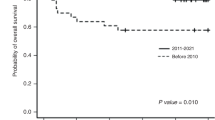
Abstract
Background: Transplantation-associated thrombotic microangiopathy (TA-TMA) confers high mortality after allogeneic hematopoietic stem cell transplantation (HSCT). While characteristics are established in pediatric populations, real-world data on adults—particularly regarding standardized diagnostics and risk stratification—remain scarce. Methods: This multicenter, retrospective cohort study analyzed 113 Asian adult patients diagnosed per international consensus criteria (median onset: 60 days post-HSCT). Outcomes were assessed using Jodele and harmonized risk tools, treatment responses, and biomarker profiles (cytokines, aGVHD, proteinuria, sC5b-9, D-dimer). Results: The 6-month post-TMA and 1-year post-HSCT survival rates were 30.5% and 31.6%, respectively. High-risk classification by Jodele criteria (33.6% of patients) predicted significantly inferior 6-month survival (10.5% vs. 38.3%, p = 0.001). All patients met harmonized high-risk criteria. Immune reconstitution was severely impaired, especially in higher Jodele risk categories. Proteinuria (HR = 3.55, p = 0.027), multi-organ dysfunction syndrome (MODS; HR = 0.06, p < 0.001), and elevated IL-10 (HR = 0.24, p = 0.025) were independent predictors of reduced survival. Eculizumab yielded higher response rates than plasma exchange (57.1% vs. 31.3%). Conclusion: This study validates harmonized diagnostic criteria and Jodele risk stratification in Asian adults with TA-TMA, and identifies proteinuria, MODS, and IL-10 as prognostic biomarkers. The universal high-risk classification underscores disease severity in this population. Early complement inhibition and cytokine-targeted therapies merit further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Jodele S, Sabulski A. Reeling in complement in transplant-associated thrombotic microangiopathy: You’re going to need a bigger boat. Am J Hematol. 2023;98:S57–S73. https://doi.org/10.1002/ajh.26872.
Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–53. https://doi.org/10.1182/blood-2014-03-564997.
Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transpl. 2021;56:1805–17. https://doi.org/10.1038/s41409-021-01283-0.
Yang J, Xu X, Han S, Qi J, Li X, Pan T, et al. Comparison of multiple treatments in the management of transplant‑related thrombotic microangiopathy: a network meta‑analysis. Ann Hematol. 2023;102:31–39. https://doi.org/10.1007/s00277-022-05069-2.
Ye Y, Zheng W, Wang J, Hu Y, Luo Y, Tan Y, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35:821–7. https://doi.org/10.1002/hon.2310.
Vasu S, Zhao Q, Miller EG, Elder P, Langenberg L, Cataland S, et al. High incidence of severe TA-TMA increases mortality in adult allogeneic transplant recipients: a prospective MIDAS Consortium study. Blood. 2025;146:638–46. https://doi.org/10.1182/blood.2025028390.
Abou-Ismail MY, Kapoor S, Sridhar DC, Nayak L, Ahuja S. Thrombotic microangiopathies: An illustrated review. Res Pract Thrombosis Haemost. 2022;6:e12708. https://doi.org/10.1002/rth2.12708.
Jiang S, Qi J, Pan T, Yao Z, Lu S, Han Y, et al. KLF4 overexpression protects against complement-mediated endothelial injury in transplant-associated thrombotic microangiopathy. Haematologica. 2025. https://doi.org/10.3324/haematol.2025.287676.
Schoettler ML, French K, Harris A, Bryson E, Deeb L, Hudson Z, et al. D-dimer and sinusoidal obstructive syndrome-novel poor prognostic features of thrombotic microangiopathy in children after hematopoietic cellular therapy in a single institution prospective cohort study. Am J Hematol. 2024;99:370–9. https://doi.org/10.1002/ajh.27186.
Acosta-Medina AA, Sridharan M, Go RS, Moyer AM, Leung N, Willrich MAV, et al. Clinical Outcomes and Treatment Strategies of Adult Transplant-Associated Thrombotic Microangiopathy: External Validation of Harmonizing Definitions and High-Risk Criteria. Am J Hematol. 2025;100:830–9. https://doi.org/10.1002/ajh.27651.
Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora’s box. Bone Marrow Transpl. 2017;52:1355–60. https://doi.org/10.1038/bmt.2017.39.
Hematopoietic Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association. Chinese consensus on the diagnosis and management of transplant-associated thrombotic microangiopathy (2021). Zhonghua Xue Ye Xue Za Zhi. 2021;42:177–84.
Schoettler ML, Carreras E, Cho B, Dandoy CE, Ho VT, Jodele S, et al. Harmonizing Definitions for Diagnostic Criteria and Prognostic Assessment of Transplantation-Associated Thrombotic Microangiopathy: A Report on Behalf of the European Society for Blood and Marrow Transplantation, American Society for Transplantation and Cellular Therapy, Asia-Pacific Blood and Marrow Transplantation Group, and Center for International Blood and Marrow Transplant Research. Transpl Cell Ther. 2023;29:151–63. https://doi.org/10.1016/j.jtct.2022.11.015.
Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink-Cross A, Myers KC, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135:1049–57. https://doi.org/10.1182/blood.2019004218.
Dvorak CC, Higham C, Shimano KA. Transplant-Associated Thrombotic Microangiopathy in Pediatric Hematopoietic Cell Transplant Recipients: A Practical Approach to Diagnosis and Management. Front Pediatr. 2019;7:133 https://doi.org/10.3389/fped.2019.00133. eCollection 2019.
Schoettler ML, Bhatt H, Vasu S. A systematic review of diagnostic, prognostic, and risk blood and urine biomarkers of transplant-associated thrombotic microangiopathy. Front Immunol. 2023;13:1064203. https://doi.org/10.3389/fimmu.2022.1064203.
Yoon SH, Kang SH, Kim H, Choi ES, Im HJ, Koh K-N. Incidence, risk factors, and outcomes of transplant-associated thrombotic microangiopathy in pediatric patients after allogeneic hematopoietic cell transplantation: a single-institution prospective study. Bone Marrow Transpl. 2025;60:447–57. https://doi.org/10.1038/s41409-024-02506-w.
Lax N, Davidovits M, Chodick G, Bernfeld Y, Peled O. Eculizumab is efficacious and safe in pediatric patients with various forms of hemolytic uremic syndrome: a retrospective clinical experience of a tertiary center. Front Pharmacol. 2025;16:1535407. https://doi.org/10.3389/fphar.2025.1535407.
Jodele S, Dandoy CE, Aguayo-Hiraldo P, Lane A, Teusink-Cross A, Sabulski A, et al. A prospective multi-institutional study of eculizumab to treat high-risk stem cell transplantation–associated TMA. Blood. 2024;143:1112–23. https://doi.org/10.1182/blood.2023022526.
Mizuno K, Dandoy CE, Teusink-Cross A, Davies SM, Vinks AA, Jodele S. Eculizumab precision-dosing algorithm for thrombotic microangiopathy in children and young adults undergoing HSCT. Blood Adv. 2022;6:1454–63. https://doi.org/10.1182/bloodadvances.2021006523.
Yang L-P, Zhao P, Wu Y-J, Fu H-X, He Y, Mo X-D, et al. Treatment outcome and efficacy of therapeutic plasma exchange for transplant-associated thrombotic microangiopathy in a large real-world cohort study. Bone Marrow Transpl. 2022;57:554–61. https://doi.org/10.1038/s41409-022-01581-1.
Higham CS, Shimano KA, Kharbanda S, Chu J, Cisneros GS, Winestone LE, et al. Cyclophosphamide and Thiotepa Increases Risk of Transplant-Associated Thrombotic Microangiopathy. Transpl Cell Ther. 2024;30:931.e931–931.e910. https://doi.org/10.1016/j.jtct.2024.06.020.
Ma S, Bhar S, Guffey D, Kim RB, Jamil M, Amos CI, et al. Prospective Clinical and Biomarker Validation of the American Society for Transplantation and Cellular Therapy Consensus Definition for Transplantation-Associated Thrombotic Microangiopathy. Transpl Cell Ther. 2023;29:685.e681–685.e687. https://doi.org/10.1016/j.jtct.2023.08.015.
Schoettler ML, Ofori J, Bryson E, Spencer K, Qayed M, Stenger E, et al. Real-World Application of Recently Proposed ASTCT/CIBMTR/EBMT/APBMT Consensus Risk Stratification for Transplantation-Associated Thrombotic Microangiopathy in Children. Transpl Cell Ther. 2024;30:929.e921–929.e926. https://doi.org/10.1016/j.jtct.2024.06.017.
Schoettler ML, Gavriilaki E, Carreras E, Bo-Kyoung C, Dandoy CE, Ho VT, et al. An ASTCT, CIBMTR, EBMT, and APBMT Consensus Statement Defining Response Criteria for Hematopoietic Cell Transplantation Associated Thrombotic Microangiopathy (TA-TMA) Directed Therapy. Transpl Cell Ther. 2025. https://doi.org/10.1016/j.jtct.2025.05.028. Online ahead of print.
Styczynski J, Velden Wvd, Fox CP, Engelhard D, Camara Rdl, Cordonnier C, et al. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–11. https://doi.org/10.3324/haematol.2016.144428.
Ljungman P, Alain S, Chemaly RF, Einsele H, Galaverna F, Hirsch HH, et al. Recommendations from the 10th European Conference on Infections in Leukaemia for the management of cytomegalovirus in patients after allogeneic haematopoietic cell transplantation and other T-cell-engaging therapies. Lacet Infect Dis. 2025;25:e451–e462. https://doi.org/10.1016/S1473-3099(25)00069-6.
Law N, Logan C, Taplitz R. EBV Reactivation and Disease in Allogeneic Hematopoietic Stem Cell Transplant (HSCT) Recipients and Its Impact on HSCT Outcomes. Viruses. 2024;16:1294 https://doi.org/10.3390/v16081294.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transpl. 2016;22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001.
Jagasia M, Perales M-A, Schroeder MA, Ali H, Shah NN, Chen Y-B, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135:1739–49. https://doi.org/10.1182/blood.2020004823.
Wright MN, Dankowski T, Ziegler A. Unbiased split variable selection for random survival forests using maximally selected rank statistics. Stat Med. 2017;36:1272–84. https://doi.org/10.1002/sim.7212.
Schoettler ML, Williams KM. Application of the Consensus Definition of Transplant Associated Thrombotic Microangiopathy (TA-TMA) Requires a More Expansive Lens to Accurately Diagnose and Risk Stratify Patients—Beware Reliance on Schistocytes Alone. Transpl Cell Ther. 2024;30:818–20. https://doi.org/10.1016/j.jtct.2024.05.027.
Petinati N, Davydova Y, Nikiforova K, Bigildeev A, Belyavsky A, Arapidi G, et al. T Cell and Cytokine Dynamics in the Blood of Patients after Hematopoietic Stem Cell Transplantation and Multipotent Mesenchymal Stromal Cell Administration. Transpl Cell Ther. 2023;29:109.e101–109.e110. https://doi.org/10.1016/j.jtct.2022.10.030.
Zhu P, Yang L, Wu Y, Shi J, Lai X, Liu L, et al. Graft CD8 T-cell-based risk system predicts survival in antithymocyte globulin-based myeloablative haploidentical peripheral blood stem cell transplantation. Clin Transl Immunol. 2024;13:e1484. https://doi.org/10.1002/cti2.1484.
Orofino G, Xue E, Doglio M, Noviello M, Tassi E, Cristante M, et al. Dynamics of polyclonal immuno-reconstitution after allogeneic transplant with post-transplant cyclophosphamide and letermovir. Bone Marrow Transpl. 2023;58:1104–11. https://doi.org/10.1038/s41409-023-02046-9.
Jodele S, Dandoy CE, Sabulski A, Koo J, Lane A, Myers KC, et al. Transplantation-Associated Thrombotic Microangiopathy Risk Stratification: Is There a Window of Opportunity to Improve Outcomes?. Trnaspl Cell Ther. 2022;28:392.e391–392.e399. https://doi.org/10.1016/j.jtct.2022.04.019.
Castelli M, Micò MC, Grassi A, Algarotti A, Lussana F, Finazzi MC, et al. Safety and efficacy of narsoplimab in pediatric and adult patients with transplant-associated thrombotic microangiopathy: a real-world experience. Bone Marrow Transpl. 2024;59:1161–8. https://doi.org/10.1038/s41409-024-02305-3.
Chumnumsiriwath P, Vittayawacharin P, Ramos-Perez J, Jeyakumar D, Lee BJ, Doh J, et al. Narsoplimab for refractory transplantation-associated thrombotic microangiopathy (TA-TMA) in adult patients receiving allogeneic hematopoietic stem cell transplantation (AHSCT). Bone Marrow Transpl. 2025;60:1065–9. https://doi.org/10.1038/s41409-025-02595-1.
Schoettler ML, Lisac R, Ofori J, Frost E, Liang W, Parikh S, et al. Eculizumab is Associated With Increased Infection Rates and Infection Related Mortality in Children With Thrombotic Microangiopathy After HCT. Am J Hematol. 2025. https://doi.org/10.1002/ajh.70022.
Dandoy CE, Rotz S, Alonso PB, Klunk A, Desmond C, Huber J, et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv. 2021;5:1–11. https://doi.org/10.1182/bloodadvances.2020003455.
Jodele S, Dandoy CE, Danziger-Isakov L, Myers KC, El-Bietar J, Nelson A, et al. Terminal Complement Blockade after Hematopoietic Stem Cell Transplantation Is Safe without Meningococcal Vaccination. Biol Blood Marrow Transpl. 2016;22:1337–40. https://doi.org/10.1016/j.bbmt.2016.03.032.
Zhang R, Zhou M, Qi J, Miao W, Zhang Z, Wu D, et al. Efficacy and Safety of Eculizumab in the Treatment of Transplant-Associated Thrombotic Microangiopathy: A Systematic Review and Meta-Analysis. Front Immunol. 2021;11:564647. https://doi.org/10.3389/fimmu.2020.564647.
Rudakova TA, Vlasova JY, Paina OV, Slesarchuk OA, Gorodnova MA, Schegoleva TS, et al. Time course of organ and hematological response to complement blockage in transplant-associated thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Front Med. 2025;12:1551066. doi: 0.3389/fmed.2025.1551066.
Matsui H, Arai Y, Imoto H, Mitsuyoshi T, Tamura N, Kondo T, et al. Risk factors and appropriate therapeutic strategies for thrombotic microangiopathy after allogeneic HSCT. Blood Adv. 2020;4:3169–79. https://doi.org/10.1182/bloodadvances.2020002007.
Jodele S, Gavriilaki E. Translating biomarker insights into practice: a path forward in TA-TMA management. Front Med. 2025;12:1550365. https://doi.org/10.3389/fmed.2025.1550365.
Zhang P, Fleming P, Andoniou CE, Waltner OG, Bhise SS, Martins JP, et al. IL-6-mediated endothelial injury impairs antiviral humoral immunity after bone marrow transplantation. J Clin Investig. 2024;134:e174184. https://doi.org/10.1172/JCI174184.
Damme KFAV, Hoste L, Declercq J, Leeuw ED, Maes B, Martens L, et al. A complement atlas identifies interleukin-6-dependent alternative pathway dysregulation as a key druggable feature of COVID-19. Sci Transl Med. 2023;15:eadi0252. https://doi.org/10.1126/scitranslmed.adi0252.
Mongelos MA, Sosa FN, Pineda GE, Fiorentino G, Santiago A, Abelleyro MM, et al. Assessment of interleukin-10 promoter variant (-1082A/G) and cytokine production in patients with hemolytic uremic syndrome. Front Pediatr. 2023;11:1210158. https://doi.org/10.3389/fped.2023.1210158.
Pineda GE, Rearte B, Todero MF, Bruballa AC, Bernal AM, Fernandez-Brando RJ, et al. Absence of interleukin-10 reduces progression of shiga toxin-induced hemolytic uremic syndrome. Clin Sci. 2021;135:575–88. https://doi.org/10.1042/CS20200468.
Acknowledgements
We extend our gratitude to the Zhejiang Province Bone Marrow Transplant Collaboration Group for their coordination and data sharing, which made this multicenter retrospective study possible. We also thank all the patients whose data were included in this research.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82300240 and 82170205) and the Natural Science Foundation of Zhejiang Province (LMS25H080005).
Author information
Authors and Affiliations
Contributions
Y.W. designed the study and wrote the paper. XL.Y. data collecting, analyzing data, revising paper. L.C., X.L., L.L. recruiting patients, data collecting. P.Z. analyzing data, format figures. L.Y., J.S., J.Y., Y.Z., G.O., L.N., H.X., Y.X., L.T. recruiting patients, data collecting. Z.C., H.H., YING.L., Y.L. funding acquisition, project administration, review, and validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Yuan, X., Chen, L. et al. Validation of risk stratification and novel prognostic biomarkers in asian adult HSCT recipients with TA-TMA: A multicenter real-world study. Bone Marrow Transplant (2026). https://doi.org/10.1038/s41409-025-02777-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41409-025-02777-x