disease-free relapse-free survival in matched unrelated donor hematopoietic stem cell transplantation with anti-thymocyte globulin (ATG) compared to matched related donor without ATG. Transpl Cell Ther. 2021;27:621.e1–621.e3.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl J Med. 2016;374:43–53.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
DeFilipp Z, Alousi AM, Pidala JA, Carpenter PA, Onstad LE, Arai S, et al. Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv. 2021;5:4278–84.
Jiang J, Sigmund AM, Zhao Q, Elder P, Vasu S, Jaglowski S, et al. Impact of chronic graft-versus-host disease on non-relapse mortality and survival. Leuk Lymphoma. 2024;65:1698–705.
Akahoshi Y, Spyrou N, Hogan WJ, Ayuk F, DeFilipp Z, Weber D, et al. Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. 2023;7:4479–91.
Carpenter PA, Gooley TA, Boiko JR, Lee CJ, Burroughs L, Mehta RS, et al. Decreasing Chronic Graft-Versus-Host Disease rates in all populations. Blood Adv. 2024;8:5829–37.
Author information
Authors and Affiliations
Contributions
DP, CP, WB, IM, OP, and ZP contributed to the design of the study, analysis and interpretation of the data. DP wrote the original draft of the manuscript. CP performed statistical analyses. CR, NK, DM, US, RZ, KEG, EF, DB, TL, HLW, CK, HS, and GB contributed to the collection and interpretation of data. All authors reviewed, critically revised, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
DP received honoraria from Novartis and Takeda. NK received honoraria from Sanofi and Neovii. DM received research grants from Novartis, Sanofi and CSL Behring, consulting fees from Novartis, Incyte, Sanofi, Jazz Pharmaceuticals, and Mallinckrodt. RZ received honoraria from Novartis, Incyte, Sanofi, Medac and Mallinckrodt. KEH received travel support and honoraria from Beigene, Sanofi, Johnson&Johnson and Servier. IM received honoraria from Novartis, Sanofi, J&J. OP has received honoraria or travel support from Alexion, Gilead, Jazz, MSD, Neovii, Novartis, Pfizer and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Apogepha, Alexion, Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Orca Bio, Priothera, Sanofi, Shionogi and SOBI. OP acknowledges the support of José Carreras Leukämie-Stiftung (3 R/2019, 23 R/2021), Deutsche Krebshilfe (70113519), Deutsche Forschungsgemeinschaft (PE 1450/7-1, PE 1450/9-1, PE 1450/10-1, PE 1450/11-1) and Stiftung Charité BIH (BIH_PRO_549, Focus Group Vascular Biomedicine). ZP received honoraria from Therakos, Sanofi and Novartis.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the scientific board of the Transplant Complications Working Party of EBMT. As per EBMT data collection policies, written informed consent was obtained from all patients for the use of their data. All methods were conducted in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pulanic, D., Peczynski, C., Boreland, W. et al. Chronic Graft-versus-Host disease trends over 30 years - a study by the EBMT transplant complications working party. Bone Marrow Transplant 60, 1436–1444 (2025). https://doi.org/10.1038/s41409-025-02697-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02697-w