Abstract
Chronic lower back pain (LBP) is the leading cause of disability worldwide. Due to its close relationship with intervertebral disc (IVD) degeneration (IVDD), research has historically focused more on understanding the mechanism behind IVDD while clinical efforts prioritize pain management. More recently, there has been a shift toward understanding LBP as a distinct pathological entity. This review synthesizes current knowledge on discogenic LBP, combining known pathophysiology, molecular mechanisms, risk factors, diagnostic challenges, and available experimental models. IVDD is a complex, multifactorial process involving biochemical, mechanical, and inflammatory changes within the disc, leading to structural breakdown and potential discogenic pain. Key mechanisms include extracellular matrix degradation, upregulation of inflammatory mediators, immune cell infiltration, and aberrant nerve and vascular ingrowth. However, not all cases of IVDD result in LBP, highlighting the need for further investigation into the cellular, molecular, and biomechanical factors contributing to symptom development. Current diagnostic tools and experimental models for studying discogenic LBP remain limited, impeding the development of targeted treatments. Existing therapies primarily focus on symptom management rather than addressing underlying disease mechanisms.
Similar content being viewed by others
Introduction
Chronic lower back pain (LBP) is the greatest cause of disability burden worldwide, affecting an estimated 619 million people.1 This figure is projected to increase to 843 million by 2050, largely due to population aging and expansion.1 Of patients reporting chronic LBP, approximately 40% are attributed to intervertebral disc (IVD) degeneration, and as a result, IVD degeneration (IVDD) has traditionally been the focus of research rather than LBP itself.2 However, in clinical practice, it is LBP that often takes precedence over IVDD. Consequently, there has been a shift towards exploring LBP as an entity distinct from its link to IVDD. This transition underscores the importance of delving into the intricacies of LBP as a multifaceted condition with its own unique mechanisms and clinical implications. In our recent study, we tackled the question of the cellular and molecular differences between degenerated asymptomatic and LBP-inducing IVDs.3 In this review we will take a wider look at this problem and elaborate on what is known to date and what open questions remain to be answered.
IVD anatomy, degeneration process, and discogenic low back pain
IVD unique structure and anatomy
The IVD is an organ situated between two vertebral bodies, comprising the nucleus pulposus (NP) at its center, the annulus fibrosus (AF) encircling the NP, and a thin layer of fibrocartilage that separates the NP from the vertebral body endplates. Each component serves distinct functions essential for spinal function and biomechanics.
The NP, primarily responsible for shock absorption and flexibility, is composed mainly of water absorbing extracellular matrix (ECM) and a small population of cells. The ECM within the NP is predominantly made up of collagens and proteoglycans, with collagen II and aggrecan being the primary constituents.4 Collagen II functions to form irregular networks of fibers in the IVD matrix to provide disc hydration, structure, and elasticity.5 Aggrecan is credited with the ability to retain water and thus is thought to be largely responsible for preserving water content, osmotic pressure, and biomechanical properties within the NP.4 Although the cells within the NP constitute only about 1% of its total volume, they play a crucial role in maintaining disc homeostasis.6 These cells are responsible for synthesizing ECM proteins, cytokines, growth factors, and proteases that contribute to the NP’s integrity and functionality.
Surrounding the NP is a ring of highly organized connective tissue known as the AF, which provides structural stability to the disc. In humans, the AF is organized into 15 to 25 stacked layers known as lamellae, which are composed primarily of collagen I.7 Within each lamella, collagen fibers are aligned in parallel with an orientation that shifts by about 60° between each adjacent layer to enhance the disc’s resistance to compressive forces.7 Between the lamellae are AF cells, which are responsible for producing ECM, including proteoglycans, elastic fibers, and glycoproteins.
A transition zone, known as the inner AF, sees a gradual shift in ECM properties and cell composition that bridges the gap from the organized outer AF to the less rigid NP. Cells within the transition zone exhibit a rounder morphology, in contrast to the spindle-shaped cells characteristic of the AF. Additionally, the ECM composition exhibits a reduction in the ratio of collagen I to collagen II as one approaches the NP. This gradual transition facilitates a smooth gradation in mechanical properties between the rigid outer AF and the flexible NP.8
At the rostro-caudal boundaries of the IVD are endplates composed of a bone-cartilage bilayer connecting the disc to adjacent vertebrae. These endplates are vital for maintaining disc homeostasis, with their damage or calcification linked to IVDD.9 Given the IVD’s largely avascular nature, exchange of nutrients and waste predominantly takes place through the endplate and the outer edge of the AF.10
Intervertebral disc degeneration process, causes, and symptoms
The relationship between LBP and IVDD has long been known, but the exact mechanism linking these phenomena has yet to be identified. IVDD is a multifactorial disease, and progression is challenging to stop once degenerative changes begin. Among various contributing factors, age is thought to be the most influential factor on IVDD. Signs of IVDD have been observed in pre-pubescent children and exist in about 20% of teenagers, with the percentage steadily increasing with age.11 As the disc ages, the homeostatic balance between anabolic and catabolic processes within the ECM gradually becomes disrupted, resulting in a net reduction of ECM content and quality within the IVD.12,13 This imbalance can be further exacerbated by injury to the disc, whether from acute, chronic, or even micro damage.14
During IVDD, changes in the cellular phenotype of IVD cells, especially in the NP, are observed.15 These changes are triggered by various forms of cell stress, including mechanical, metabolic, and oxidative stress.16,17 IVD-resident cells, namely NP cells (NPC) and AF cells (AFC), which are phenotypically unique, balance the breakdown and production of the disc’s matrix under healthy conditions.5 However, during IVDD, the metabolic balance within the disc is disrupted, resulting in a shift toward net catabolism.18 At the molecular level, early indicators of IVDD manifest through the decreased production and accelerated breakdown of the disc’s ECM, including critical components like proteoglycans and collagen II.19 Upregulation of Matrix Metalloproteases (MMPs) and ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) family are a likely culprit for this change in ECM composition. MMPs, ADAMTSs, and other proteases designed to degrade ECM are typically expressed at low levels to maintain normal tissue turnover.20,21 However, as IVDD develops, proteases including MMP3 and MMP13, ADAMTS1 and ADAMTS4 become upregulated thus increasing the rate of ECM degradation.20,21
Though the IVD is considered an immune-privileged site, recent single-cell RNA sequencing (scRNA-seq) studies have identified small subsets of native immune cells within the disc.22,23,24 In our own study, human cells injected to a healthy rat IVD did not induce immune response or rejection.25 In the early stages of degeneration, immune cells were found to adopt anti-inflammatory and tissue repair roles, whereas in later stages, macrophages tend to exhibit a more proinflammatory phenotype.24 Specifically, fragmented ECM components are recognized by resident immune cells as damage-associated molecular patterns (DAMPs), triggering additional immune cell infiltration into the IVD. These immune cells, including macrophages and neutrophils, release cytokines such as TNF-α and IL-1, further amplifying the inflammatory response within the disc. Whether immune cells enter the disc freely or through pre-existing fissures, has yet to be determined.
Inflammation within the IVD is a key contributor to the development and progression of IVDD.26 While inflammation can have both positive and negative effects on disease progression, in the context of IVDD, it is seen as detrimental.26 Changes in the IVD microenvironment induce cell stress, prompting the expression of pro-inflammatory factors, including cytokines, chemokines, and neurotrophins.27 Interestingly, many of these factors have a dual role in not only initiating an inflammatory response but also in recruiting immune cells, thereby perpetuating the inflammatory cascade within the disc.27 Indeed, expression of inflammatory markers such as TNFs and interleukins have been found to be significantly upregulated in degenerated discs and are believed to exacerbate the degenerative process by further attracting inflammatory cells into the disc microenvironment.27 The influx of pro-inflammatory cytokines into the IVD induces changes in collagen makeup and loss of proteoglycans.17 IL-1β and TNF-α downregulate the production of collagen II and aggrecan. As IVDD progresses, collagen II is replaced by collagen I, which is more organized and decreases the effectiveness of nutrient diffusion to NPCs. A decrease in aggrecan levels leads to dehydration and increased stiffness of the disc, altering the biomechanical properties of the NP.17 As the NP becomes dehydrated, its capacity to absorb shock and evenly distribute pressure across the disc diminishes. Consequently, the AF is subjected to greater compressive and shear forces, escalating the risk of disc damage. Damage during this phase often extends to the endplates, impairing nutrient supply and waste removal. This impairment can trigger cellular stress, potentially leading to cell senescence, damage, or even death, along with a reduction in the pH of the disc.28 We have shown that annular injury and consequent IVDD can also lead to changes in pH in a porcine model.29,30,31 Research indicates that a lower pH environment within the disc significantly contributes to tissue inflammation and cellular stress, leading to an increase in MMP3, Interleukin (IL)-6, IL-8, Tumor Necrosis Factor (TNF) Alpha, Nerve Growth Factor (NGF), and Connective Tissue Growth Factor (CTGF) secretion.3 This acidic environment may also exacerbate ECM degradation by altering matrix metabolism, affecting cell activity, and even inducing cell death.28
IVD degeneration induces discogenic low back pain
Due to the multifaceted nature of LBP, it can be difficult to pinpoint the exact source of pain. In fact, a majority of LBP cases do not have a clear pathoanatomical cause, which makes developing treatments and therapies especially challenging.32,33,34 Besides the IVD, many other structures have been associated with LBP development, including the vertebral endplate, spasm or strain of spinal muscles, spondylosis, and other spinal conditions such as spinal stenosis and disc herniation.10,32,35,36,37,38
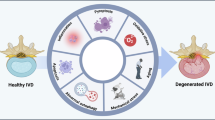
The development of LBP has long been considered synonymous with IVDD, with up to 40% of LBP cases attributed to this condition.39,40 However, studies have found that depending on a patient’s age, between 30% and 95% of IVDD cases are asymptomatic.41 Discogenic LBP is a multifactorial disease, with various factors implicated in its development, including hyperinnervation, vascularization, and inflammation of the IVD (Fig. 1).8
In healthy discs only the outer AF is innervated, leaving the inner AF and NP aneural.42 For a degenerated disc to exhibit discogenic pain, innervation is required, but the exact locations of this innervation and the factors driving pain development are not well understood. Studies have shown an increased innervation extent and depth within degenerated discs is associated with LBP, suggesting that hyperinnervation may be a key factor.42 In our study, we have demonstrated the link between nociceptors and discogenic pain induction in in vitro and in vivo models.3
Healthy IVDs are also predominantly avascular in adults. During childhood, the disc is moderately vascularized; however, by age 17, this vasculature dies off.11 Despite this, it has been noted that as IVDD progresses, neovascularization of the outer layers of the AF occurs.43
Unfortunately, despite its high prevalence and disability burden, the exact mechanism governing the development of IVDD has yet to be elucidated. Our lack of understanding of both degeneration and discogenic LBP has limited our ability to develop effective preventative treatments, and thus, current treatments for LBP focus on treating a patient’s symptoms rather than the disease mechanism.
The goal of the current review and its structure
Our current understanding and treatments for LBP are limited. A significant amount of work has been done to elucidate the biology and pathology of IVDD. However, the question of how and why LBP develops in some cases has yet to be determined.
IVDD and LBP are chronic conditions that are widely considered a natural part of aging.11,44,45 However, the chronicity of IVDD makes it more challenging to study relative to acute diseases, necessitating the use of long-term studies that require increased funds, supplies, and staff. In addition, chronic diseases often include more variables for consideration, such as genetics, sex, environmental, and lifestyle factors.
Current treatments for LBP are limited, primarily aiming to manage symptoms through analgesics or, in severe cases, surgical intervention, which treat the symptoms but not the mechanism of disease. This approach is largely due to a limited understanding of the specific processes by which discogenic LBP occurs, highlighting a critical need for advancements in our understanding and treatment of this condition.
Several reviews have examined different aspects of LBP; however, a comprehensive synthesis of key elements relevant to research and treatment approaches remains lacking. Some reviews provide a broad overview of LBP but do not discuss the models and methods used to study the condition.46 Others focus on LBP while omitting discussion of the molecular mechanisms that may contribute to its development.47 Additionally, some reviews address the mechanisms underlying LBP in humans, but do not include any animal models that can provide increased insight into exact molecular mechanisms or assess current treatment strategies that can be used to address them.48
To address this gap in literature, we have identified key advancements, tools and methodologies through targeted searches to synthesize a comprehensive narrative review of discogenic LBP and its relationship with IVDD. This review will discuss the current clinical definition of discogenic LBP and its relationship with IVDD, emphasizing our understanding of molecular mechanisms and risk factors that may contribute to its development. It will also outline available methodologies, experimental models, and current treatments. Further, this review aims to integrate our current understanding of cellular and molecular component in IVDD induction3 and methodologies to create a comprehensive resource for researchers in the field by providing a structured overview of existing literature while identifying challenges and areas for further investigation. The integration of biological, clinical, and translational perspectives presented in this review may facilitate the development of more effective, targeted treatments for LBP.
This review specifically focuses on discogenic LBP, and the methods currently employed to study it. However, it must be acknowledged that other non-discogenic pathologies can and often do contribute to LBP.
Specifically, this review seeks to define the current methods for diagnosing discogenic LBP and the challenges faced by research and clinical communities. Additionally, it explores the etiology of discogenic LBP, examining established and potential mechanisms underlying its development. It further discusses the models and evaluation methods used to study LBP and IVDD. Finally, the review explores current treatment strategies and potential future therapeutic approaches.
Defining and diagnosing discogenic pain
Despite the high prevalence of LBP in the population today, the definition of “discogenic” pain alone is vague and varies even amongst clinicians. Often referred to as “axial” back pain, this type of pain is typically viewed as a combination of tears in the AF, introduction of nociceptive nerve endings, and cytokine irritation causing an inflammatory cascade, all exacerbated by mechanical loading. These factors create an environment that creates a variable pain presentation, consisting of stiffness, midline LBP, and radicular/neuropathic symptoms. As such, no universally accepted definition of discogenic LBP exists. However, clinicians often use a combination of consistent symptoms, physical examination findings, imaging studies, and other diagnostic modalities to identify the pathologic process in an individual with LBP.
Perhaps the most widely accepted clinical presentation of discogenic LBP is an active individual in their 2nd through 5th decade of life who presents predominantly axial LBP with or without intermittent radicular symptoms.49,50,51 These symptoms are classically exacerbated by activities that load the anterior column, including sitting or standing, and they typically experience relief when supine. Motion or physical activity that loads the disc in a standing or flexed position, including weightlifting activities, running, or sports, may be particularly painful. Identification of any “red flag” findings must be evaluated since occult trauma, infection, or malignancy may present in similar, nonspecific ways.52 Physical examination is typically unremarkable, as discogenic LBP is unlikely to cause neurologic deficits in the absence of compression (e.g., herniated disc). Exacerbation of pain with forward flexion is classically thought to be driven by the disc, whereas painful lumbar extension is thought to be driven by the facet joints and posterior elements, so-called “facetogenic pain.” However, the specificity for painful lumbar flexion and hyperextension is rather poor, and this finding alone is insufficient to diagnose discogenic pain accurately.52
Confirmation of discogenic LBP may be done through either non-invasive or invasive modalities. Non-invasive modalities include history taking, physical examination, and imaging studies. X-ray remains the first line imaging study in evaluating persistent LBP and provides an overall view of global alignment and lumbar pathology.53 Findings such as transitional anatomy, spondylolisthesis or spondylolysis, and loss of disc height may be quickly identified on a lateral lumbar X-ray and provide the clinician with important clues to the etiology of the patient’s pain.52 However, there remains a poor correlation between imaging findings and reported symptoms, as upwards of 30% of patients with axial LBP have normal X-rays. As such, the present indication for plain film X-rays for LBP includes mechanical symptoms without any “red flag” findings that persist for greater than 1 month.
Judicious use of Magnetic Resonance Imaging (MRI) is required to help tease out the diagnosis of discogenic LBP, given that degenerative disc changes and varying degrees of spinal stenosis are found in asymptomatic individuals.50 T1- and T2-weighted images on MRI provide excellent visualization of the IVD, neural elements, and surrounding soft tissues, but this abundance of detail has led to drastic overuse in modern medicine, particularly amongst non-spine specialists.54 Many MRI findings within the IVD, including height loss, annular fissures, loss of high signal intensity within the disc on T2-weight imaging, anterolisthesis/retrolisthesis, and bulging of the NP, are not associated with pain.55,56,57 Some degree of disc degeneration has been reported in 44%–90% of individuals from the 2nd to 7th decades of life.55,56,58,59 Assessment of the disc may be reported as a Pfirrmann score, which grades the disc based on the signal intensity within the NP on axial T2-weighted images and on disc height on sagittal images.60 However, this grading is typically used in a research setting, as clinicians may simply refer to a degenerated disc as a so-called “black disc”.61 Nevertheless, MRI findings must be carefully assessed in the setting of presenting symptoms in order to determine their clinical significance. Annular tears may be identified by a High-Intensity Zone (HIZ) of the posterior AF. HIZ are assessed on a sagittal T2 weighted MRI of the lumbar spine and are defined by an area of high signal intensity (white) along the posterior annulus with a low signal intensity (black) border that has been shown to have a correlation with LBP and findings of provocative discography.62
Recently, new MRI techniques have been introduced in an attempt to clarify the degenerative process within the IVD through quantitative methods. Quantitative MRI utilizes T2 relaxation time as a quantitative assessment for intervertebral disc degeneration. By evaluating subtle changes in disc structure and composition, this study shows early promise as a means of evaluating regional changes in the AF.53,63,64 T1-Rho is another MRI technique introduced to detect early disc degeneration based on loss of proteoglycans and shows some promise in its ability to detect early disc degeneration.65,66 Similarly, delayed gadolinium-enhanced MRI of cartilage (dGemeric) is an MRI study protocol that provides quantitative analysis of cartilage and has been applied to the assessment of IVDs.67 Endplate cartilage integrity and disc glycosaminoglycan (GAG) concentration can also theoretically be assessed via sodium MRI.68 Quantitative chemical exchange saturation transfer (qCEST) MRI is another emerging modality capable of detecting pH changes in IVDs and has been indirectly correlated with pain.29,30,31 While these novel quantitative metrics may offer clinicians valuable insight into the early degenerative process, further research is needed to provide a full understanding of these imaging modalities before their clinical relevance may be determined.
Perhaps the most controversial invasive diagnostic modality for discogenic back pain is provocative discography. This procedure has been foundational in research and the clinical evaluation of discogenic pain.69 Discography is performed by injection of iodine-based contrast into the subject disc in question, and the dye pattern, volume of injection, and patient’s pain response are recorded. However, despite its long history of use, its validity has never been clearly established. Perhaps the most significant limitations of this study are the reliability of the patient’s response and operator variability. Multiple studies have attempted to establish the positive predictive value of provocative discography and have been plagued by an unacceptably high false positive rate given the multimodal nature of LBP from a psychosomatic, social, and neurologic standpoint.61,69,70,71 In addition, the safety of discography remains a significant concern, as iatrogenic disc degeneration has been postulated to be a potential consequence of provocative discography. Other significant risks include discitis, disc herniation, nerve damage, or allergic reactions to the contrast medium.61,72 A 2014 guideline update for the performance of fusion procedure for degenerative disease of the lumbar spine provided a Grade C recommendation for the use of discography in patient selection. This consensus noted that while discography may be considered a diagnostic option in the management of LBP, it should not be used as a stand-alone diagnostic tool.73 Recent advances in imaging techniques such as qCEST offer promising, less invasive alternatives for evaluating discogenic pain.29,30,31 Taken in sum, clinicians must carefully weigh the risks and benefits of discography as a diagnostic tool and ensure careful selection of patients with whom they feel may benefit from this study.
Etiology of discogenic pain
Historically, research into IVDD and LBP has centered around environmental lifestyle factors that induced histological changes within the disc. More recently, however, with the rise of genetic testing, research has begun to explore other factors posing a potential risk of IVDD and LBP development such as genetic factors, sex, high BMI, smoking, and ergonomic factors. However, the exact relationship between these factors on discogenic LBP has yet to be fully elucidated. As a result, understanding discogenic LBP etiology and why it only occurs in a subset of IVDD patients is not understood.
Genetics, environmental, and lifestyle risk factors for discogenic pain
Biological sex as a risk factor
IVDD and LBP have widely been considered diseases stemming from environmental and lifestyle factors and thus was studied without consideration of how sex may impact the development, presentation, and progression of the disease. Recent studies into how sex differences affect these conditions have unveiled complex interactions involving hormonal fluctuations, anatomical distinctions, and life stages such as pregnancy and menopause that may play a role in LBP development.74,75 Sex has long been shown to impact LBP development with females having a higher global prevalence than males across all age groups and differences becoming more pronounced at older ages.1,75
MRI studies examining sex differences in IVDD and LBP revealed that females have lower NP-T1ρ values and more severe disc degeneration than males at any given age.76,77 The NP-T1ρ value is a marker of proteoglycan content and disc health, suggesting that, on average, males may maintain healthier disc composition into older age.76,77
A landmark study by Mosley et al. highlighted stark sex-based differences in the experience and biological markers of IVDD and LBP in a rat model.78,79 While minor differences were noted in the onset and progression of IVDD between sexes, the study revealed profound disparities in pain perception and the expression of pain-related markers.78,79 Male rats exhibited a significant reduction in pain sensitivity (as measured by paw withdrawal) starting at 2 weeks post-injury and persisting to sacrifice at 6 weeks post-injury.79 In comparison, females not only showed no significant changes in pain sensitivity post-injury but also had significantly more variability both at baseline and after injury, which could not be attributed solely to baseline differences or the estrus cycle.79 Furthermore, only males showed significant upregulation of pain markers in DRGs.79 These findings suggest that sex plays a critical and complex role in the manifestation of LBP, underpinned by biological and possibly genetic differences.
This sex-specific variability is particularly important to consider, given that many studies investigating LBP using animal models often overlook sex as a biological variable, opting to only use one sex or disregard sex in mixed-sex cohorts. However, the distinct responses observed between male and female animals emphasize the necessity of considering sex as a crucial factor in pain research, prompting a more nuanced exploration of the underlying mechanisms driving sex-related differences in LBP manifestation.
Genetic predisposition
While a diverse array of studies has explored the role of genetics in IVDD, there remains a notable gap in understanding how genetic predisposition may influence the development of LBP. Early studies involving mono- and dizygotic twins have highlighted a strong association between genetic background and the development of IVDD.80,81,82 More recently, the advent of genetic testing and genome-wide association studies (GWAS) have emerged as promising tools to identify genes and polymorphisms associated with LBP. A range of genes, including SLC13A1 and FSCN3, as well as specific polymorphisms such as rs12310519 in SOX5 and rs2228570 and rs7975232 in VDR, have been implicated in LBP.83,84,85,86
Baumbauer et al. conducted a study integrating genomic and pain testing, identifying the rs4680 genotype (GG) of catecholamine-O-methyltransferase (COMT), an enzyme involved in catecholamine metabolism, as being associated with an increased risk of transitioning from acute to chronic back pain.87 Additionally, patients with the rs4680 genotype exhibited heightened sensitivity to cold and mechanical stimuli.87
Beyond single nucleotide polymorphisms (SNPs) located in protein-coding regions of the genome, SNPs located in non-exon coding regions have also been implicated in LBP. Variants such as intergenic variant rs7833174, situated between CCDC26 and GSDMC, and intronic variant rs4384683, found within the DCC gene, have been associated with LBP.84
Furthermore, while numerous gene polymorphisms have been linked to IVDD, including those affecting ECM proteins, cytokines, and ECM degradation enzymes, their roles in contributing to LBP remain uncertain.88 For instance, polymorphisms within the IL-1 gene have demonstrated associations with both increased and decreased susceptibility to LBP.89,90
The genetic landscape influencing LBP is complex, encompassing both exonic and non-exonic variants, and involves multiple biological pathways. Despite advancements in genetic research, the precise mechanisms by which genetic predisposition contributes to LBP are not fully elucidated. Continued research integrating genomic data with clinical phenotypes is crucial to uncover the nuanced genetic factors at play and develop personalized treatment strategies for those affected by LBP.
Lifestyle factors
Lifestyle factors are some of the most important factors, outside of age, that can predict IVDD development. Factors such as physical activity, body weight, age, posture, occupational hazards, nutrition, stress, sleeping habits, and smoking have all been linked to an increased risk of LBP.75
Back pain is a significant issue for athletes and former athletes, particularly those involved in specific sports. LBP is especially prevalent among gymnasts. A meta-analysis by Trompeter et al. revealed that both male and female gymnasts have a high lifetime prevalence of LBP, exceeding 65%, with male gymnasts experiencing an even higher rate of over 80%.91 Other sports with a high risk of long-term LBP (affecting over 60% of participants) include rowing, weightlifting, and wrestling.91 The excessive spinal loading and high training volumes associated with these sports are key risk factors for overuse injuries, leading to chronic pain and degeneration.
Gymnastics sees a high prevalence of LBP among athletes under the age of 18. The sport demands extreme spinal movements such as flexion, torsion, and hyperextension, combined with high-impact activities.92 A study on adolescent female gymnasts found that 45% experienced LBP, with higher rates among those who had reached menarche. Post-menarche skeletal development, including increases in weight and height, contributes to additional spinal stress.
Excessive axial loading of the spine is another major factor in chronic LBP. Sports like powerlifting, weightlifting, and CrossFit, which involve heavy lifting, commonly lead to spinal injuries such as IVD bulges, herniations, and muscle strains.93 Weightlifters have a notably high lifetime incidence of LBP, exceeding 20%.
Repetitive lumbar flexion also contributes to LBP in athletes. In rowers, those with LBP exhibit distinct kinematic patterns compared to their pain-free counterparts. Rowers with LBP show increased lumbar spine range of motion (ROM) and decreased hip ROM during the catch phase of a stroke, leading to greater posterior pelvic tilt and reduced engagement of spinal extensor muscles.94 Conversely, healthy rowers maintain a more neutral pelvic tilt, greater hip ROM during the catch phase, and better co-activation of trunk and spine extensor muscles, resulting in improved spine stabilization during the pull.94 The increased load exerted onto the spine when the erector muscles are not properly engaged can be attributed to the flexion relaxation phenomenon. This biomechanical phenomenon occurs because of spinal flexion and is characterized by a sudden reduction in the myoelectrical activity of the paraspinal muscles, supporting the spine.95 Due to lack of engagement of the trunk extensors, the bending load is transferred onto passive tissues, including ligaments and IVDs, making the individual more susceptible to disc injury.95
While overexertion and improper spine loading can lead to LBP, sedentary behaviors are also linked to chronic LBP. Sedentary lifestyles, characterized by increased sitting time and decreased physical activity, are associated with higher obesity rates.96 Prolonged sitting has been shown to be a significant risk factor for LBP development in adults, likely due to postural changes in the spine over time, resulting in muscle atrophy and disc degeneration. Additionally, sedentary behaviors closely correlate with obesity, which further influences LBP development. Excess body weight increases the load on spinal tissues, potentially causing overload and damage. Obesity can also exacerbate LBP through other mechanisms, such as increases in systemic inflammation. Hypertrophied adipose tissue is characterized by chronic upregulation of pro-inflammatory cytokines like TNF, IL-6, and IL-1B, leading to long-term low-grade inflammation.97 Engaging in any form of exercise (while avoiding over-exercise) is proposed as a potential conservative treatment for patients with LBP who led sedentary lifestyles. Exercise can alleviate symptoms by reducing body fat, lowering inflammatory markers, and increasing trunk muscle strength.97
Chronic LBP is a significant cause of disability and impaired quality of life, particularly prevalent in older adults. Among individuals over 60 years of age, the prevalence of LBP ranges from 40% to 70%. The severity of LBP increases with age, with those over 80 experiencing pain that is three times more severe than that of younger individuals.98 In the aging population, LBP has no single pathology and often arises from multiple sources, including disc degeneration, facet joint pain, lumbar degenerative spondylosis, and other issues in the areas surrounding the lumbar spine.98
The role of cytokines, inflammation, and vascularization in discogenic LBP
One of many factors contributing to the onset and propagation of IVDD is the activation of inflammatory pathways through pro-inflammatory molecules known as cytokines. The pathways can be activated through both intracellular and extracellular mechanisms.
Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are critical activators of the inflammatory response in IVDD. PAMPs, which arise from microbial infections, viruses, and fungi, are less often associated with IVDD propagation.99,100,101 DAMPs are more commonly responsible for triggering the inflammatory cascades associated with IVDD and include, but are not limited to, cellular stress or injury, ECM degradation products, mitochondrial dysfunction, and mechanical stress.102 DAMPs are molecules that are released by stressed or damaged cells and can originate from intracellular components such as mitochondrial DNA, ATP, uric acid crystals, and HMGB1.103,104,105,106 They can also be released from extracellular matrix fragments, including hyaluronan fragments and fibronectin.107,108,109 Additional ECM fragments have been reported in the literature as DAMPs. Fragments from collagen II have been found in multiple studies to induce cytokine and MMP production.110,111,112,113,114,115 Also, aggrecan has been reported to induce chemokine CCL2 release by recruiting immune cells to the disc.116 Other less common proteoglycans, including fragments from biglycan, decorin, and versican, have also been identified as DAMPs.117,118,119,120,121,122,123 Additionally, cell stress and death via mechanical overloading, environmental stress, cellular aging, and senescence release intracellular DAMPs, further adding to inflammatory signaling.
DAMPs are recognized as danger signals by specialized receptors known as pattern recognition receptors (PRRs).103,124 These receptors can be expressed by immune cells such as macrophages and dendritic cells, as well as non-immune cells, including those that make up the NP and AF in the disc.99,125,126,127 The DAMP molecules contribute to the upregulation of cytokines such as IL-1, IL-6, IL-8, IL-17, TNF-α, and interferon-γ (IFN-γ) and have long been linked to IVDD.27,128,129,130,131,132,133,134 DAMPs initiate the activation of intracellular multi-protein complexes known as inflammasomes by binding to PRRs, such as the toll-like receptor (TLR).135 Upon DAMP binding, the TLR recruits intracellular adaptor proteins, primarily Myeloid Differentiation Primary Response Protein 88 (MyD88).136 MyD88 acts as a bridge, connecting the activated receptor complex to downstream signaling molecules. It undergoes a change in shape and recruits IL-1 receptor-associated kinase 4 (IRAK4), which then phosphorylates and activates IRAK1. Activated IRAK1 dissociates from the receptor complex and interacts with downstream signaling molecules, leading to the activation of the Transforming Growth Factor-beta-Activated Kinase 1 (TAK1) complex.135 TAK1, a serine/threonine kinase, activates multiple downstream signaling pathways, including nuclear factor kappa-light-chain-enhancer activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways.99,137 Downstream, NF-κB translocates to the nucleus, inducing the transcription of pro-IL-1β and inactive NLRP3, a component of the inflammasome, making it a potential therapeutic target.138 The NLRP3 protein is then produced and released from the nucleus, along with the inactive pro-IL-1β.139 Once the inactive NLRP3 protein is released, a secondary signal induces a conformational change in the protein, allowing it to oligomerize. It then recruits the adaptor protein ASC, and recruits pro-caspase-1, forming the NLRP3 inflammasome.140 Upon activation, NLRP3 facilitates the conversion of procaspase-1, an inactive precursor, to its active form, caspase-1. Caspase-1, in turn, cleaves pro-IL-1β into its mature, bioactive form. Once mature, IL-1β is secreted from the cell and binds to its receptor, IL-1 receptor type I (IL-1RI), on the surface of IVD cells.139
The binding of IL-1β to IL-1RI initiates a conformational change in the receptor, allowing the recruitment of the IL-1 receptor accessory protein (IL-1RAcP).99,141 Together, IL-1RI and IL-1RAcP form a high-affinity signaling complex that, similarly to the TLR, recruits the MyD88/IRAK/TRAF6 complex.99,141,142 TAK1 is then activated and ultimately results in activation of the NF-κB and MAPK signaling pathways.99
Certain DAMPs can also be responsible for upregulating TNF-α via the NF-κB pathway, however, not through activation of the inflammasome. Instead, DAMPs bind to cell surface PRRs such as those of the TLR family or RAGE.143,144 Activation of PRRs leads to downstream recruitment and activation of the IKK complex, which is responsible for releasing NF-κB dimers upon phosphorylation. These dimers (typically p50 and p65), are then able to translocate into the nucleus and bind to the promoter region of the TNF-α gene, thus upregulating TNF-α production.145 TNF-α is then secreted out of the cell, where it can bind to surface receptors and activate other signaling pathways that contribute to inflammation.135,146 The chronic activation of these pathways leads to progressive ECM destruction, increased cellular senescence, disc cell death, and impairment of biomechanical function, all of which are characteristic features of IVDD (Fig. 2).147,148
Key mediators of IVDD, namely IL-1β and TNF-α, are typically produced by immune cells but are also secreted by IVD cells.99 Low levels of these cytokines are present in non-degenerative disc cells, indicating their role in tissue reconstruction. However, higher levels of expression are observed in IVDD, correlating positively with age and the degree of disc degeneration.125 Notably, TNF-α is more abundant in NP cells compared to AF cells, and receptors like TNFR1, TNFR2, TACE, and IL-1RI are expressed in NP tissues.149 Overexpression of IL-1β and TNF-α in degenerated IVDs may be a causative factor in IVDD.99
Given the central role of IL-1β and TNF-α in the inflammatory processes of IVDD, targeting these cytokines with specific inhibitors or regulatory molecules offers potential therapeutic benefits.99 Anti-IL-1 and anti-TNF therapies may alleviate disc degeneration and improve patient outcomes by reducing inflammation and slowing down the degenerative process.99
Angiogenesis, the formation of new blood vessels, is a notable characteristic of symptomatic IVDD.43 Typically avascular, IVDs become sites of increased vascularization during degeneration, which is considered critical for symptomatic IVDD.150 During inflammation, a macrophage-related cascade results in the secretion of high levels of pro-inflammatory cytokines, further promoting angiogenesis.151 Mechanistically, the hypoxic conditions in degenerating discs lead to the upregulation of hypoxia-inducible factor 1-alpha (HIF-1α), a regulator of angiogenesis that stimulates the transcription of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), angiopoietin-1, and platelet-derived growth factor (PDGF).152,153 These factors promote endothelial cell proliferation, migration, and differentiation, leading to the formation of new blood vessels.153 Endothelial cells in existing vessels respond to the secretion of these angiogenic factors by migrating towards hypoxic, nutrient-deprived areas within the disc, forming endothelial sprouts that eventually establish a network of neovascularization.154
The SDF1 (CXCL12) /CXCR4 axis also facilitates angiogenesis in degenerated discs by activating the PI3K/AKT pathway.155 SDF1 is a chemokine that is upregulated in response to tissue injury, inflammation, and hypoxia. SDF1 binds to the CXCR4 receptor in endothelial cells, promoting cell migration, proliferation, and survival.155 Additionally, SDF1 recruits immune cells such as macrophages and T cells, further contributing to tissue inflammation. Crosstalk between SDF1/CXCR4 and VEGF signaling amplifies angiogenesis.156 TNF-α increases the expression of VEGF, IL-8, and bFGF in endothelial cells, promoting angiogenesis.157
While angiogenesis can improve nutrient delivery to NPCs and facilitate the phagocytosis of dead cells by immune cells, it also promotes inflammation and may exacerbate disc degeneration.150 There are conflicting views on the role of HIF-1 α in IVDD; some believe it worsens the condition, while others argue it helps by improving nutrient supply and immune cell infiltration.152 Hypoxia plays an important role in angiogenesis, aiming to balance the activation of the HIF-1α/VEGF axis to maintain cellular function. Overall, the role of angiogenesis in IVDD is complex and multifaceted, involving a delicate balance between beneficial and detrimental effects.
Neurotrophins and innervation
In a healthy IVD, innervation is predominantly limited to the outer third of the outer AF, with a majority of the disc being aneural. However, in the context of IVDD, the innervation pattern undergoes significant changes, with nerves extending deeper into the disc, particularly into the normally aneural NP. This phenomenon is prominently observed in cases of chronic LBP.158,159,160 Previous studies have identified vascularization of the disc as a pathway for LBP development; however, that does not explain why pain is exhibited only in some degenerated discs.161
Neurotrophic factors are defined as a family of biomolecules that support the growth, survival, and differentiation of neurons and encompasses a wide range of subfamilies including neurotrophins, neuropeptides, and TGF-β family factors.162
Most well-known of these neurotrophic factors are neurotrophins, which are a small subset of structurally similar neurotrophic factors comprised of NGF, BDNF, NT-3, and NT-4 (Table 1). These essential proteins, well known for their roles in neuronal development, survival, and maintenance, have emerged as key players in disc innervation dynamics. While traditionally associated with nervous tissue, neurotrophins have been found to be expressed in non-neural cells within the disc microenvironment, indicating their broader involvement in tissue homeostasis and response to injury.163,164,165 The exact neurotrophins or factors that induce neural ingrowth into the disc remain unknown, but multiple studies have observed upregulation of neurotrophic factors such as Neural Growth Factor (NGF) and Brain-Derived Neurotrophic Factor (BDNF) in degenerated IVD tissues and LBP samples, suggesting that neurotrophins likely play a key role in LBP development.165,166
Neurotrophins exert their effects by binding to receptors on the surface of neurons, namely the Tropomyosin Receptor Kinase (Trk) receptors and the p75 neurotrophin receptor, initiating signaling pathways that promote neuronal survival, growth, and differentiation.160,167 Interestingly, neurotrophins can bind to both Trk receptors and the p75 neurotrophin receptors, which fall into two completely distinct classes of receptors. First, Trk class receptors, including TrkA (for NGF), TrkB (for BDNF, NT-4, and NT-5), and TrkC (for NT-3), are high-affinity receptors that, upon neurotrophin binding, undergo dimerization and autophosphorylation of their tyrosine kinase domains.168,169,170,171 This activation initiates downstream signaling cascades, such as the Ras/MAPK pathway and the PI3K/Akt pathway, eventually leading to axon outgrowth, branching, and guidance toward the source of neurotrophins in the target tissue.168,169,170,171,172,173,174
Additionally, neurotrophin signaling involves the p75 neurotrophin receptor (p75NTR), a low-affinity receptor capable of binding all neurotrophins with lower specificity but at a broader range. This receptor can act independently or in conjunction with Trk.175,176,177,178 Unlike Trk receptors, p75NTR can modulate both independent and cooperative signaling with Trk receptors, influencing pathways such as Akt signaling and cellular responses such as apoptosis.179 NGF and BDNF are two of the most common neurotrophins studied in IVDD and LBP and are involved in the growth, development, maintenance, and survival of neurons. In the context of IVDD and LBP, both are produced by stressed NPCs and immune cells within the disc and act as a chemotactic factor that guides the outgrowth of axons from peripheral neurons toward the target tissue.174,180,181,182,183 In addition to their role in promoting the outgrowth of axons, NGF and BDNF are also involved in the synthesis of neurotransmitters and neuropeptides, including substance P and CGRP.174,181,182 Both NGF and BDNF trigger a complex cascade that results in transcriptional changes, axonal sprouting, and neuronal sensitization, contributing to the progression of pain observed in IVDD.184,185,186
NGF initiates intracellular signaling pathways by binding to its high-affinity receptor TrkA, which is prominently expressed on nociceptive nerve fibers within degenerated intervertebral discs. Upon NGF binding, TrkA receptors dimerize and undergo autophosphorylation of their tyrosine kinase domains, creating docking sites for adaptor proteins like Src homology 2 domain containing (Shc) and growth factor receptor-bound protein2 (Grb2).187 This event leads to the activation of the MAPK/ERK pathway, where Grb2 recruits son of sevenless (SOS) to activate Ras. Activated Ras triggers a phosphorylation cascade involving RAF, MEK, and ultimately ERK. Phosphorylated ERK translocates to the nucleus where it phosphorylates transcription factors such as cAMP response element-binding protein (CREB), thereby regulating gene expression related to neuronal survival, growth, and plasticity.188
Concurrently, NGF-TrkA binding activates phosphoinositide 3-kinase (PI3K), which phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 recruits and activates Akt (protein kinase B) at the plasma membrane, leading to downstream effects that promote cell survival, inhibit apoptosis, and regulate protein synthesis.189,190 Additionally, NGF-TrkA signaling activates phospholipase C-gamma (PLC-γ), which hydrolyzes PIP2 to generate inositol trisphosphate (IP3) and diacylglycerol (DAG).191 IP3 induces calcium release from intracellular stores, modulating cellular processes including synaptic transmission, while DAG activates protein kinase C (PKC), involved in various signal transduction pathways. Together, these pathways regulate genes involved in neuronal survival, axonal sprouting, and nerve sensitization.192,193 Pathway activation promotes axonal growth and branching by increasing the expression of cytoskeletal proteins, enhancing local protein production, and regulating microtubule and actin assembly.192 Nerve sensitization is also promoted through an increase in expression and traffic of ion channels such as TRPV1 to the cell membrane and promoting the release of neuropeptides that amplify the pain signal.192
NGF has been extensively studied as a possible cause of innervation due to various studies identifying it as being upregulated in degenerated IVD tissue.166,194 Interestingly, a study by Freemont et al. found that LBP-causing discs had microvascular vessels within the inner AF and NP that produced NGFβ, while non-painful discs did not. This finding aligns with our hypothesis that vascular ingrowth causes neural ingrowth.195 Interestingly, Freemont determined that all vessels expressing NGFβ originated from the endplate, suggesting a possible role for the endplate in innervation and LBP.195
Similarly to NGF, BDNF is a neurotrophin known for its ability to induce nerve growth and has been found to be expressed by IVD cells across all stages of degeneration.165,166 Research indicates that stimulation with pro-inflammatory cytokines such as IL-1β can induce BDNF secretion by IVD cells. Once secreted, BDNF binds to its receptor TrkB, leading to dimerization and autophosphorylation, thus activating the receptor.165 The activated TrkB receptor then engages several downstream signaling pathways, including PLC-γ, PI3K, MAPK, and ERK. Specifically, TrkB activates PLC-γ via phosphorylation of the Y816 residue, increasing intracellular calcium levels and activating calcium/calmodulin-dependent protein kinase II (CaMKII).196 Consequently, this activates the CREB transcription factor, which promotes gene expression related to neuronal survival and plasticity.197 Furthermore, TrkB activation also stimulates the PI3K pathway by recruiting Shc, which activates the Grb2/SOS complex.198 This leads to the activation of downstream molecules such as Ras and Akt, promoting cell survival and growth by inhibiting apoptotic pathways.198 In the MAPK pathway, activated Ras initiates a kinase cascade involving Raf, MEK, and ERK. ERK then translocates to the nucleus, activating transcription factors that support neuronal differentiation and survival.199 The activation of these pathways culminates in the activation of transcription factors such as CREB and mTOR, which drive the expression of genes and the translation of proteins essential for neuronal growth, differentiation, and synaptic plasticity (Fig. 3).200
In addition to their role in producing cytokines, immune cells such as T-cells, B-cells, and monocytes have been found to produce neurotrophins, including NGF, BDNF, NT-4 and NT-5, as well as their receptors, TrkA, TrkB, and TrkC.201 These neurotrophins are produced downstream of TLRs such as TLR2 and TLR4, which activate inflammatory pathways like IL-1β and NF-κB.202 Stress on NPCs has been suggested as an alternative pathway for the upregulation of neurotrophins within the IVD. Multiple studies have identified NPCs as being able to produce neurotrophins and induce nociceptor growth and innervation in vitro.3,183,203 A recent study by Jiang et al. found that compared to non-stressed NPCs, stressed NPCs, that is, NPCs exposed to pro-inflammatory conditions induced significantly more axon ingrowth in vitro.3 These results suggest that NPC stress likely plays a role in inducing innervation of the disc, whether by expression of neurotrophins and neo-innervation factors or by secretion of protective factors that prevent innervation from occurring. Indeed, both neurotrophic and pain factors have been found to be positively regulated by cytokines such as IL-1, IL-6, and TNF-α, which are commonly found to be upregulated during IVDD.166 These findings suggest that IVDD and, in turn, expression of pro-inflammatory cytokines may induce innervation and pain.166
Another commonly studied group of neurotrophic factors are GDNF family ligands (GFLs) which is composed of GDNF as well as neurturin (NRTN), artemin (ARTN), persephin (PSPN). Due to its structure, this family falls into the TGF-β superfamily which includes non-traditionally neurotrophic factor subfamilies such as TGF-β, BMPs, activins, and inhibin. Regarding discogenic LBP, GFLs are the most studied factors and have been linked to neurite outgrowth, neuroprotection, neuronal survival, etc.204 All four of these ligands can exert their effects though three different receptor systems. The first and most well-understood is through the GDNF family receptor alpha (GFRα). GFLs bind with the four characterized GFRα, however, each GFL binds one of the GFRα as its highest affinity receptor.204 Specifically, GFRα1 interacts with GDNF, GFRα2 with NRTN, GFRα3 with ARTN, and GFRα4 with PSPN.204 Once the GFLs bind to one of the GFRα, it forms a complex with tyrosine kinase Rearranged During Transfection (RET), which is then able to activate RET via dimerization and transphosphorylation of its tyrosine residues. The activated RET then induces a downstream signaling cascade through the PI3K/Akt and MAPK pathways. Another pathway used by GFLs is low affinity binding with NCAM, which increases the binding affinity of the GFLs with GFRα1.205 The GFL-NCAM- GFRα1 complex can then activate Src-like kinase Fyn and focal adhesion kinase FAK and induce downstream signaling.205 Finally, GFLs can also interact with syndecan-3, which is a heparin sulfate proteoglycan, in the absence of co-receptors.206 Once formed, GFL-syndecan-3 complexes can act as both co-receptors, bringing the GFL to GFRα and inducing downstream RET signaling, and independently, directly activating Src-dependent signaling.205 Though GDNF has been long shown to be expressed in degenerated IVD, recent studies have also found GFLs NRTN, ARTN, and PSPN expressed in cells derived from degenerated IVD suggesting they may play a role in disc innervation and LBP development.207,208 Furthermore, stimulation with IL-1β was found to upregulate expression of these ligands and their co-receptors both at the mRNA and protein level.208
In addition to neurotrophic factors, neuropeptides are small proteinaceous substance that function as neurotransmitters and neuromodulators, influencing neurotropic activity.209 While they are mainly associated with neurons, some neuropeptides have also been expressed in non-neural cells, including within the IVD.166,210 These neuropeptides bind to G-protein coupled receptors (GPCRs) with high affinity and, unlike classic neurotransmitters, are active at much lower concentrations and have slower reuptake by synapses, allowing them to exert prolonged effects through various downstream pathways (Table 1).
One of the most well-known neuropeptides associated with pain is Substance P (SubP). SubP is not only endogenously expressed by IVD cells but is also found in a greater percentage of cells in degenerated IVD samples (Table 1).166,210 SubP is derived from the alternative splicing of the preprotachykinin A (TAC1) gene and functions both as a neurotransmitter and neuromodulator. Typically stored in vesicles within neurons, SubP is released in response to various stimuli, including inflammation.211 Studies have also found that SubP is produced by IVD cells themselves, particularly under conditions of degeneration.212 Once released, SubP binds to one of four neurokinin receptors, with the highest affinity for the neurokinin-1 receptor (NK1R).213 The binding of SubP to NK1R activates multiple pathways, including the IP3/DAG pathway and the cAMP pathway, leading to various cellular responses.213 Additionally, SubP can induce cytokine production and, in conjunction with nitric oxide, promote vasodilation.214 High levels of SubP have been observed in the spinal cords of rats with chronic pain, highlighting its role in neuroinflammation.
Another neuropeptide commonly associated with pain and nociception is Calcitonin Gene-Related Protein (CGRP; Table 1).215 CGRP exists in two major isoforms: CALCA, mainly found in the central and peripheral nervous systems, and CALCB, primarily expressed in the enteric nervous system.215 Despite their structural similarities, these isoforms have distinct biological functions.215 CGRP’s receptor complex requires three subunits for optimal function: the calcitonin receptor-like receptor (CALCRL), a GPCR capable of binding any calcitonin family ligand; the receptor activity-modifying protein 1 (RAMP1), which is CGRP-specific; and the receptor component protein (RCP), which facilitates coupling of the CGRP-CALCRL-RAMP1 complex with downstream signaling pathways, including cAMP.215 Activation of this receptor complex leads to an increase in intracellular cAMP, which in turn activates various targets involved in pain signaling and vasodilation.215
SubP and CGRP are often co-released and co-localized in neural cells, but they use different receptors—SubP through NK1R and CGRP through the CGRP receptor complex.216 Both SubP and CGRP are upregulated in response to pro-inflammatory cytokines like IL-1β and TNF-α, suggesting a role in the inflammatory processes associated with IVDD.216 Additionally, neuropeptide Y (NPY) is another neuropeptide that plays a role in nociception and pain modulation.217 NPY acts through GPCRs, particularly the Y2 receptor (Y2R), which has been found to be upregulated in degenerated human IVD tissue, indicating a potential role in disc pathology.218,219
Transient Receptor Potential (TRP) ion channels are another group of receptors implicated in pain and nociception within the IVD.220 The TRP channel family is diverse, responding to various stimuli such as temperature, mechanical forces, and osmotic pressure.220 Although TRP channels are primarily associated with neurons, they have also been found in IVD cells, with higher expression levels in degenerated discs.220 Surprisingly, 26 of the 28 TRP channels expressed in humans have been found to be expressed by IVD at the mRNA level suggesting these receptors may play a greater role in IVD homeostasis and that previously predicted.221
Of the TRP family, TRPV1 and TRPV4 have been directly linked to low back pain (LBP). TRPV1, mainly expressed in nociceptive neurons, is activated by noxious stimuli like low pH and inflammatory mediators, both prevalent in degenerating IVDs. (Table 1). In contrast, TRPV4 is more widely expressed and is involved in detecting osmotic changes and mechanical stimuli, which are crucial for IVD homeostasis.221 It plays an important role in detecting osmotic changes and mechanical stimuli, which are both important measures for IVD homeostasis. Indeed, studies into the function of TRPV4 in the IVD have found that activation of the channel in IVD derived cells has been linked to increased production of IL-6.222,223 Activation of TRPV1 and TRPV4 channels leads to an influx of calcium ions (Ca2+), triggering action potentials and transmitting pain signals to the central nervous system.
In addition to neuropeptides and ion channels, other proteins like Netrin-1 (NTN1), an axon guidance molecule, have been implicated in LBP (Table 1). NTN1, expressed by degenerated nucleus pulposus cells, has been shown to promote neuronal outgrowth, suggesting a role in pathological innervation within the disc.224 Moreover, the highly innervated endplate, specifically the bone marrow and periosteum, may also contribute to pathological innervation, further linking these molecular mechanisms to the development of pain in IVDD.
Peripheral and central sensitization
Over time, as the LBP transitions from acute to chronic, sensitization of both the peripheral and central nervous systems occur.225 Sensitization induces neuroplastic changes, recruiting non-nociceptive pathways. This recruitment includes the involvement of low-threshold mechanoreceptor inputs to pain pathways and the conversion of nociceptive-specific neurons to wide dynamic range neurons that can respond to both painful and non-painful stimuli.226
Recent studies have identified sensitization of the peripheral and central nervous systems as playing a significant role in chronic LBP.3 Peripheral sensitization refers to neuropathic pain mediated by amplified nociceptor stimulation at normally sub-threshold levels for pain.227 In discogenic LBP, inflammatory cells respond to ECM degradation and destruction of the disc structure via the release of cytokines such as IL-1β, IL-1α, and TNF-α that increase the synthesis of NGF.228 Elevated levels of neurotrophins, like BDNF and NGF, have shown evidence of leading to the ingrowth of sympathetic nerve fibers that penetrate through the outer AF and endplates into the inner AF and NP.165,167 These infiltrating nerves express substance P, TrkA receptors, and increase the production of BDNF with increasing IVDD.165,167 Furthermore, as nerves penetrate the IVD, cytokines released into the disc space activate nociceptors, increase inflammatory marker receptors, and upregulate the activation of Cav2.2 voltage-gated calcium channels that cause calcium influx into the cell and may play a key role in mechanoresponse.165,167,229 Inflammatory factors such as IL-1β, IL-6, and TNF-α also can influence sodium channels such as TRPA1, TRPV1, NaV1.7, NaV1.8, and NaV1.9.230 Over time, an increase in neural discharge results in sensitization of ingrowing sympathetic nerves to fire at previously sub-threshold levels. It is believed that these neurons are important in pain propagation and the feeling of chronic LBP, as observed in a rat model by Murata et al. which demonstrated that when ingrowing sympathetic nerves were ablated, there was a decrease in pain response.231 The barrage of pain signaling induces progressive amplification from consistent input, also known as “wind-up,” resulting in an increase in synaptic efficacy and neuronal excitability.232
Central sensitization, however, refers to changes in the properties of CNS nociceptors to respond to stimuli that previously would not have caused a response, increasing the potential for hyperalgesia and allodynia.233 Excessive nociceptive signals from the peripheral nervous system within the dorsal horn of the spinal cord can result in transcriptional and translational changes in second order neurons, thus causing lingering hyperexcitability known as central sensitivity. In addition, inhibitory controls also tend to be affected, thus creating an imbalance between excitation and relaxation.230
In addition, neuroinflammation has been identified as also playing a role in chronic pain development. During acute injury, local macrophages, glial cells, and injured neurons signal immune cells to release pro-inflammatory cytokines, such as IL-1β, IL-6, IL-12, and TNF-α, inducing neuroinflammation.234 The influx of inflammatory markers at the site of injury leads to the discharge of nociceptors that propagate the signals up to the spinal cord.234 However, in a recent study on neuroinflammation in a rat model of IVDD, researcher found that neuroinflammation may last for far longer than initially predicted with rats exhibiting increased satellite glial cells and active macrophages up to eight weeks post injury.235 Furthermore, signs of neuroinflammation were identified not only in the DRGs but also in the dorsal horn of the spinal cord.235
Modeling discogenic pain
Ex vivo models using organ cultures
Cell monoculture is the most basic and long-standing model for studying IVDD and has played a vital role in our understanding (Table 2). Like many cell types, studies on IVD cells have found that the method of cell culture significantly impacts cell morphology, gene expression, and cell behavior. Indeed, multiple studies comparing cells in situ, monolayer, and 3D culture of IVD-derived cells have identified significant differences in cell morphology.236,237,238,239 In vivo, NPCs were found to have a rounded shape and be surrounded by a distinct capsule, while the cells of the AF took on an elongated appearance following the direction of the collagen fibers of the disc.238 Studies examining IVD cells in vitro found that, initially, the morphology observed in native discs is preserved after cell isolation and seeding, with AFCs maintaining a spindle-shaped fibroblast-like morphology and NPCs adopting a polygonal appearance.237 However, morphological changes were found to occur shortly after initial seeding, with some studies observing changes as soon as three days in monolayer culture.240 Gene expression analysis studies of IVD-derived cells in monolayer culture over time demonstrate a loss of aggrecan, collagen I, and collagen II expression, resembling patterns observed in articular cartilage monolayer culture.237,239
Given that IVDD and LBP are chronic diseases, experiments involving long-term culture are required, making monoculture non-ideal for long term study. To address these limitations, researchers have turned to 3D culture of cells, which has been found to preserve some of the features lost in monolayer culture. Compared to monolayer culture, 3D culture has been shown to significantly improve cell viability, matrix production, and phenotypic stability. However, these models still have their limitations and may not fully recapitulate the complex microenvironment of the native IVD. Various materials, including alginate and collagen scaffolds, have been employed to create 3D environments.
Organ-on-chip systems
More recently, the development of organ-on-chip systems and microfluidic chips has become increasingly used and is an excellent method for small-scale modeling and studying cellular crosstalk (Table 2). Given that IVDD is a chronic disease, and that IVD-derived cell morphology and functionality are rapidly lost in monolayer culture, strides have been made to improve the long-term culture of the IVD and their derived cells. Various versions of IVD-on-chip utilizing microfluidic technology have been developed to provide sufficient nutrient transport and waste removal.
Dai et al. developed a “disc on chip” microfluidic chip, enabling long-term culture of whole mouse IVD in vitro.241 Unlike static culture methods, the IVD cultured in the “disc-on-chip” system retained matrix organization, cellular phenotype, cell viability, and overall structural integrity comparable to native discs for 10 to 21 days post-seeding.241 Various studies have identified that mechanical stimulation is essential for maintaining IVD health and function.242,243 McKinley et al. developed a multiaxial strain AFC-on-chip system combining microfluidic technology with multiaxial strain to allow for long-term study of strain on IVDD.244 More recently, Xie et al. combined the developments of McKinley and Dai to create a mouse IVD mechanical loading chip system with dynamic media flow.245 The combination of a continuous nutrient supply plus appropriate mechanical loading resulted in structural integrity, collagen breakdown and alignment, catabolic enzyme activity, and cell alignment comparable to native IVD and significantly improved compared to static culture up to 21 days.245 Though promising, these systems still lack the ability to preserve disc morphology for long-term study and thus require additional optimization.
In addition to enhancing long-term culture of IVD cells in vitro, microfluidic organ-on-chip systems have emerged as valuable tools for studying cellular crosstalk within the IVD microenvironment. These systems offer a unique advantage for investigating cellular interactions by fluidically isolating cells while enabling their interaction.
Given the recognized role of immune involvement in the progression and development of pain in IVDD, researchers have utilized microfluidic platforms to delve into these aspects. Son et al. developed an NP-monocyte chip to investigate monocyte extravasation when exposed to IL-1β-stimulated NP cells.246
More recently, NP cells have been identified as a potential source of factors involved in neurogenesis and initiating innervation of the disc. Jiang et al. developed an iPSC-derived nociceptor-nucleus pulposus cell microfluidics model using the Xona chip (Xona Microfluidics) which studied nociceptor-NPC interaction.3 Similarly, Zheng et al. utilized standard neuron device (Xona Microfluidics) to explore innervation in response to conditioned media from degenerated NP cells.224 Hwang et al. developed a microfluidics chip designed to examine the paracrine interaction between AFC, NPC, and endothelial cells and neurons via a microfluidic gradient system.247,248
Animal models
Animal models serve as invaluable tools for studying pain, including discogenic pain, as it is currently impossible to fully recapitulate the pain signaling process in vitro. A diverse array of animal models, ranging from small organisms like zebrafish, mice, and rats to larger mammals such as pigs, dogs, and sheep, have been developed to investigate IVDD (Table 3).29,30,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273 However, very few specifically study discogenic pain. Studies focusing on discogenic pain in animals are almost exclusively performed in mice and rats.272,273,274,275 Several factors contribute to the predominance of murine models in discogenic pain research. Firstly, a rich body of literature exists on IVDD, pain mechanisms, and overall anatomy in murine models, along with a plethora of well-established pain tests, making them a reliable and widely utilized model for discogenic pain research. Additionally, their small size and ease of handling and training make them ideal for conducting biobehavioral pain assessments. Moreover, murine models are cost-effective to procure, house, and maintain compared to larger animals. Furthermore, the availability of diverse pre-existing genotypes and the ease of generating new genetic models make murine models ideal for pain research. In murine models, disc puncture method of IVDD induction is the preferred choice in the field due to its ease of induction, low cost and procedure simplicity. This model most commonly targets the lumbar or caudal discs. Some labs, like ours, prefer the lumbar discs to maximize clinical relevance, while others opt for the caudal levels due to their greater accessibility.78,79,224,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293 Other methods of IVDD induction have been developed, including surgical induction, injection of noxious substances such as TNF-α or Freud’s adjuvant, and the use of custom spine-stretching apparatuses, however, these alternatives are less popular due to the simplicity and effectiveness of the needle puncture method.3,294,295,296,297,298,299
While murine models have been instrumental in characterizing discogenic pain, their translational potential to humans is limited. Larger animal models, such as dogs and pigs, hold promise as a translational model due to established BBT methods in other fields of study.300,301 Establishing such models in the context of LBP holds promise for advancing our understanding of the etiology of discogenic back pain and expediting the translation of potential treatments from preclinical studies to clinical applications.
Methods of pain assessment
Imaging
MR imaging has long been used to identify IVDD; however, it remains unable to identify IVDD pain. Recent developments in MR imaging have allowed for the detection of biochemical changes within the disc, such as changes in pH, GAG content, water content, and more. These changes are present in the IVD before the appearance of morphological changes, making them particularly useful for early detection of IVDD and for diagnosing possible discs exhibiting pain. Advances in MR imaging have resulted in the development of chemical exchange saturation transfer (CEST) imaging and, more recently, qCEST MRI which can noninvasively identify biochemical changes within the disc associated with both degeneration and pain (Table 4).29,30,31
CEST is an MRI technique that indirectly detects exchangeable protons in the water pool by pre-saturation at different frequency offsets. CEST MRI can image endogenous or exogenous compounds containing protons that exhibit a suitable exchange rate with bulk water. Compared to 1H MR spectroscopy, CEST techniques offer higher in-plane spatial resolution and higher sensitivity for the target molecules.302 Recent advances in MRI technologies have allowed researchers to assess pH changes in the body non-invasively.28,303 Of note, CEST has been studied to measure pH-dependent MRI signal changes.304,305,306,307 This technique exploits the pH-sensitive chemical exchange between water protons and solute protons in certain molecules. Previous studies have applied CEST to detect GAG concentration and pH changes in the IVDs of pigs and humans.303,308 However, the CEST signal can be affected by other contributing factors, including water relaxation parameters and solute concentration.309 To eliminate these confounding factors, qCEST was developed to measure the exchange rate independently from T1- and T2-signals and the solute concentration.310,311,312 In the NP, the hydroxyl proton of GAGs can serve as an endogenous agent for qCEST to measure the pH-sensitive exchange rate with water protons. It has recently been shown that the exchange rate measured using qCEST is closely correlated to pH values found in the IVD.31 Though not currently part of the standard of care, the qCEST is a promising method of diagnosing discogenic pain. Unlike other methods, qCEST MRI is non-invasive and can provide definitive, quantitative results, making it ideal for implementation in a clinical setting.
Biobehavioral testing
In animal models, biobehavioral testing (BBT) has long been the gold standard for pain assessment, as animals cannot effectively communicate their pain to researchers. Due to the labor-intensive nature of these tests and the active involvement required from researchers, development of BBTs assays have primarily been in small, easy to handle models, such as mice and rats. While many tests assess pain by applying stimuli to the hind paw or tail, some have been adapted for other regions, including the back.313,314,315 Generally, these tests fall into four main categories: 1) mechanical hyperalgesia, 2) thermal hyperalgesia, 3) movement evoked hypersensitivity, and 4) spontaneous pain-related behaviors.
Tests for mechanical hypersensitivity
Mechanical hypersensitivity tests measure responses to noxious stimuli and include the von Frey (VF), Randall-Selitto (RS), and vocal threshold tests (Table 5). Of all the BBTs used to quantify LBP-induced allodynia and hyperalgesia, the VF is the most common, involving the application of calibrated filaments to the palmar surface of the hind paw.3,275,313,316,317,318 Some modified versions of VF have been developed for application on the lower back.313,314,315 The wide spread use and validity of the test makes it extremely popular for allodynia and hyperalgesia induced allodynia and hyperalgesia, however, due to the variability of animal response to stimuli, experience is required to ensure accurate data collection. Furthermore, some critics argue that interpretation of animal responses is subjective, thus potentially introducing observer bias.319 However, using blinded testers and ensuring the same tester conducts all assessments throughout the experiment can substantially mitigate this issue, enhancing consistency and reliability of the data.
The Randall-Selitto and vocal threshold tests, like the von Frey, assess mechanical hypersensitivity by applying increasing controlled pressure to a localized area, typically the hind paw.320 These methods rely on specialized equipment to provide precise quantification of mechanical nociceptive responses. However, compared to the VF, they require more specialized and expensive equipment, making them less commonly used. Additionally, both tests necessitate animal restraint during assessments, which can introduce variability or stress-related effects into the data.321,322 However, unlike the multiple filaments used in VF tests, these tests use a single probe which allow for increased standardization between stimulus applications.
Tests for thermal hypersensitivity
Pain also induces increased sensitivity to both hot and cold stimuli, making thermal sensitivity assessments an important component of comprehensive pain evaluation. Thermal hypersensitivity tests are typically categorized into heat and cold sensitivity, including tests such as Hargreaves, acetone, hot/cold plate, and hot/cold water (Table 5).
Among these, the acetone test is the most used, involving the application of a drop of acetone onto the plantar surface of the paw to elicit a cooling sensation and a subsequent nocifensive response.323 This test is favored for its simplicity, low cost, and rapid administration. However, interpretation of test responses can be subjective and analysis is time-consuming. These limitations can be significantly reduced by providing appropriate tester training and ensuring the same tester conducts all assessments.
Cold water and cold plate assays, like the acetone test, elicits nocifensive responses to cold stimuli, while hot water and hot plate assays use high-temperature stimuli in. These assays measure responses such as tail flicking, paw licking or jumping, reflecting thermal pain sensitivity thresholds.324,325,326
The Hargreaves test, another commonly used assay, measures the latency to paw withdrawal in response to focused heat.327 Its simplicity and effectiveness have contributed to its widespread adoption; however, the lack of precise temperature control can lead to variability in results. Additionally, inconsistent heat application increases the risk of tissue injury at the site of stimulation, making careful calibration and monitoring essential for reliable data collection.
Tests for movement-evoked hypersensitivity
Less commonly, movement evoked hypersensitivity tests assess pain indirectly by evaluating animal’s response to challenging or potentially pain-evoking movements such as twisting, stretching or bending. These include, tail suspension, grip strength, rotarod, and lateral bending maze tests (Table 5).
The most common of these tests is grip strength assessments, which assesses animal sensitivity by stretching the spinal to identifying potential discomfort or weakness.328 Similarly, the tail suspension test evaluates spinal sensitivity by leveraging gravity to induce stretching of the spine.313,329 These assays are favored for its relative simplicity and minimal need for specialized equipment, however, they require technical consistency and careful handling to prevent stress-related artifacts, ensuring reliable and reproducible results.
In contrast, the rotarod tests assess balance and motor coordination by measuring the time an animal can remain on a rotating rod, reflecting pain-induced motor impairments.273,293,330,331,332 However, unlike other assays, rotarod testing carries high risk of animal injury, making it less ideal.
The lateral bending maze test evaluates flexibility and discomfort associated with movement-induced pain.333 Unlike other movement-evoked hypersensitivity assays, the lateral bending maze provides a more controlled environment, reducing external variables and allowing for more precise assessments of movement-related pain responses.
Tests for spontaneous pain-related behaviors
Finally, assays that monitor spontaneous behaviors – such as weight bearing, catwalk/gait analysis, and open field – offer valuable insight into pain-related alterations in natural behaviors and locomotion (Table 5).
Weight-bearing assays measure the distribution of weight on the limbs, allowing for the detection of asymmetries indicative of pain or discomfort.333,334,335,336 Slight shifts in the weight distribution can reflect compensatory behaviors following injury or intervention, thus indirectly assessing pain response. However, this test, however, requires highly sensitive equipment and some tester interpretation during analysis.
Catwalk and gait analysis systems record and analyze the animal’s walking pattern, assessing stride length, paw placement, and coordination changes resulting from pain.285,333,337,338,339 However, these tests are difficult for the animals to perform, as rodents do not typically walk in a straight line, but rather hop, or run, and insufficient step cycles will result in inaccurate data.
Open field tests provide insights into exploratory behavior, anxiety levels, and overall activity, which can be influenced by pain-induced alterations in mood or motivation.333,340,341 Unlike many of the other assays, the open field test provides a broad evaluation of both locomotor and behavioral activity levels and requires minimal training to perform. However, it is not ideal for detecting subtle changes which may occur in the early stages of LBP development and can easily be confounded by other variables such as anxiety, time of testing, and environmental conditions. Additionally, because results are indirect, data interpretation can be complex.
Biobehavioral testing provides valuable insights into pain mechanisms and responses in animal models, facilitating the development of effective pain management treatments. By utilizing a combination of these tests, researchers can comprehensively evaluate LBP development and uncover potential physiological and neurological changes associated with LBP.
Current and future treatments
Current treatment guidelines for chronic LBP and degenerative disc disease involve symptom relief through intervention via medication and surgical therapy. Although these methods are useful for mitigating pain associated with IVDD, they are unable to reverse or halt the causes and progression of disc degeneration.342 Proposed clinical trials and hypotheses for future treatments look to address these shortcomings. Although there is optimism surrounding the potential of IVDD treatments for patient pain relief, further work needs to be done.
Pain medications
A common practice for patients suffering from chronic LBP is the utilization of pain relief medication. Although effective at relieving pain in the short term, medications are not a long-term solution for patients suffering from chronic LBP as they do not address the root cause of pain and can lead to side effects from long-term use.343,344
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) work by inhibiting cyclooxygenase (COX) enzymes, which normally function to convert arachidonic acid molecules into prostaglandins, causing swelling and an inflammatory response that leads to pain.345,346,347 Prostaglandins that are produced during a pro-inflammatory response increase blood flow to affected tissues. Further, prostaglandins, such as prostaglandin E2, cause pain by acting on peripheral sensory neurons and within the CNS.348 In the IVD, there is limited blood supply, which can limit the effectiveness of NSAID use. Despite this, NSAIDs have been found to be effective at providing short-term relief for patients with chronic LBP.349 NSAIDs are particularly desirable for patients because many forms are available over the counter, which makes it easier for patients to obtain them compared to other pain relief medications. As a whole, NSAIDs are widely tolerated by patients but do carry a risk of side effects, such as an increased risk of cardiovascular disease, gastrointestinal ulcers, and renal failure.346,349,350
Opioid medications
For patients experiencing significant levels of pain that cannot be alleviated through over-the-counter medication use, opioid pain medications are available via prescription. Most opioids work by eliciting analgesia through the mimicking of naturally produced opioid peptides in the body and binding to opioid receptors.351,352 Opioid receptors are GPCRs that are embedded in the cell membrane of cells found throughout the body.353 When stimulated, opioid receptors activate GPCR pathways, converting guanosine triphosphate (GTP) to guanosine diphosphate (GDP) and inhibiting adenylate cyclase, leading to increased levels of cyclic adenosine monophosphate (cAMP) intracellularly.354 Higher levels of cAMP lead to K+ channel hyperpolarization and the closure of Ca2+ channels, activating MAPKs.354 As a result of pathway activation, neurotransmitter release is reduced, decreasing the transmission of pain signals along both afferent and efferent pathways.354
Different opioid receptors exist, such as MOP (μ), DOP (δ), and KOP (κ) receptors, which each interact with different opioids that are naturally produced in the body and opioid medications.354 As a result, certain medications have higher affinities for specific receptors. Opioids act on opioid receptors by binding and stabilizing the active conformation of the receptor, leading to pathway activation and pain relief.354
Although these medications do provide patients with considerable pain relief, opioids have a substantial risk of addiction and chronic opioid use for patients.355 Furthermore, recent findings have found that patients who utilize opioids for symptom relief from chronic LBP are at an elevated risk of chronic opioid use compared to patients who use opioids for relief from other musculoskeletal diseases.356
Steroid injection
A more invasive medication available to patients is the use of epidural steroid injections. Corticosteroids are one of the more commonly used steroids, eliciting pain relief by mimicking cortisol.342,357 This prevents the release of arachidonic acid from cells and blocks the COX pathway, ultimately reducing inflammation.342,357 Current clinical trials are investigating whether the route of steroid administration for discogenic LBP can influence the quality of pain relief experienced by the patient. One such trial is investigating the efficacy of lumbar transforaminal epidural steroid injections.358 Despite this, there are many side effects that corticosteroids can cause, which may require further medications to be prescribed to reduce side effect severity.359 As a result, corticosteroids are recommended for short-term use and at low dosages.359 Furthermore, steroid injections for chronic LBP have not been shown to provide long-term relief for patients.360
Other medications in clinical trials
Other medications are also being investigated for future use in IVDD pain management. Pamidronate is a bisphosphonate that is classically used for treating hypercalcemia of malignancy and Paget disease of bone.361 Pamidronate works to inhibit bone resorption by osteoclasts.361,362 Bisphosphonates are a class of medications that mimic inorganic pyrophosphate and bind hydroxyapatite crystals.361 When osteoclasts utilize hydroxyapatite during bone resorption, pamidronate is left unbound, where it can act on farnesyl pyrophosphate synthase within the osteoclasts and induce apoptosis.361 Pamidronate has shown evidence of being effective at managing pain associated with bone diseases, and as such, researchers are looking to see if these benefits can be harnessed for use in discogenic LBP.361,362
Abaloparatide is a synthetic analog of parathyroid hormone-related protein (PTHrP). In an animal model of back pain in mice, researchers found that parathyroid hormone use effectively decreased disc degeneration.363 Abaloparatide has been used in the treatment of osteoporosis to increase bone mineral density and to slow and prevent disease progression.364
Alpha-2-Macroglobulin (A2M) is a naturally occurring macromolecule found in the extracellular matrix and acts as a protease inhibitor.365 One such protein that A2M targets and prevents the formation of is the cartilage degradation product, Fibronectin-Aggrecan complex (FAC).366 FAC has been identified as a potential cause of pain in patients with IVDD.366 As such, a clinical trial has been proposed to concentrate a patient’s A2M within their blood plasma and return the concentrate to the patient to target FAC.366 A2M prevents damage by FAC and other proteases by forming a tetrameric cage to encapsulate the protease and prevent further breakdown product formation.365
Surgery
Surgical intervention is considered for patients afflicted by severe symptoms of chronic LBP who do not experience relief from non-invasive treatment measures. Current surgical interventions include spinal fusion and total disc replacement.342 Despite the high rate of surgical treatment in chronic LBP patients, recent findings suggest that surgical intervention has little evidence of being beneficial for the patient.367
Spinal fusion
Spinal fusion is a popular surgical intervention for chronic LBP mediated by IVDD.342 Spinal fusion surgeries require the removal of the degenerated disc, cleaning of the IVD space, and implantation of a cage into the vacated IVD space to return the IVD space to its proper height.342 The spine is further stabilized with screws, plates, or rods to aid in the fusion process between adjacent vertebrae.342 There are many techniques regarding hardware placement into the vertebra that raise questions regarding the best technique for each patient’s unique circumstances.368 Questions include considerations for the type of hardware used, the insertion angles of the hardware, and the vertebral bony landmarks to which the hardware is anchored.368 Furthermore, this procedure can take place in either a minimally invasive or open surgery setting, each coming with its own costs and benefits.342 Minimally invasive surgical techniques are beneficial because of a decreased skin incision size, leading to less muscle and soft tissue injury during the surgical process and recovery.342 However, these procedures require specialized surgical equipment, and an increased time of operation compared to open surgery.342 Spinal fusion is considered successful when bone tissue is produced, connecting the vertebrae above and below the removed IVD. As a technique, spinal fusion is beneficial for pain relief in some patients while sacrificing spine mobility.369 Some studies have reported long-term consequences in patients who undergo spinal fusion, such as degeneration of the adjacent IVD.370 Furthermore, additional studies have reported few long-term benefits for patients who underwent the procedure and pointed to evidence of some patients requiring the use of opioids and other medications post-operatively for continued pain management.371
Total disc replacement
Total disc replacement is a surgical intervention for IVDD in patients with chronic LBP with the goal of relieving patient pain while retaining range of motion in the spine.372,373 In a total disc replacement, the entire IVD is removed and replaced with an artificial disc composed of metal endplates that fuse to the patient’s adjacent vertebral bodies and a plastic spacer to maintain height between the vertebrae.373 Clinical trials are being conducted to investigate the development of new artificial discs comparing their effectiveness to that of current models and spinal fusion techniques.374,375,376 In contrast to spinal fusion, total disc replacement enables the patient to retain a higher range of motion.373 Also, when directly comparing spinal fusion to total disc replacement, statistically significant findings were observed for short-term pain relief, disability, and quality of life, however, it was not considered clinically significant for the patient.373
Platelet rich plasma
PRP is currently being studied as a potential treatment for musculoskeletal disorders, such as chronic LBP caused by IVDD.377,378 PRP refers to a portion of the patient’s blood obtained during a peripheral blood draw and is rich in platelets and growth factors, like PDGF, TGF, VEGF, EGF, FGF, CTGF, IGF-1.379,380 Previous research into IVDD has demonstrated that the use of growth factors commonly found in patients’ blood has yielded positive results in vitro and in vivo when studying effects on extracellular matrix synthesis and IVD cell proliferation.380 It has been demonstrated that PRP can provide similar benefits in a cell culture setting and in animal models, showing evidence of IVD height restoration and extracellular matrix production.380 Despite this, evidence surrounding the use of PRP in humans is highly debated. There is still much to understand about PRP and its effects, including limitations on a consensus PRP preparation method and the best route of PRP administration in a clinical setting.379
Stem cell therapies
The utilization of stem cells for regenerative therapy in IVDD has shown encouraging evidence that they can be implemented in future treatments.381,382,383 Many hypotheses about stem cells and IVDD revolve around the transplantation of stem cells into the IVD and allowing for cell growth and proliferation to rebuild the IVD structure and replace the dead and dying cells. There is significant debate on the ideal stem cell type as well as methods to control the proliferation and growth of these cells in vivo. Current stem cell clinical trials are being conducted in humans using bone marrow-mesenchymal stem cells (BM-MSCs), umbilical cord-derived mesenchymal stem cells, and allogeneic mesenchymal stem cells.384,385,386,387 Research is also being conducted on the efficacy of NPCs and induced pluripotent stem cell-derived notochordal cells to determine if these cell populations may be better suited for treating discogenic LBP. Despite optimism regarding the potential of stem cell treatments, clinical trials have demonstrated that cells struggle to survive in the environment of the degenerated disc.381,382,383 As a result, further research needs to be done to better understand how stem cells can be utilized to treat IVDD and chronic LBP.
Other minimally invasive therapies
Multiple clinical trials are underway looking into the use of injectable therapies to address discogenic LBP. Injectable therapies are hypothesized to be superior to surgical procedures due to their being less invasive in nature. Furthermore, the volume of tissue removal required and potential irreversible anatomical changes that result from surgical procedures like spinal fusions result in surgeries being viewed a last resort option. Injectables, such as morselized NP tissue allografts from cadaveric donors and cell-free hydrogel-based microgels aim to introduce therapeutic molecules and cells into the affected disc to relieve pain and halt the degeneration process.388,389 While these approaches show promise, injectable therapies remain in the early stages of development, with much still to be tested in regard to efficacy and long term durability. In addition, researchers are investigating combination strategies, utilizing the regenerative potential of cell therapies with the structural and bioactive support of biomaterials.
Conclusion
LBP and IVDD are and will continue to be among the most prevalent musculoskeletal conditions in the world. Though a significant amount of research has been performed and had advanced our understanding of on IVDD and LBP, we have only scratched the surface of what mechanisms genetically, lifestyle-wise, molecularly, etc., are causing LBP to occur. However, recent advancements in diagnostic imaging, biological, and molecular techniques offer great promise for deepening our understanding and developing innovative therapeutic approaches.
References
Collaborators, G. B. D. L. B. P. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of didisease study 2021. Lancet Rheumatol. 5, e316–e329 (2023).
Simon, J., McAuliffe, M., Shamim, F., Vuong, N. & Tahaei, A. Discogenic low back pain. Phys. Med Rehabil. Clin. N. Am. 25, 305–317 (2014).
Jiang, W. et al. Intervertebral disc human nucleus pulposus cells associated with back pain trigger neurite outgrowth in vitro and pain behaviors in rats. Sci. Transl. Med. 15, eadg7020 (2023).
Melrose, J. & Roughley, P. in The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease (eds Irving M. Shapiro & Makarand V. Risbud) 53–77 (Springer Vienna, 2014).
Dou, Y., Sun, X., Ma, X., Zhao, X. & Yang, Q. Intervertebral disk degeneration: the microenvironment and tissue engineering strategies. Front. Bioeng. Biotechnol. 9, 592118 (2021).
Rider, S. M., Mizuno, S. & Kang, J. D. Molecular mechanisms of intervertebral disc degeneration. Spine Surg. Relat. Res. 3, 1–11 (2019).
Waxenbaum, J. A., Reddy, V. & Futterman, B. Anatomy, Back, Intervertebral Discs. in StatPearls [Internet]. (StatPearls Publishing, Treasure Island (FL), 2023). Available from: https://www.ncbi.nlm.nih.gov/books/NBK470583/.
Mohd Isa, I. L., Teoh, S. L., Mohd Nor, N. H. & Mokhtar, S. A. Discogenic low back pain: anatomy, pathophysiology and treatments of intervertebral disc degeneration. Int. J. Mol. Sci. 24, 208 (2022).
Fields, A. J., Ballatori, A., Liebenberg, E. C. & Lotz, J. C. Contribution of the endplates to disc degeneration. Curr. Mol. Biol. Rep. 4, 151–160 (2018).
Lotz, J. C., Fields, A. J. & Liebenberg, E. C. The role of the vertebral end plate in low back pain. Glob. Spine J. 3, 153–164 (2013).
Boos, N. et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Philos. Pa 1976) 27, 2631–2644 (2002).
An, H. S., Masuda, K. & Inoue, N. Intervertebral disc degeneration: biological and biomechanical factors. J. Orthop. Sci. 11, 541–552 (2006).
Antoniou, J. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 98, 996–1003 (1996).
Alkhatib, B. et al. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur. Cell Mater. 28, 98–110 (2014).
Zhao, C. Q., Wang, L. M., Jiang, L. S. & Dai, L. Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 6, 247–261 (2007).
Xiao, H., Wang, K., Peng, L. & Yin, Z. Laquinimod attenuates oxidative stress-induced mitochondrial injury and alleviates intervertebral disc degeneration by inhibiting the NF-kappaB signaling pathway. Int. Immunopharmacol. 131, 111804 (2024).
Vergroesen, P. P. et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr. Cartil. 23, 1057–1070 (2015).
Yang, H. et al. Secreted factors from intervertebral disc cells and infiltrating macrophages promote degenerated intervertebral disc catabolism. Spine (Philos. Pa 1976) 44, E520–E529 (2019).
Sivan, S. S. et al. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur. Spine J. 23, S344–S353 (2014).
Vo, N. V. et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 13, 331–341 (2013).
Zhang, Y. et al. Melatonin modulates IL-1beta-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY) 11, 10499–10512 (2019).
Panebianco, C. J., Dave, A., Charytonowicz, D., Sebra, R. & Iatridis, J. C. Single-cell RNA-sequencing atlas of bovine caudal intervertebral discs: discovery of heterogeneous cell populations with distinct roles in homeostasis. FASEB J. 35, e21919 (2021).
Rohanifar, M. et al. Single cell RNA-sequence analyses reveal uniquely expressed genes and heterogeneous immune cell involvement in the rat model of intervertebral disc degeneration. Appl. Sci 12, 8244 (2022).
Wang, D. et al. Single-cell transcriptomics reveals heterogeneity and intercellular crosstalk in human intervertebral disc degeneration. iScience 26, 106692 (2023).
Später, T. et al. Retention of human iPSC-derived or primary cells following xenotransplantation into rat immune-privileged sites. Bioengineering 10, 1049 (2023).
Molinos, M. et al. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 12, 20141191 (2015).
Johnson, Z. I., Schoepflin, Z. R., Choi, H., Shapiro, I. M. & Risbud, M. V. Disc in flames: roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur. Cell Mater. 30, 104–116 (2015).
Liang, C. Z. et al. The relationship between low pH in intervertebral discs and low back pain: a systematic review. Arch. Med. Sci. 8, 952–956 (2012).
Bez, M. et al. Molecular pain markers correlate with pH-sensitive MRI signal in a pig model of disc degeneration. Sci. Rep. 8, 17363 (2018).
Sheyn, D. et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 9, 7506–7524 (2019).
Zhou, Z. et al. Quantitative chemical exchange saturation transfer MRI of intervertebral disc in a porcine model. Magn. Reson. Med. 76, 1677–1683 (2016).
Hartvigsen, J. et al. What low back pain is and why we need to pay attention. Lancet 391, 2356–2367 (2018).
Maher, C., Underwood, M. & Buchbinder, R. Non-specific low back pain. Lancet 389, 736–747 (2017).
Henschke, N. et al. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum. 60, 3072–3080 (2009).
Trainor, T. J. & Trainor, M. A. Etiology of low back pain in athletes. Curr. Sports Med. Rep. 3, 41–46 (2004).
Middleton, K. & Fish, D. E. Lumbar spondylosis: clinical presentation and treatment approaches. Curr. Rev. Musculoskelet. Med. 2, 94–104 (2009).
Walter, K. L. & O’Toole, J. E. Lumbar spinal stenosis. JAMA 328, 310 (2022).
Awadalla, A. M. et al. Management of lumbar disc herniation: a systematic review. Cureus 15, e47908 (2023).
Schwarzer, A. C. et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Philos. Pa 1976) 19, 801–806 (1994).
de Schepper, E. I. et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Philos. Pa 1976) 35, 531–536 (2010).
Brinjikji, W. et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 36, 2394–2399 (2015).
Groh, A. M. R., Fournier, D. E., Battie, M. C. & Seguin, C. A. Innervation of the human intervertebral disc: a scoping review. Pain. Med. 22, 1281–1304 (2021).
Ratsep, T., Minajeva, A. & Asser, T. Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur. Spine J. 22, 2474–2480 (2013).
Xu, J., Shao, T., Lou, J., Zhang, J. & Xia, C. Aging, cell senescence, the pathogenesis and targeted therapies of intervertebral disc degeneration. Front. Pharm. 14, 1172920 (2023).
Dowdell, J. et al. Intervertebral disk degeneration and repair. Neurosurgery 80, S46–S54 (2017).
Jha, R. et al. Updates on pathophysiology of discogenic back pain. J. Clin. Med. 12, 6907 (2023).
De Simone, M. et al. Discogenic low back pain: anatomic and pathophysiologic characterization, clinical evaluation, biomarkers, AI, and treatment options. J. Clin. Med. 13, 5915 (2024).
Chiu, A. P. et al. Human molecular mechanisms of discogenic low back pain: a scoping review. J. Pain. 27, 104693 (2025).
Cooke, P. M. & Lutz, G. E. Internal disc disruption and axial back pain in the athlete. Phys. Med Rehabil. Clin. N. Am. 11, 837–865 (2000).
Deyo, R. A. & Weinstein, J. N. Low back pain. N. Engl. J. Med. 344, 363–370 (2001).
Koes, B. W., van Tulder, M. W., Ostelo, R., Kim Burton, A. & Waddell, G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine (Philos. Pa 1976) 26, 2504–2513 (2001).
Wang, J. C., Lamartina, C. & AOSpine International (Firm). AOSpine masters series. Volume 8, Back pain. (Thieme, 2017).
Fujii, K. et al. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR 3, e10180 (2019).
Bridwell, K. H., Bridwell, K. H. & Gupta, M. C. Bridwell and DeWald’s textbook of spinal surgery. Fourth edition edn, (Wolters Kluwer Health, 2019).
Boden, S. D., Davis, D. O., Dina, T. S., Patronas, N. J. & Wiesel, S. W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 72, 403–408 (1990).
Jensen, M. C. et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N. Engl. J. Med. 331, 69–73 (1994).
Stadnik, T. W. et al. Annular tears and disk herniation: prevalence and contrast enhancement on MR images in the absence of low back pain or sciatica. Radiology 206, 49–55 (1998).
Weishaupt, D., Zanetti, M., Hodler, J. & Boos, N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology 209, 661–666 (1998).
Jarvik, J. J., Hollingworth, W., Heagerty, P., Haynor, D. R. & Deyo, R. A. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine (Philos. Pa 1976) 26, 1158–1166 (2001).
Pfirrmann, C. W., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Philos. Pa 1976) 26, 1873–1878 (2001).
Brayda-Bruno, M. et al. Advances in the diagnosis of degenerated lumbar discs and their possible clinical application. Eur. Spine J. 23, S315–S323 (2014).
Schellhas, K. P., Pollei, S. R., Gundry, C. R. & Heithoff, K. B. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine (Philos. Pa 1976) 21, 79–86 (1996).
Marinelli, N. L., Haughton, V. M., Munoz, A. & Anderson, P. A. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Philos. Pa 1976) 34, 520–524 (2009).
Marinelli, N. L., Haughton, V. M. & Anderson, P. A. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. AJNR Am. J. Neuroradiol. 31, 1278–1282 (2010).
Blumenkrantz, G. et al. In vivo 3.0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn. Reson. Med. 63, 1193–1200 (2010).
Johannessen, W. et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Philos. Pa 1976) 31, 1253–1257 (2006).
Vaga, S. et al. Quantitative assessment of intervertebral disc glycosaminoglycan distribution by gadolinium-enhanced MRI in orthopedic patients. Magn. Reson. Med. 59, 85–95 (2008).
Shapiro, E. M., Borthakur, A., Gougoutas, A. & Reddy, R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn. Reson. Med. 47, 284–291 (2002).
Lindblom, K. Diagnostic puncture of intervertebral disks in sciatica. Acta Orthop. Scand. 17, 231–239 (1948).
Walker, J. III., El Abd, O., Isaac, Z. & Muzin, S. Discography in practice: a clinical and historical review. Curr. Rev. Musculoskelet. Med. 1, 69–83 (2008).
Carragee, E. J. et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine (Philos. Pa 1976) 25, 1373–1380 (2000).
Willems, P. Decision making in surgical treatment of chronic low back pain: the performance of prognostic tests to select patients for lumbar spinal fusion. Acta Orthop. Suppl. 84, 1–35 (2013).
Eck, J. C. et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: discography for patient selection. J. Neurosurg. Spine 21, 37–41 (2014).
Bizzoca, D. et al. Gender-related issues in the management of low-back pain: a current concepts review. Clin. Pr. 13, 1360–1368 (2023).
Wang, Y. X., Wang, J. Q. & Kaplar, Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quant. Imaging Med. Surg. 6, 199–206 (2016).
Bonnheim, N. B. et al. ISSLS Prize in Bioengineering Science 2023: age- and sex-related differences in lumbar intervertebral disc degeneration between patients with chronic low back pain and asymptomatic controls. Eur. Spine J. 32, 1517–1524 (2023).
Lou, C. et al. Association between menopause and lumbar disc degeneration: an MRI study of 1566 women and 1382 men. Menopause 24, 1136–1144 (2017).
Mosley, G. E. et al. Sex differences in rat intervertebral disc structure and function following annular puncture injury. Spine (Philos. Pa 1976) 44, 1257–1269 (2019).
Mosley, G. E. et al. Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci. Rep. 10, 15120 (2020).
Battie, M. C., Videman, T., Levalahti, E., Gill, K. & Kaprio, J. Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine (Philos. Pa 1976) 33, 2801–2808 (2008).
Livshits, G. et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann. Rheum. Dis. 70, 1740–1745 (2011).
Sambrook, P. N., MacGregor, A. J. & Spector, T. D. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 42, 366–372 (1999).
Zorkoltseva, I. V. et al. Multi-trait exome-wide association study of back pain-related phenotypes. Genes 14, 1962 (2023).
Suri, P. et al. Genome-wide meta-analysis of 158 000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet. 14, e1007601 (2018).
Zhang, H. et al. Association of single nucleotide polymorphism rs2228570 with lumbar disc degeneration: a case-control study and meta-analysis. J. Pain. Res. 14, 2001–2012 (2021).
Cauci, S. et al. Low back pain and FokI (rs2228570) polymorphism of vitamin D receptor in athletes. BMC Sports Sci. Med Rehabil. 9, 4 (2017).
Baumbauer, K. M. et al. Contribution of COMT and BDNF genotype and expression to the risk of transition from acute to chronic low back pain. Clin. J. Pain. 36, 430–439 (2020).
Martirosyan, N. L. et al. Genetic alterations in intervertebral disc disease. Front. Surg. 3, 59 (2016).
Solovieva, S. et al. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain 109, 8–19 (2004).
Biczo, A. et al. Genetic variants of interleukin 1B and 6 are associated with clinical outcome of surgically treated lumbar degenerative disc disease. BMC Musculoskelet. Disord. 23, 774 (2022).
Trompeter, K., Fett, D. & Platen, P. Prevalence of back pain in sports: a systematic review of the literature. Sports Med. 47, 1183–1207 (2017).
Sweeney, E. A., Daoud, A. K., Potter, M. N., Ritchie, L. & Howell, D. R. Association between flexibility and low back pain in female adolescent gymnasts. Clin. J. Sport Med. 29, 379–383 (2019).
Oliva, F. A., Vitaterna, M. N. & Maffulli, M. C. M. Low back pain in weightlifters: personalised exercise protocols for elite athletes. Muscles, Ligaments Tendons J. 13, 187–200 (2023).
Nugent, F. J. et al. The relationship between rowing-related low back pain and rowing biomechanics: a systematic review. Br J. Sports Med. (2021). https://doi.org/10.1136/bjsports-2020-102533
Murillo, C. et al. High-density electromyography provides new insights into the flexion relaxation phenomenon in individuals with low back pain. Sci. Rep. 9, 15938 (2019).
Baradaran Mahdavi, S., Riahi, R., Vahdatpour, B. & Kelishadi, R. Association between sedentary behavior and low back pain; a systematic review and meta-analysis. Health Promot Perspect. 11, 393–410 (2021).
da Cruz Fernandes, I. M., Pinto, R. Z., Ferreira, P. & Lira, F. S. Low back pain, obesity, and inflammatory markers: exercise as potential treatment. J. Exerc Rehabil. 14, 168–174 (2018).
Wong, A. Y. L., Karppinen, J. & Samartzis, D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord. 12, 14 (2017).
Wang, Y. et al. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Biomed. Pharmacother. 131, 110660 (2020).
Hise, A. G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Ma, M., Jiang, W. & Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 57, 752–771 (2024).
Ohnishi, T., Iwasaki, N. & Sudo, H. Causes of and molecular targets for the treatment of intervertebral disc degeneration: a review. Cells 11, 394 (2022).
Dela Cruz, C. S. & Kang, M. J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 41, 37–44 (2018).
Nakahira, K., Hisata, S. & Choi, A. M. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid. Redox Signal 23, 1329–1350 (2015).
Murphy, K., Lufkin, T. & Kraus, P. Development and degeneration of the intervertebral disc-Insights from across species. Vet. Sci. 10, 540 (2023).
Quero, L. et al. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res. Ther. 15, R94 (2013).
Schmidli, M. R. et al. Fibronectin fragments and inflammation during canine intervertebral disc disease. Front. Vet. Sci. 7, 547644 (2020).
Roh, J. S. & Sohn, D. H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 18, e27 (2018).
Klatt, A. R. et al. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J. Orthop. Res. 27, 65–70 (2009).
Ruettger, A., Schueler, S., Mollenhauer, J. A. & Wiederanders, B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J. Biol. Chem. 283, 1043–1051 (2008).
Fichter, M. et al. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J. Orthop. Res. 24, 63–70 (2006).
Tchetina, E. V. et al. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 26, 247–258 (2007).
Yasuda, T. Type II collagen peptide stimulates Akt leading to nuclear factor-kappaB activation: its inhibition by hyaluronan. Biomed. Res. 35, 193–199 (2014).
Lambert, C., Borderie, D., Dubuc, J. E., Rannou, F. & Henrotin, Y. Type II collagen peptide Coll2-1 is an actor of synovitis. Osteoarthr. Cartil. 27, 1680–1691 (2019).
Lees, S. et al. Bioactivity in an aggrecan 32-mer fragment is mediated via toll-like receptor 2. Arthritis Rheumatol. 67, 1240–1249 (2015).
Babelova, A. et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J. Biol. Chem. 284, 24035–24048 (2009).
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Hsieh, L. T. et al. Biglycan- and sphingosine kinase-1 signaling crosstalk regulates the synthesis of macrophage chemoattractants. Int. J. Mol. Sci. 18, 595 (2017).
Zeng-Brouwers, J., Beckmann, J., Nastase, M. V., Iozzo, R. V. & Schaefer, L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 35, 132–142 (2014).
Merline, R. et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci. Signal 4, ra75 (2011).
Wang, W. et al. Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch. Med. Res. 40, 321–323 (2009).
Tang, M. et al. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Rep. 13, 2851–2864 (2015).
Li, D. & Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target Ther. 6, 291 (2021).
Song, C. et al. An in-depth analysis of the immunomodulatory mechanisms of intervertebral disc degeneration. JOR Spine 5, e1233 (2022).
Suresh, R. & Mosser, D. M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 37, 284–291 (2013).
Feng, P. et al. Immune exposure: how macrophages interact with the nucleus pulposus. Front. Immunol. 14, 1155746 (2023).
Krock, E. et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 43, 487–500 (2019).
Rand, N., Reichert, F., Floman, Y. & Rotshenker, S. Murine nucleus pulposus-derived cells secrete interleukins-1-beta, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine (Philos. PA 1976) 22, 2598–2601 (1997) 2.
Burke, J. G. et al. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine (Philos. PA 1976) 28, 2685–2693 (2003).
Burke, J. G. et al. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine (Philos. Pa 1976) 27, 1402–1407 (2002).
Gabr, M. A. et al. Interleukin-17 synergizes with IFNgamma or TNFalpha to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J. Orthop. Res. 29, 1–7 (2011).
Wang, S. S. et al. IL-17A enhances ADAMTS-7 expression through regulation of TNF-alpha in human nucleus pulposus cells. J. Mol. Histol. 46, 475–483 (2015).
Cuellar, J. M. et al. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 10, 212–218 (2010).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2, 17023 (2017).
Ahmed, H. et al. Role of adaptor protein myeloid differentiation 88 (MyD88) in post-subarachnoid hemorrhage inflammation: a systematic review. Int. J. Mol. Sci. 22, 4185 (2021).
Xu, Y. R. & Lei, C. Q. TAK1-TABs complex: a central signalosome in inflammatory responses. Front. Immunol. 11, 608976 (2020).
Glaeser, J. D. et al. NF-kappaB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration. Spine J. 20, 1480–1491 (2020).
Chao-Yang, G., Peng, C. & Hai-Hong, Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr. Cartil. 29, 793–801 (2021).
Sun, X. et al. The NLRP3 Inflammasome and Its Role in T1DM. Front. Immunol. 11, 1595 (2020).
Zarezadeh Mehrabadi, A. et al. The roles of interleukin-1 receptor accessory protein in certain inflammatory conditions. Immunology 166, 38–46 (2022).
Fan, H. et al. Necroptosis of nucleus pulposus cells involved in intervertebral disc degeneration through MyD88 signaling. Front. Endocrinol. (Lausanne) 13, 994307 (2022).
Lambert, C. et al. The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front. Med. (Lausanne) 7, 607186 (2020).
Na, H. S. L., Haneef, X. & Liu, K. W. Old and new damage-associated molecular patterns (DAMPs) in autoimmune diseases. Rheumatol. Autoimmun. 2, 185–197 (2022).
Yu, H., Lin, L., Zhang, Z., Zhang, H. & Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther. 5, 209 (2020).
Ahmed, A. S. et al. NF-kappaB-associated pain-related neuropeptide expression in patients with degenerative disc disease. Int. J. Mol. Sci. 20, 658 (2019).
Guo, Q. et al. NF-kappaB in biology and targeted therapy: new insights and translational implications. Signal Transduct. Target Ther. 9, 53 (2024).
Garcia-Garcia, V. A., Alameda, J. P., Page, A. & Casanova, M. L. Role of NF-kappaB in ageing and age-related diseases: lessons from genetically modified mouse models. Cells 10, 1906 (2021).
Zhang, G. Z. et al. NF-kappaB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 54, e13057 (2021).
David, G., Ciurea, A. V., Iencean, S. M. & Mohan, A. Angiogenesis in the degeneration of the lumbar intervertebral disc. J. Med. Life 3, 154–161 (2010).
Koroth, J. et al. Macrophages and intervertebral disc degeneration. Int. J. Mol. Sci. 24, 1367 (2023).
Li, Y. et al. The potential role and trend of HIF‑1alpha in intervertebral disc degeneration: friend or foe? (Review). Mol. Med. Rep. 23, 239 (2021).
Yamakawa, M. et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ. Res. 93, 664–673 (2003).
Kwon, W. K., Moon, H. J., Kwon, T. H., Park, Y. K. & Kim, J. H. The role of hypoxia in angiogenesis and extracellular matrix regulation of intervertebral disc cells during inflammatory reactions. Neurosurgery 81, 867–875 (2017).
Zhang, H. et al. SDF1/CXCR4 axis facilitates the angiogenesis via activating the PI3K/AKT pathway in degenerated discs. Mol. Med. Rep. 22, 4163–4172 (2020).
Yang, H. et al. Synergistic effect of VEGF and SDF-1alpha in endothelial progenitor cells and vascular smooth muscle cells. Front. Pharm. 13, 914347 (2022).
Giraudo, E. et al. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J. Biol. Chem. 273, 22128–22135 (1998).
Freemont, A. J. et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 350, 178–181 (1997).
Binch, A. L. et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res. Ther. 17, 370 (2015).
Ashton, I. K., Roberts, S., Jaffray, D. C., Polak, J. M. & Eisenstein, S. M. Neuropeptides in the human intervertebral disc. J. Orthop. Res. 12, 186–192 (1994).
He, M. et al. Overexpression of TIMP3 inhibits discogenic pain by suppressing angiogenesis and the expression of substance P in nucleus pulposus. Mol. Med. Rep. 21, 1163–1171 (2020).
Landreth, G. in Basic Neurochemistry: Molecular, Cellular and Medical Aspects (Lippincott Williams & Wilkins, 1999).
Iannone, F. et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatol. (Oxf.) 41, 1413–1418 (2002).
Rihl, M. et al. Involvement of neurotrophins and their receptors in spondyloarthritis synovitis: relation to inflammation and response to treatment. Ann. Rheum. Dis. 64, 1542–1549 (2005).
Purmessur, D., Freemont, A. J. & Hoyland, J. A. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 10, R99 (2008).
Binch, A. L. et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res. Ther. 16, 416 (2014).
Garcia-Cosamalon, J. et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain?. J. Anat. 217, 1–15 (2010).
Lee, F. S. & Chao, M. V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA 98, 3555–3560 (2001).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu Rev. Neurosci. 24, 677–736 (2001).
Bibel, M. & Barde, Y. A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14, 2919–2937 (2000).
Kruttgen, A., Saxena, S., Evangelopoulos, M. E. & Weis, J. Neurotrophins and neurodegenerative diseases: receptors stuck in traffic?. J. Neuropathol. Exp. Neurol. 62, 340–350 (2003).
Minnone, G., De Benedetti, F. & Bracci-Laudiero, L. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 18, 1028 (2017).
Covaceuszach, S. et al. The conundrum of the high-affinity NGF binding site formation unveiled?. Biophys. J. 108, 687–697 (2015).
McGregor, C. E. & English, A. W. The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and val66Met. Front. Cell Neurosci. 12, 522 (2018).
Reichardt, L. F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 (2006).
Barker, P. A. p75NTR: a study in contrasts. Cell Death Differ. 5, 346–356 (1998).
Sajanti, A. et al. A comprehensive p75 neurotrophin receptor gene network and pathway analyses identifying new target genes. Sci. Rep. 10, 14984 (2020).
Chen, Y. et al. Multiple roles of the p75 neurotrophin receptor in the nervous system. J. Int. Med. Res. 37, 281–288 (2009).
Ceni, C. et al. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J. Cell Sci. 123, 2299–2307 (2010).
Hoyle, G. W., Mercer, E. H., Palmiter, R. D. & Brinster, R. L. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron 10, 1019–1034 (1993).
Aloe, L., Rocco, M. L., Bianchi, P. & Manni, L. Nerve growth factor: from the early discoveries to the potential clinical use. J. Transl. Med. 10, 239 (2012).
Bracci-Laudiero, L. M., L. NGF and Immune Regulation. 1849–1876 (Springer, 2014).
Richardson, S. M. et al. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PLoS One 7, e47735 (2012).
Davis, B. M., Goodness, T. P., Soria, A. & Albers, K. M. Over-expression of NGF in skin causes formation of novel sympathetic projections to trkA-positive sensory neurons. Neuroreport 9, 1103–1107 (1998).
Bracci-Laudiero, L. & De Stefano, M. E. NGF in early embryogenesis, differentiation, and pathology in the nervous and immune systems. Curr. Top. Behav. Neurosci. 29, 125–152 (2016).
Causing, C. G. et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 18, 257–267 (1997).
Benito-Gutierrez, E., Garcia-Fernandez, J. & Comella, J. X. Origin and evolution of the Trk family of neurotrophic receptors. Mol. Cell Neurosci. 31, 179–192 (2006).
Triaca, V. et al. hNGF peptides elicit the NGF-TrkA signalling pathway in cholinergic neurons and retain full neurotrophic activity in the DRG assay. Biomolecules 10, 216 (2020).
Zha, K. et al. Nerve growth factor (NGF) and NGF receptors in mesenchymal stem/stromal cells: Impact on potential therapies. Stem Cells Transl. Med. 10, 1008–1020 (2021).
Daragmeh, J., Barriah, W., Saad, B. & Zaid, H. Analysis of PI3K pathway components in human cancers. Oncol. Lett. 11, 2913–2918 (2016).
Molloy, N. H., Read, D. E. & Gorman, A. M. Nerve growth factor in cancer cell death and survival. Cancers3, 510–530 (2011).
Mantyh, P. W., Koltzenburg, M., Mendell, L. M., Tive, L. & Shelton, D. L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 115, 189–204 (2011).
Turney, S. G. et al. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol. Biol. Cell 27, 500–517 (2016).
Barker, P. A., Mantyh, P., Arendt-Nielsen, L., Viktrup, L. & Tive, L. Nerve growth factor signaling and its contribution to pain. J. Pain. Res. 13, 1223–1241 (2020).
Freemont, A. J. et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 197, 286–292 (2002).
Jin, W. Regulation of BDNF-TrkB signaling and potential therapeutic strategies for parkinson’s disease. J. Clin. Med. 9, 257 (2020).
Esvald, E. E. et al. CREB family transcription factors are major mediators of BDNF transcriptional autoregulation in cortical neurons. J. Neurosci. 40, 1405–1426 (2020).
Gupta, V. K., You, Y., Gupta, V. B., Klistorner, A. & Graham, S. L. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 14, 10122–10142 (2013).
Bonni, A. et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Moya-Alvarado, G. et al. BDNF/TrkB signaling endosomes in axons coordinate CREB/mTOR activation and protein synthesis in the cell body to induce dendritic growth in cortical neurons. Elife 12, e77455 (2023).
Vega, J. A., Garcia-Suarez, O., Hannestad, J., Perez-Perez, M. & Germana, A. Neurotrophins and the immune system. J. Anat. 203, 1–19 (2003).
Krock, E. et al. Nerve growth factor is regulated by Toll-Like receptor 2 in human intervertebral discs. J. Biol. Chem. 291, 3541–3551 (2016).
Abe, Y. et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Philos. Pa 1976) 32, 635–642 (2007).
Airaksinen, M. S. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3, 383–394 (2002).
Saarma, M. in Encyclopedia of Neuroscience (2009).
Kotliarova, A. & Sidorova, Y. A. Glial cell line-derived neurotrophic factor family ligands, players at the interface of neuroinflammation and neuroprotection: focus onto the glia. Front. Cell Neurosci. 15, 679034 (2021).
Yamada, J. et al. Expression of glial cell line-derived neurotrophic factor in the human intervertebral disc. Spine (Philos. Pa 1976) 45, E768–E775 (2020).
Iwasaki, T. et al. Expression of glial-cell-line-derived neurotrophic factor family ligands in human intervertebral discs. Int. J. Mol. Sci. 24, 15874 (2023).
Burbach, J. P. What are neuropeptides?. Methods Mol. Biol. 789, 1–36 (2011).
Kepler, C. K. et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Philos. Pa 1976) 38, E1291–E1299 (2013).
Suvas, S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 199, 1543–1552 (2017).
Zheng, J. et al. Reactive oxygen species mediate low back pain by upregulating substance P in intervertebral disc degeneration. Oxid. Med. Cell Longev. 2021, 6681815 (2021).
Johnson, M. B., Young, A. D. & Marriott, I. The therapeutic potential of targeting substance P/NK-1R interactions in inflammatory CNS disorders. Front. Cell Neurosci. 10, 296 (2016).
Freidin, M. & Kessler, J. A. Cytokine regulation of substance P expression in sympathetic neurons. Proc. Natl. Acad. Sci. USA 88, 3200–3203 (1991).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Kim, Y. J. & Granstein, R. D. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav. Immun. Health 18, 100361 (2021).
Solway, B., Bose, S. C., Corder, G., Donahue, R. R. & Taylor, B. K. Tonic inhibition of chronic pain by neuropeptide Y. Proc. Natl. Acad. Sci. USA 108, 7224–7229 (2011).
Dombrowski, M. E. et al. Rabbit annulus fibrosus cells express neuropeptide Y, which is influenced by mechanical and inflammatory stress. Neurospine 17, 69–76 (2020).
Sun, K. et al. Neuropeptide Y prevents nucleus pulposus cells from cell apoptosis and IL‑1beta‑induced extracellular matrix degradation. Cell Cycle 20, 960–977 (2021).
Kameda, T. et al. Expression and activity of TRPA1 and TRPV1 in the intervertebral disc: association with inflammation and matrix remodeling. Int. J. Mol. Sci. 20, 1767 (2019).
Sadowska, A. et al. Differential regulation of TRP channel gene and protein expression by intervertebral disc degeneration and back pain. Sci. Rep. 9, 18889 (2019).
Easson, G. W. D., Savadipour, A., Gonzalez, C., Guilak, F. & Tang, S. Y. TRPV4 differentially controls inflammatory cytokine networks during static and dynamic compression of the intervertebral disc. JOR Spine 6, e1282 (2023).
Easson, G. W. D. et al. Modulation of TRPV4 protects against degeneration induced by sustained loading and promotes matrix synthesis in the intervertebral disc. FASEB J. 37, e22714 (2023).
Zheng, B. et al. Netrin-1 mediates nerve innervation and angiogenesis leading to discogenic pain. J. Orthop. Transl. 39, 21–33 (2023).
Krames, E. S. The role of the dorsal root ganglion in the development of neuropathic pain. Pain. Med. 15, 1669–1685 (2014).
Diwan, A. D. & Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 6, e1231 (2023).
Li, W. et al. Peripheral and central pathological mechanisms of chronic low back Pain: a narrative review. J. Pain. Res. 14, 1483–1494 (2021).
Scholz, J. & Woolf, C. J. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368 (2007).
Poillot, P., Snuggs, J. W., Le Maitre, C. L. & Huyghe, J. M. L-type voltage-gated calcium channels partly mediate Mechanotransduction in the intervertebral disc. JOR Spine 5, e1213 (2022).
Vergne-Salle, P. & Bertin, P. Chronic pain and neuroinflammation. Jt. Bone Spine 88, 105222 (2021).
Murata, Y., Olmarker, K., Takahashi, I., Takahashi, K. & Rydevik, B. Effects of lumbar sympathectomy on pain behavioral changes caused by nucleus pulposus-induced spinal nerve damage in rats. Eur. Spine J. 15, 634–640 (2006).
Herrero, J. F., Laird, J. M. & Lopez-Garcia, J. A. Wind-up of spinal cord neurones and pain sensation: much ado about something?. Prog. Neurobiol. 61, 169–203 (2000).
Latremoliere, A. & Woolf, C. J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 10, 895–926 (2009).
Cairns, B. E., Arendt-Nielsen, L. & Sacerdote, P. Perspectives in pain research 2014: neuroinflammation and glial cell activation: the cause of transition from acute to chronic pain?. Scand. J. Pain. 6, 3–6 (2015).
Lai, A. et al. Annulus fibrosus injury induces acute neuroinflammation and chronic glial response in dorsal root ganglion and spinal cord-an in vivo rat discogenic pain model. Int. J. Mol. Sci. 25, 1762 (2024).
Gruber, H. E. & Hanley, E. N. Jr Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet. Disord. 1, 1 (2000).
Wang, J. Y., Baer, A. E., Kraus, V. B. & Setton, L. A. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine (Philos. Pa 1976) 26, 1747–1751 (2001).
Horner, H. A. et al. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine (Philos. Pa 1976) 27, 1018–1028 (2002).
Chou, A. I., Reza, A. T. & Nicoll, S. B. Distinct intervertebral disc cell populations adopt similar phenotypes in three-dimensional culture. Tissue Eng. Part A 14, 2079–2087 (2008).
Chen, Y. F. et al. Insights into the hallmarks of human nucleus pulposus cells with particular reference to cell viability, phagocytic potential and long process formation. Int. J. Med. Sci. 10, 1805–1816 (2013).
Dai, J. et al. Microfluidic disc-on-a-chip device for mouse intervertebral disc-pitching a next-generation research platform to study disc degeneration. ACS Biomater. Sci. Eng. 5, 2041–2051 (2019).
Owen, P. J., Hangai, M., Kaneoka, K., Rantalainen, T. & Belavy, D. L. Mechanical loading influences the lumbar intervertebral disc. A cross-sectional study in 308 athletes and 71 controls. J. Orthop. Res. 39, 989–997 (2021).
Xiang, P., Luo, Z. P. & Che, Y. J. Insights into the mechanical microenvironment within the cartilaginous endplate: an emerging role in maintaining disc homeostasis and normal function. Heliyon 10, e31162 (2024).
McKinley, J. P. et al. Design of a flexing organ-chip to model in situ loading of the intervertebral disc. Biomicrofluidics 16, 054111 (2022).
Xie, W. et al. Intervertebral disc-on-a-chipMF: a new model for mouse disc culture via integrating mechanical loading and dynamic media flow. Adv. Mater.Technol. 8, 2300606 (2023).
Son, H. G. et al. Intervertebral disc organ-on-a-chip: an innovative model to study monocyte extravasation during nucleus pulposus degeneration. Lab Chip 23, 2819–2828 (2023).
Hwang, M. H. et al. Spine-on-a-chip: human annulus fibrosus degeneration model for simulating the severity of intervertebral disc degeneration. Biomicrofluidics 11, 064107 (2017).
Hwang, M. H., Son, H. G., Kim, J. & Choi, H. In vitro model of distinct catabolic and inflammatory response patterns of endothelial cells to intervertebral disc cell degeneration. Sci. Rep. 10, 20596 (2020).
Kague, E. et al. 3D assessment of intervertebral disc degeneration in zebrafish identifies changes in bone density that prime disc disease. Bone Res. 9, 39 (2021).
Chengguo, S. U. et al. Effect of Tuina along “bladder meridian” alleviating intervertebral disc degeneration by regulating the transforming growth factor-beta1/Smad signaling pathway in a rabbit model. J. Tradit. Chin. Med. 43, 991–1000 (2023).
Yang, K. et al. Comparisons between needle puncture and chondroitinase ABC to induce intervertebral disc degeneration in rabbits. Eur. Spine J. 31, 2788–2800 (2022).
Zhang, Y., Drapeau, S., Howard, S. A., Thonar, E. J. & Anderson, D. G. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Philos. Pa 1976) 36, 372–377 (2011).
Peredo, A. P. et al. Tension-activated nanofiber patches delivering an anti-inflammatory drug improve repair in a goat intervertebral disc herniation model. Sci. Transl. Med. 15, eadf1690 (2023).
Zhang, Y. et al. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Philos. Pa 1976) 36, 1519–1527 (2011).
Yuan, Q. et al. Autologous mesenchymal stromal cells combined with gelatin sponge for repair intervertebral disc defect after discectomy: a preclinical study in a goat model. Front. Biosci. (Landmark Ed. 27, 131 (2022) .
Woiciechowsky, C. et al. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J. Tissue Eng. Regen. Med. 8, 811–820 (2014).
Vadala, G. et al. Novel stepwise model of intervertebral disc degeneration with intact annulus fibrosus to test regeneration strategies. J. Orthop. Res. 36, 2460–2468 (2018).
Daly, C. D. et al. A comparison of two ovine lumbar intervertebral disc injury models for the evaluation and development of novel regenerative therapies. Glob. Spine J. 8, 847–859 (2018).
Oehme, D. et al. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J. Neurosurg. Spine 20, 657–669 (2014).
Melrose, J. et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Philos. Pa 1976) 37, 18–25 (2012).
Fuller, E. S., Shu, C., Smith, M. M., Little, C. B. & Melrose, J. Hyaluronan oligosaccharides stimulate matrix metalloproteinase and anabolic gene expression in vitro by intervertebral disc cells and annular repair in vivo. J. Tissue Eng. Regen. Med 12, e216–e226 (2018).
Shu, C. C. et al. Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine 1, e1037 (2018).
Willems, N. et al. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet. Res 12, 10 (2016).
Tellegen, A. R. et al. Intradiscal application of a PCLA-PEG-PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: what’s in it for humans?. J. Tissue Eng. Regen. Med. 12, 642–652 (2018).
Tellegen, A. R. et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Control Release 286, 439–450 (2018).
Rudnik-Jansen, I. et al. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 19, 905–919 (2019).
Platenberg, R. C., Hubbard, G. B., Ehler, W. J. & Hixson, C. J. Spontaneous disc degeneration in the baboon model: magnetic resonance imaging and histopathologic correlation. J. Med. Primatol. 30, 268–272 (2001).
Nuckley, D. J. et al. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J. Orthop. Res. 26, 1283–1288 (2008).
Wang, J. et al. Correlation between motor behavior and age-related intervertebral disc degeneration in cynomolgus monkeys. JOR Spine 5, e1183 (2022).
Shi, C. et al. Development of an in vivo mouse model of discogenic low back pain. J. Cell Physiol. 233, 6589–6602 (2018).
Yang, G., Chen, L., Gao, Z. & Wang, Y. Implication of microglia activation and CSF-1/CSF-1Rpathway in lumbar disc degeneration-related back pain. Mol. Pain. 14, 1744806918811238 (2018).
Wawrose, R. A. et al. Percutaneous lumbar annular puncture: a rat model to study intervertebral disc degeneration and pain-related behavior. JOR Spine 5, e1202 (2022).
Glaeser, J. D. et al. Optimization of a rat lumbar IVD degeneration model for low back pain. JOR Spine 3, e1092 (2020).
Olmarker, K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine (Philos. Pa 1976) 33, 850–855 (2008).
Barbe, M. F. et al. Characterization of pain-related behaviors in a rat model of acute-to-chronic low back pain: single vs. multi-level disc injury. Front Pain. Res. (Lausanne) 5, 1394017 (2024).
Lee, S., Millecamps, M., Foster, D. Z. & Stone, L. S. Long-term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury-induced low back pain. J. Orthop. Res. 38, 1238–1247 (2020).
Millecamps, M., Lee, S., Foster, D. Z. & Stone, L. S. Disc degeneration spreads: long-term behavioural, histologic and radiologic consequences of a single-level disc injury in active and sedentary mice. Eur. Spine J. 30, 2238–2246 (2021).
Nojima, D. et al. Efficacy of anti-NaV1.7 antibody on the sensory nervous system in a rat model of lumbar intervertebral disc injury. Yonsei Med. J. 57, 748–753 (2016).
Miyagi, M. et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Philos. Pa 1976) 36, 2260–2266 (2011).
Chen, X. et al. Reactive oxygen species induced upregulation of TRPV1 in dorsal root ganglia results in low back pain in rats. J. Inflamm. Res. 17, 2245–2256 (2024).
Sadamasu, A. et al. Upregulation of NaV1.7 in dorsal root ganglia after intervertebral disc injury in rats. Spine (Philos. Pa 1976) 39, E421–E426 (2014).
Li, Y. et al. Activation of satellite cells in the dorsal root ganglia in a disc-punctured rat model. J. Orthop. Sci. 16, 433–438 (2011).
Kim, J. S. et al. The rat intervertebral disk degeneration pain model: relationships between biological and structural alterations and pain. Arthritis Res. Ther. 13, R165 (2011).
Lai, A. et al. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J. Orthop. Res. 33, 755–764 (2015).
Miyagi, M. et al. Assessment of pain behavior in a rat model of intervertebral disc injury using the CatWalk gait analysis system. Spine (Philos. Pa 1976) 38, 1459–1465 (2013).
Suzuki, H. et al. Injection of ultra-purified stem cells with sodium alginate reduces discogenic pain in a rat model. Cells 12, 505 (2023).
Zhang, H., La Marca, F., Hollister, S. J., Goldstein, S. A. & Lin, C. Y. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. J. Neurosurg. Spine 10, 522–530 (2009).
Liao, Z. et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9, 4084–4100 (2019).
Chen, C. et al. Autologous fibroblasts induce fibrosis of the nucleus pulposus to maintain the stability of degenerative intervertebral discs. Bone Res. 8, 7 (2020).
Martin, J. T. et al. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J. Orthop. Res. 31, 1276–1282 (2013).
Ohnishi, T. et al. In vivo mouse intervertebral disc degeneration model based on a new histological classification. PLoS One 11, e0160486 (2016).
Au, T. Y. K. et al. Transformation of resident notochord-descendent nucleus pulposus cells in mouse injury-induced fibrotic intervertebral discs. Aging Cell 19, e13254 (2020).
Millecamps, M. & Stone, L. S. Delayed onset of persistent discogenic axial and radiating pain after a single-level lumbar intervertebral disc injury in mice. Pain 159, 1843–1855 (2018).
Lee, M. et al. Complete Freund’s adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth. Analg. 109, 1287–1296 (2009).
Sasaki, N., Kikuchi, S., Konno, S., Sekiguchi, M. & Watanabe, K. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine (Philos. PA 1976) 32, 413–416 (2007).
Yang, L. et al. Effective modulation of inflammation and oxidative stress for enhanced regeneration of intervertebral discs using 3D porous hybrid protein nanoscaffold. Adv. Mater. 35, e2303021 (2023).
Ishiguro, H. et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 87, 118–129 (2019).
Moon, C. S., Lim, T. H., Hong, J., Sul, D. & Kim, N. Assessment of a discogenic pain animal model induced by applying continuous shear force to intervertebral discs. Pain. Physician 26, E181–E189 (2023).
Xie, W. et al. A mouse coccygeal intervertebral disc degeneration model with tail-looping constructed using a suturing method. Anim. Model Exp. Med. https://doi.org/10.1002/ame2.12501 (2025).
Matyas, J. R., Gutmann, A., Randev, J., Hurtig, M. & Bertram, J. E. Intra-articular anaesthesia mitigates established pain in experimental osteoarthritis: a preliminary study of gait impulse redistribution as a biomarker of analgesia pharmacodynamics. Osteoarthr. Cartil. 21, 1365–1373 (2013).
Castel, D., Willentz, E., Doron, O., Brenner, O. & Meilin, S. Characterization of a porcine model of post-operative pain. Eur. J. Pain. 18, 496–505 (2014).
Wu, B. et al. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 3, 19 (2016).
Melkus, G., Grabau, M., Karampinos, D. C. & Majumdar, S. Ex vivo porcine model to measure pH dependence of chemical exchange saturation transfer effect of glycosaminoglycan in the intervertebral disc. Magn. Reson Med. 71, 1743–1749 (2014).
Zhou, J., Payen, J. F., Wilson, D. A., Traystman, R. J. & van Zijl, P. C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 9, 1085–1090 (2003).
Sun, P. Z. et al. Relaxation-compensated fast multislice amide proton transfer (APT) imaging of acute ischemic stroke. Magn. Reson Med. 59, 1175–1182 (2008).
Sun, P. Z., Zhou, J., Sun, W., Huang, J. & van Zijl, P. C. Detection of the ischemic penumbra using pH-weighted MRI. J. Cereb. Blood Flow. Metab. 27, 1129–1136 (2007).
Zhou, J. & van Zijl, P. C. Defining an acidosis-based ischemic penumbra from pH-weighted MRI. Transl. Stroke Res. 3, 76–83 (2011).
Liu, Q. et al. Detection of low back pain using pH level-dependent imaging of the intervertebral disc using the ratio of R1rho dispersion and -OH chemical exchange saturation transfer (RROC). Magn. Reson Med. 73, 1196–1205 (2015).
Vinogradov, E., Sherry, A. D. & Lenkinski, R. E. CEST: from basic principles to applications, challenges and opportunities. J. Magn. Reson 229, 155–172 (2013).
Wu, R., Xiao, G., Zhou, I. Y., Ran, C. & Sun, P. Z. Quantitative chemical exchange saturation transfer (qCEST) MRI - omega plot analysis of RF-spillover-corrected inverse CEST ratio asymmetry for simultaneous determination of labile proton ratio and exchange rate. NMR Biomed. 28, 376–383 (2015).
Wu, R., Longo, D. L., Aime, S. & Sun, P. Z. Quantitative description of radiofrequency (RF) power-based ratiometric chemical exchange saturation transfer (CEST) pH imaging. NMR Biomed. 28, 555–565 (2015).
Sun, P. Z., Xiao, G., Zhou, I. Y., Guo, Y. & Wu, R. A method for accurate pH mapping with chemical exchange saturation transfer (CEST) MRI. Contrast Media Mol. Imaging 11, 195–202 (2016).
Millecamps, M., Tajerian, M., Naso, L., Sage, H. E. & Stone, L. S. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain 153, 1167–1179 (2012).
Corey, S. M., Vizzard, M. A., Bouffard, N. A., Badger, G. J. & Langevin, H. M. Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PLoS One 7, e29831 (2012).
Khosravi, H. et al. Back mechanical sensitivity assessment in the rat for mechanistic investigation of chronic back pain. J. Vis. Exp. https://doi.org/10.3791/63667 (2022).
Lai, A. et al. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 16, 420–431 (2016).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Millecamps, M., Czerminski, J. T., Mathieu, A. P. & Stone, L. S. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 15, 2524–2537 (2015).
Deuis, J. R., Dvorakova, L. S. & Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 10, 284 (2017).
Randall, L. O. & Selitto, J. J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int Pharmacodyn. Ther. 111, 409–419 (1957).
Bigelow, L. J., Pope, E. K., MacDonald, D. S., Rock, J. E. & Bernard, P. B. Getting a handle on rat familiarization: the impact of handling protocols on classic tests of stress in Rattus norvegicus. Lab Anim. 57, 259–269 (2023).
Sensini, F. et al. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci. Rep. 10, 17281 (2020).
Yoon, C., Wook, Y. Y., Sik, N. H., Ho, K. S. & Mo, C. J. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59, 369–376 (1994).
Ramabadran, K., Bansinath, M., Turndorf, H. & Puig, M. M. Tail immersion test for the evaluation of a nociceptive reaction in mice. Methodol. Consid. J. Pharm. Methods 21, 21–31 (1989).
Brenner, D. S., Golden, J. P. & Gereau, R. W. T. A novel behavioral assay for measuring cold sensation in mice. PLoS One 7, e39765 (2012).
Pizziketti, R. J., Pressman, N. S., Geller, E. B., Cowan, A. & Adler, M. W. Rat cold water tail-flick: a novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. Eur. J. Pharm. 119, 23–29 (1985).
Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 (1988).
Munier, J. J. et al. Simultaneous monitoring of mouse grip strength, force profile, and cumulative force profile distinguishes muscle physiology following surgical, pharmacologic and diet interventions. Sci. Rep. 12, 16428 (2022).
Steru, L., Chermat, R., Thierry, B. & Simon, P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. (Berl.) 85, 367–370 (1985).
Miyagi, M. et al. ISSLS prize winner: disc dynamic compression in rats produces long-lasting increases in inflammatory mediators in discs and induces long-lasting nerve injury and regeneration of the afferent fibers innervating discs: a pathomechanism for chronic discogenic low back pain. Spine (Philos. Pa 1976) 37, 1810–1818 (2012).
Pattison, L. A. et al. Digging deeper into pain: an ethological behavior assay correlating well-being in mice with human pain experience. Pain 165, 1761–1773 (2024).
Piel, M. J., Kroin, J. S., van Wijnen, A. J., Kc, R. & Im, H. J. Pain assessment in animal models of osteoarthritis. Gene 537, 184–188 (2014).
Leimer, E. M. et al. Behavioral compensations and neuronal remodeling in a rodent model of chronic intervertebral disc degeneration. Sci. Rep. 9, 3759 (2019).
Kim, H. T., Uchimoto, K., Duellman, T. & Yang, J. Automated assessment of pain in rats using a voluntarily accessed static weight-bearing test. Physiol. Behav. 151, 139–146 (2015).
Park, E. H. et al. Disc degeneration induces a mechano-sensitization of disc afferent nerve fibers that associates with low back pain. Osteoarthr. Cartil. 27, 1608–1617 (2019).
Xu, L. et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthr. Cartil. 24, 1587–1595 (2016).
Hamers, F. P., Lankhorst, A. J., van Laar, T. J., Veldhuis, W. B. & Gispen, W. H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18, 187–201 (2001).
Fukui, D. et al. Gait abnormality due to spinal instability after lumbar facetectomy in the rat. Eur. Spine J. 24, 2085–2094 (2015).
Huang, Y. et al. Associations of lumber disc degeneration with paraspinal muscles myosteatosis in discogenic low back pain. Front. Endocrinol. (Lausanne) 13, 891088 (2022).
Urban, R., Scherrer, G., Goulding, E. H., Tecott, L. H. & Basbaum, A. I. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152, 990–1000 (2011).
Castel, D., Sabbag, I., Nasaev, E., Peng, S. & Meilin, S. Open field and a behavior score in PNT model for neuropathic pain in pigs. J. Pain. Res. 11, 2279–2293 (2018).
Xin, J. et al. Treatment of intervertebral disc degeneration. Orthop. Surg. 14, 1271–1280 (2022).
Kuijpers, T. et al. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur. Spine J. 20, 40–50 (2011).
Jones, C. M. P. et al. Analgesia for non-specific low back pain. BMJ 385, e080064 (2024).
Enthoven, W. T., Roelofs, P. D., Deyo, R. A., van Tulder, M. W. & Koes, B. W. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst. Rev. 2, CD012087 (2016).
Qureshi, O. & Dua, A. COX Inhibitors. in StatPearls [Internet]. (StatPearls Publishing, Treasure Island (FL), 2024). Available from: https://www.ncbi.nlm.nih.gov/books/NBK549795/.
Smith, C. J. et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 95, 13313–13318 (1998).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler Thromb. Vasc. Biol. 31, 986–1000 (2011).
Roelofs, P. D., Deyo, R. A., Koes, B. W., Scholten, R. J. & van Tulder, M. W. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database Syst. Rev. 2008, CD000396 (2008).
Vonkeman, H. E. & van de Laar, M. A. Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin. Arthritis Rheum. 39, 294–312 (2010).
Pathan, H. & Williams, J. Basic opioid pharmacology: an update. Br. J. Pain. 6, 11–16 (2012).
Bovill, J. G. Mechanisms of actions of opioids and non-steroidal anti-inflammatory drugs. Eur. J. Anaesthesiol. Suppl. 15, 9–15 (1997).
Knapp, C. M. in Encyclopedia of the Human Brain 729-739 (Elsevier, 2002).
Lambert, D. G. Opioids and opioid receptors; understanding pharmacological mechanisms as a key to therapeutic advances and mitigation of the misuse crisis. BJA Open 6, 100141 (2023).
Webster, L. R. Risk factors for opioid-use disorder and overdose. Anesth. Analg. 125, 1741–1748 (2017).
Moshfegh, J., George, S. Z. & Sun, E. Risk and risk factors for chronic opioid use among opioid-naive patients with newly diagnosed musculoskeletal pain in the neck, shoulder, knee, or low back. Ann. Intern Med. 170, 504–505 (2019).
Carassiti, M. et al. Epidural steroid injections for low back pain: a narrative review. Int. J. Environ. Res. Pub. Health 19, 231 (2021).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04930211 (2021).
Vyvey, M. Steroids as pain relief adjuvants. Can. Fam. Physician 56, 1295–1297 (2010).
Katz, J. N., Zimmerman, Z. E., Mass, H. & Makhni, M. C. Diagnosis and management of lumbar spinal stenosis: a review. JAMA 327, 1688–1699 (2022).
Ballard, T. & Chargui, S. in StatPearls (2024).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT01799616 (2013).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03708926 (2018).
Akel, M., Patel, P. & Parmar, M. Abaloparatide. in StatPearls [Internet]. (StatPearls Publishing. Treasure Island (FL), 2024). Available from: https://www.ncbi.nlm.nih.gov/books/NBK587447/.
Vandooren, J. & Itoh, Y. Alpha-2-macroglobulin in inflammation, immunity and infections. Front. Immunol. 12, 803244 (2021).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03307876 (2016).
Evans, L., O’Donohoe, T., Morokoff, A. & Drummond, K. The role of spinal surgery in the treatment of low back pain. Med. J. Aust. 218, 40–45 (2023).
Reid, P. C., Morr, S. & Kaiser, M. G. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J. Neurosurg. Spine 31, 1–14 (2019).
Fan, H. et al. Comparison of functional outcome and quality of life in patients with idiopathic scoliosis treated by spinal fusion. Med. (Baltim.) 95, e3289 (2016).
Kumar, M. N., Jacquot, F. & Hall, H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur. Spine J. 10, 309–313 (2001).
Vraa, M. L., Myers, C. A., Young, J. L. & Rhon, D. I. More than 1 in 3 patients with chronic low back pain continue to use opioids long-term after spinal fusion: a systematic review. Clin. J. Pain. 38, 222–230 (2021).
Zhao, L., Manchikanti, L., Kaye, A. D. & Abd-Elsayed, A. Treatment of discogenic low back pain: current treatment strategies and future options-a literature review. Curr. Pain. Headache Rep. 23, 86 (2019).
Jacobs, W. et al. Total disc replacement for chronic back pain in the presence of disc degeneration. Cochrane Database Syst. Rev. CD008326 (2012). https://doi.org/10.1002/14651858.CD008326.pub2
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04004156 (2019).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03716947 (2018).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05508360 (2022).
Everts, P., Onishi, K., Jayaram, P., Lana, J. F. & Mautner, K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 21, 7794 (2020).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03197415 (2017).
Dhurat, R. & Sukesh, M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J. Cutan. Aesthet. Surg. 7, 189–197 (2014).
Li, P., Zhang, R. & Zhou, Q. Efficacy of platelet-rich plasma in retarding intervertebral disc degeneration: a meta-analysis of animal studies. Biomed. Res. Int. 2017, 7919201 (2017).
Soufi, K. H., Castillo, J. A., Rogdriguez, F. Y., DeMesa, C. J. & Ebinu, J. O. Potential role for stem cell regenerative therapy as a treatment for degenerative disc disease and low back pain: a systematic review. Int. J. Mol. Sci. 24, 8893 (2023).
Sakai, D. & Andersson, G. B. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat. Rev. Rheumatol. 11, 243–256 (2015).
Tong, W. et al. Cell therapy for the degenerating intervertebral disc. Transl. Res. 181, 49–58 (2017).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT01860417 (2013).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04759105 (2021).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04530071 (2020).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02338271 (2015).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05201287 (2021).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04727385 (2021).
Gruber, H. E., Hoelscher, G. L., Ingram, J. A. & Hanley, E. N. Jr Genome-wide analysis of pain-, nerve- and neurotrophin -related gene expression in the degenerating human annulus. Mol. Pain. 8, 63 (2012).
Sun, K. et al. CGRP regulates nucleus pulposus cell apoptosis and inflammation via the MAPK/NF-kappaB signaling pathways during intervertebral disc degeneration. Oxid. Med. Cell Longev. 2021, 2958584 (2021).
Liu, S. et al. TRPV1 channel activated by the PGE2/EP4 pathway mediates spinal hypersensitivity in a mouse model of vertebral endplate degeneration. Oxid. Med. Cell Longev. 2021, 9965737 (2021).
Peng, Y. et al. Multifunctional annulus fibrosus matrix prevents disc-related pain via inhibiting neuroinflammation and sensitization. Acta Biomater. 170, 288–302 (2023).
Krock, E., Millecamps, M., Currie, J. B., Stone, L. S. & Haglund, L. Low back pain and disc degeneration are decreased following chronic toll-like receptor 4 inhibition in a mouse model. Osteoarthr. Cartil. 26, 1236–1246 (2018).
Miyagi, M. et al. ISSLS Prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Philos. Pa 1976) 39, 1345–1354 (2014).
Margarit, C. et al. Genetic contribution in low back pain: a prospective genetic association study. Pain. Pr. 19, 836–847 (2019).
Guo, Z. et al. The role of IL-6 and TMEM100 in lumbar discogenic pain and the mechanism of the glycine-serine-threonine metabolic axis: a metabolomic and molecular biology study. J. Pain. Res. 16, 437–461 (2023).
Song, X. X., Jin, L. Y., Li, X. F., Luo, Y. & Yu, B. W. Substance P mediates estrogen modulation proinflammatory cytokines release in intervertebral disc. Inflammation 44, 506–517 (2021).
Aripaka, S. S., Bech-Azeddine, R., Jorgensen, L. M. & Mikkelsen, J. D. Transient receptor potential (TRP) channels mRNA transcripts in the lumbar intervertebral discs: biomarkers for inflammation, pain, disability, and clinical outcome. Mol. Cell Biochem. 478, 121–130 (2023).
Hiyama, A., Suyama, K., Sakai, D., Tanaka, M. & Watanabe, M. Correlational analysis of chemokine and inflammatory cytokine expression in the intervertebral disc and blood in patients with lumbar disc disease. J. Orthop. Res. 40, 1213–1222 (2022).
Lee, J. M. et al. Interleukin-1beta induces angiogenesis and innervation in human intervertebral disc degeneration. J. Orthop. Res. 29, 265–269 (2011).
Pravdyuk, N. G. et al. Immunomorphogenesis in degenerative disc disease: the role of proinflammatory cytokines and angiogenesis factors. Biomedicines 11, 2184 (2023).
Banimostafavi, E. S. et al. Determining serum levels of IL-10 and IL-17 in patients with low back pain caused by lumbar disc degeneration. Infect. Disord. Drug Targets 21, e270421185135 (2021).
Song, X. X., Jin, L. Y., Li, Q., Li, X. F. & Luo, Y. Estrogen receptor beta/substance P signaling in spinal cord mediates antinociceptive effect in a mouse model of discogenic low back pain. Front. Cell Neurosci. 16, 1071012 (2022).
Noorwali, H. et al. Link N as a therapeutic agent for discogenic pain. JOR Spine 1, e1008 (2018).
Tonelli Enrico, V. et al. The association of biomarkers with pain and function in acute and subacute low back pain: a secondary analysis of an RCT. BMC Musculoskelet. Disord. 23, 1059 (2022).
Staszkiewicz, R. et al. Usefulness of detecting brain-derived neurotrophic factor in intervertebral disc degeneration of the lumbosacral spine. Med. Sci. Monit. 29, e938663 (2023).
Aoki, Y. et al. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine (Philos. Pa 1976) 32, 857–863 (2007).
Sugiura, A. et al. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Philos. Pa 1976) 33, 2047–2051 (2008).
Caparaso, S. M., Sankaranarayanan, I., Lillyman, D. J., Price, T. J. & Wachs, R. A. Single-nuclei RNA sequencing reveals distinct transcriptomic signatures of rat dorsal root ganglia in a chronic discogenic low back pain model. bioRxiv, 2025.2002.2019.639130 (2025). https://doi.org/10.1101/2025.02.19.639130
Tian, S. et al. Nucleus pulposus cells regulate macrophages in degenerated intervertebral discs via the integrated stress response-mediated CCL2/7-CCR2 signaling pathway. Exp. Mol. Med. 56, 408–421 (2024).
Li, Z., Li, Y. & Li, Z. Low-level miR-199 contribute to neuropathic low back pain via TRPV1 by regulating the production of pro-inflammatory cytokines on macrophage. Turk. Neurosurg. 34, 299–307 (2024).
Li, Z., Zhou, Y. & Li, Z. NFKB1 signalling activation contributes to TRPV1 over-expression via repressing MiR-375 and MiR-455: a study on neuropathic low back pain. Folia Biol. 68, 105–111 (2022).
Rodrigues, P., Ruviaro, N. A. & Trevisan, G. TRPV4 role in neuropathic pain mechanisms in rodents. Antioxidants 12, 24 (2022).
Fozzato, S. et al. TRPV4 and TRPM8 as putative targets for chronic low back pain alleviation. Pflug. Arch. 473, 151–165 (2021).
Slouma, M. et al. Pro-inflammatory cytokines in patients with low back pain: a comparative study. Reumatol. Clin. (Engl. Ed. 19, 244–248 (2023).
Zhang, Y. et al. Intervertebral disc cells produce interleukins found in patients with back pain. Am. J. Phys. Med. Rehabil. 95, 407–415 (2016).
Bucknill, A. T. et al. Nerve fibers in lumbar spine structures and injured spinal roots express the sensory neuron-specific sodium channels SNS/PN3 and NaN/SNS2. Spine (Philos. Pa 1976) 27, 135–140 (2002).
Matta, A. et al. A single injection of NTG-101 reduces the expression of pain-related neurotrophins in a canine model of degenerative disc disease. Int. J. Mol. Sci. 23, 5717 (2022).
Bu, G. et al. Increased expression of netrin-1 and its deleted in colorectal cancer receptor in human diseased lumbar intervertebral disc compared with autopsy control. Spine (Philos. Pa 1976) 37, 2074–2081 (2012).
Xie, W., Strong, J. A., Ye, L., Mao, J. X. & Zhang, J. M. Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain 154, 1170–1180 (2013).
Xie, W., Zhang, J., Strong, J. A. & Zhang, J. M. Role of Na(V)1.6 and Na(V)beta4 sodium channel subunits in a rat model of low back pain induced by compression of the dorsal root ganglia. Neuroscience 402, 51–65 (2019).
Yan, J. et al. Hyperexcitability and sensitization of sodium channels of dorsal root ganglion neurons in a rat model of lumber disc herniation. Eur. Spine J. 25, 177–185 (2016).
Chuah, Y. J. et al. Scaffold-free tissue engineering with aligned bone marrow stromal cell sheets to recapitulate the microstructural and biochemical composition of annulus fibrosus. Acta Biomater. 107, 129–137 (2020).
Peng, C. et al. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science 356, 1168–1171 (2017).
Vo, N. et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J. Orthop. Res. 28, 1600–1607 (2010).
Choi, H. et al. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 70, 102–122 (2018).
Boyd, L. M. et al. Early-onset degeneration of the intervertebral disc and vertebral end plate in mice deficient in type IX collagen. Arthritis Rheum. 58, 164–171 (2008).
Hasegawa, T. et al. Regenerative effects of platelet-rich plasma releasate injection in rabbit discs degenerated by intradiscal injection of condoliase. Arthritis Res. Ther. 25, 216 (2023).
Chiang, E. R. et al. Use of allogeneic hypoxic mesenchymal stem cells for treating disc degeneration in rabbits. J. Orthop. Res. 37, 1440–1450 (2019).
Lu, S. & Lin, C. W. Lentivirus-mediated transfer of gene encoding fibroblast growth factor-18 inhibits intervertebral disc degeneration. Exp. Ther. Med. 22, 856 (2021).
Banala, R. R. et al. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 19, 896–904 (2019).
Mizrahi, O. et al. Nucleus pulposus degeneration alters properties of resident progenitor cells. Spine J. 13, 803–814 (2013).
Flouzat-Lachaniette, C. H. et al. A novel in vivo porcine model of intervertebral disc degeneration induced by cryoinjury. Int. Orthop. 42, 2263–2272 (2018).
Yoon, S. H. et al. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J. Neurosurg. Spine 8, 450–457 (2008).
Kang, R. et al. Interference in the endplate nutritional pathway causes intervertebral disc degeneration in an immature porcine model. Int. Orthop. 38, 1011–1017 (2014).
Niinimaki, J. et al. Quantitative magnetic resonance imaging of experimentally injured porcine intervertebral disc. Acta Radio. 48, 643–649 (2007).
Omlor, G. W. et al. A new porcine in vivo animal model of disc degeneration: response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine (Philos. Pa 1976) 34, 2730–2739 (2009).
Acosta, F. L. Jr. et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng. Part A 17, 3045–3055 (2011).
Lee, S. E. & Jahng, T. A. A new porcine animal model of cervical disc degeneration. Glob. Spine J. 4, s-0034-1376582-s-1370034-1376582 (2014).
Henriksson, H. B. et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Philos. Pa 1976) 34, 141–148 (2009).
Omlor, G. W. et al. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur. Spine J. 19, 601–612 (2010).
Cinotti, G. et al. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine (Philos. Pa 1976) 30, 174–180 (2005).
Holm, S. et al. Pro-inflammatory, pleiotropic, and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental porcine intervertebral disk degeneration. Vet. Pathol. 46, 1292–1300 (2009).
Detiger, S. E. et al. MRI T2* mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur. Spine J. 24, 1935–1943 (2015).
Hoogendoorn, R. J., Wuisman, P. I., Smit, T. H., Everts, V. E. & Helder, M. N. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine (Philos. Pa 1976) 32, 1816–1825 (2007).
Detiger, S. E. et al. Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J. Orthop. Res. 31, 703–709 (2013).
Peeters, M. et al. BMP-2 and BMP-2/7 heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a large animal model of mild intervertebral disc degeneration. Biores Open Access 4, 398–406 (2015).
Paul, C. P. L. et al. Changes in intervertebral disk mechanical behavior during early degeneration. J. Biomech. Eng. 140. https://doi.org/10.1115/1.4039890 (2018).
Muir, V. G. et al. Injectable radiopaque hyaluronic acid granular hydrogels for intervertebral disc repair. Adv. Health Mater. 13, e2303326 (2024).
Lim, K. Z. et al. Ovine Lumbar intervertebral disc degeneration model utilizing a lateral retroperitoneal drill bit injury. J. Vis. Exp. https://doi.org/10.3791/55753 (2017).
Pennicooke, B. et al. Annulus fibrosus repair using high-density collagen gel: an in vivo ovine model. Spine (Philos. Pa 1976) 43, E208–E215 (2018).
Ghosh, P. et al. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J. Neurosurg. Spine 16, 479–488 (2012).
Borem, R. et al. Characterization of chondroitinase-induced lumbar intervertebral disc degeneration in a sheep model intended for assessing biomaterials. J. Biomed. Mater. Res. A 109, 1232–1246 (2021).
Gu, T. et al. Human bone morphogenetic protein 7 transfected nucleus pulposus cells delay the degeneration of intervertebral disc in dogs. J. Orthop. Res. 35, 1311–1322 (2017).
Matta, A. et al. NTG-101: a novel molecular therapy that halts the progression of degenerative disc disease. Sci. Rep. 8, 16809 (2018).
Zeng, Y. et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 59, 53–65 (2015).
Li, W. et al. Blocking the function of inflammatory cytokines and mediators by using IL-10 and TGF-beta: a potential biological immunotherapy for intervertebral disc degeneration in a beagle model. Int. J. Mol. Sci. 15, 17270–17283 (2014).
Shi, Z. et al. Intervention of rAAV-hTERT-transducted nucleus pulposus cells in early stage of intervertebral disc degeneration: a study in canine model. Tissue Eng. Part A 21, 2186–2194 (2015).
Hutton, W. C. et al. Does long-term compressive loading on the intervertebral disc cause degeneration?. Spine (Philos. Pa 1976) 25, 2993–3004 (2000).
Hutton, W. C. et al. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine (Philos. Pa 1976) 23, 2524–2537 (1998).
Hiyama, A. et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J. Orthop. Res. 26, 589–600 (2008).
Pfeiffer, M. et al. Intradiscal application of hyaluronic acid in the non-human primate lumbar spine: radiological results. Eur. Spine J. 12, 76–83 (2003).
Wei, F. et al. In vivo experimental intervertebral disc degeneration induced by bleomycin in the rhesus monkey. BMC Musculoskelet. Disord. 15, 340 (2014).
Stern, W. E. & Coulson, W. F. Effects of collagenase upon the intervertebral disc in monkeys. J. Neurosurg. 44, 32–44 (1976).
Zook, B. C. & Kobrine, A. I. Effects of collagenase and chymopapain on spinal nerves and intervertebral discs of cynomolgus monkeys. J. Neurosurg. 64, 474–483 (1986).
Chen, J. X. et al. Annular defects impair the mechanical stability of the intervertebral disc. Glob. Spine J. 13, 724–729 (2023).
Russo, F. et al. A hyaluronan and platelet-rich plasma hydrogel for mesenchymal stem cell delivery in the intervertebral disc: an organ culture study. Int. J. Mol. Sci. 22 (2021). https://doi.org/10.3390/ijms220629634
de Souza Grava, A. L., Ferrari, L. F., Parada, C. A. & Defino, H. L. Pharmacologic treatment of hyperalgesia experimentally induced by nucleus pulposus. Rev. Bras. Ortop. 45, 569–576 (2010).
Lee, S. et al. Voluntary running attenuates behavioural signs of low back pain: dimorphic regulation of intervertebral disc inflammation in male and female SPARC-null mice. Osteoarthr. Cartil. 30, 110–123 (2022).
Lillyman, D. J. et al. Axial hypersensitivity is associated with aberrant nerve sprouting in a novel model of disc degeneration in female Sprague Dawley rats. JOR Spine 5, e1212 (2022).
Lee, F. S., Cruz, C. J., Allen, K. D. & Wachs, R. A. Gait assessment in a female rat Sprague Dawley model of disc-associated low back pain. Connect Tissue Res. 65, 407–420 (2024).
Pelled, G. et al. Intradiscal quantitative chemical exchange saturation transfer MRI signal correlates with discogenic pain in human patients. Sci. Rep. 11, 19195 (2021).
Schleich, C. et al. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in healthy volunteers. Spine (Philos. Pa 1976) 41, 146–152 (2016).
Haneder, S. et al. Assessment of glycosaminoglycan content in intervertebral discs using chemical exchange saturation transfer at 3.0 Tesla: preliminary results in patients with low-back pain. Eur. Radio. 23, 861–868 (2013).
Aliyev, A. et al. Age-related inflammatory changes in the spine as demonstrated by (18)F-FDG-PET:observation and insight into degenerative spinal changes. Hell. J. Nucl. Med. 15, 197–201 (2012).
Zhou, X. et al. Detection of nociceptive-related metabolic activity in the spinal cord of low back pain patients using (18)F-FDG PET/CT. Scand. J. Pain. 15, 53–57 (2017).
Gamie, S. & El-Maghraby, T. The role of PET/CT in evaluation of facet and disc abnormalities in patients with low back pain using (18)F-Fluoride. Nucl. Med. Rev. Cent. East Eur. 11, 17–21 (2008).
Sharma, D. N., Yerramneni, V. K., Srivastava, M. K., Yerragunta, T. & Akurati, S. Role of magnetic resonance imaging and 18-fluorodeoxyglucose positron emission tomography-computed tomography in identifying pain generators in patients with chronic low back pain. J. Craniovertebr Junction Spine 14, 381–387 (2023).
Piri, R. et al. PET/CT imaging of spinal inflammation and microcalcification in patients with low back pain: a pilot study on the quantification by artificial intelligence-based segmentation. Clin. Physiol. Funct. Imaging 42, 225–232 (2022).
Gornet, M. G. et al. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur. Spine J. 28, 674–687 (2019).
Wilson, L., Beall, D. P., Eastlack, R. K., Berven, S. & Lotz, J. C. The comparison of cost-effectiveness between magnetic resonance spectroscopy and provocative discography in the identification of chronic low back pain surgery candidates. Clinicoecon Outcomes Res. 17, 19–31 (2025).
Acknowledgements
This work was supported by the California Institute for Regenerative Medicine under EDUC4-12751 (Giselle Kaneda) and DISC2-14049 (Dmitriy Sheyn). Additional support was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01AR066517 (Debiao Li) and R01AR082041 (Dmitriy Sheyn).
Author information
Authors and Affiliations
Contributions
GK contributed to conceptualization, methodology, investigation, writing—original draft, writing—review and editing, visualization, and funding acquisition. LZ and JTW contributed to investigation, writing—original draft, writing—review and editing, and visualization. HB, SDK, and AT contributed to conceptualization and methodology. KS and KC contributed to investigation and writing—original draft. DL contributed to funding acquisition. DS contributed to conceptualization, methodology, investigation, writing—review and editing, and funding acquisition. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaneda, G., Zila, L., Wechsler, J.T. et al. What a pain in the back: etiology, diagnosis and future treatment directions for discogenic low back pain. Bone Res 13, 89 (2025). https://doi.org/10.1038/s41413-025-00472-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41413-025-00472-7