Abstract
The lymphatic system is widely distributed in skeletal muscles, joints, and skeletal tissues and plays a key role in maintaining immune homeostasis, regulating inflammatory responses, and tissue repair. In recent years, an increasing number of studies have shown that morphological and functional changes in lymphatic vessels are closely associated with the onset and progression of a variety of musculoskeletal disorders (MSDs), such as osteoarthritis (OA), fractures, and muscular dystrophy. However, the specific mechanisms of the lymphatic system’s role in these diseases have not been fully elucidated, and their potential clinical value remains to be thoroughly explored. In this review, we review the recent research progress on the structure, function, and pathophysiological role of the lymphatic system in the musculoskeletal system, and we focus on the association between lymphangiogenesis, dysfunction, and MSDs, and systematically summarize the therapeutic strategies targeting the lymphatic system. In addition, we summarize the limitations of current studies and propose key directions for future research, with a view to providing new ideas for basic research and clinical intervention in MSDs.
Similar content being viewed by others
Introduction
Lymphatic vessels are important channels for the return of lymphatic fluid into the bloodstream. Vascular endothelial growth factor C (VEGF-C) regulates lymphatic endothelial cells (LECs) proliferation and migration during embryogenesis, resulting in the formation of lymphatic vessels.1,2 Lymphatic vessels are found throughout the body and return to the bloodstream in the subclavian vein after collecting metabolic wastes from tissue cells for immune functions.3 Skeletal muscles, bones, and joints play an important role in human production and life, and they are governed by consciousness to enable complex and orderly behavioral movements. In healthy individuals, skeletal muscle is the most abundant tissue in the body.3 Bones are among the hardest and toughest tissues in the human body. Joints connect parts of the skeleton, hold the bones in place, and allow for flexible movement of the bones. Skeletal muscles, bones, and joints play a key role in human posture maintenance and movement.4 However, in recent years musculoskeletal disorders (MSDs) have become prevalent and have had a significant impact on patients’ quality of life and socio-economics.5 According to the World Health Organization’s 2003 Technical Report on the Burden of MSDs, 40% of people over the age of 70 years have osteoarthritis (OA), and 80% of these patients have exercise limitations.6 The Global Burden of Diseases, Injuries and Risk Factors (GBD) study indicates an overall increase in the global burden of OA from 1990 to 2019, with an estimated 18 948 965 cases of OA worldwide in 2019.7
Since the lymphatic system plays an important immune function in disease development and is closely linked to disease progression, a number of studies on diseases related to the musculoskeletal system have explored possible therapeutic options starting from the lymphatic vessels. Currently in MSDs, rheumatoid arthritis (RA) has more established therapeutic options such as anti-tumor necrosis factor (TNF) therapy, B-cell depletion therapy, vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 (VEGF-C/VEGFR-3) therapy, and inducible nitric oxide synthase (iNOS) inhibitors.8 In contrast, research on OA has not been elucidated due to technical difficulties in pathogenesis, and although some of the therapeutic strategies have been borrowed from RA findings, most of them are still in the hypothesis or preliminary experimental stage, with large research gaps.8 In addition, the pathogenesis of complex lymphatic anomalies (CLA) is still unclear, and there is a lack of effective therapeutic options.9 Progress has been made in the targeted treatment of muscle ischemia, and the identification of some key targets has provided the possibility of precise treatment.8 The pathogenesis of muscle atrophy is still under further study, and the research prospect is promising.10,11
Recent years have seen recent advances in the study of lymphatic vessels in the musculoskeletal system. Recent studies have shown that specific LECs markers lymphatic vascular endothelial hyaluronan receptor 1 (LYVE1) and podoplanin (PDPN)12 have enabled imaging of lymphatic vessels, thus revealing the pattern of distribution of lymphatic vessels in bone, joints and muscle: (1) Lymphatic vessels are absent in the modeling remodeling-active zones of the bone; (2) Lymphatic vessels are present in periosteum and surrounding tissues of normal bone and muscle lymphatic vessels; (3) Lymphatic vessels are present around joints.13 Gradually, as research techniques advanced, studies proved the existence of lymphatic vessels in bones. In the latest study, researchers determined the presence of lymphatic vessels in human bone using light film imaging of intact bone components, immunostaining, and 3D imaging.14 It is a finding that overturns the earlier perception that lymphatic vessels do not exist in bone and provides a new perspective for understanding bone tissue homeostasis and the pathogenesis of bone disease. This groundbreaking study reveals the presence of lymphatic vessels in healthy intervertebral discs (IVDs) through spatial transcriptomics and immunohistochemistry, challenging the long-held dogma that adult IVDs are avascular. The findings parallel recent advancements in musculoskeletal research, such as the discovery of lymphatic vessels in human bone (via light-sheet imaging and 3D analysis), which similarly overturned traditional views on tissue vascularization.15 In the study of musculoskeletal system-related diseases, researchers using VEGF-C/VEGFR-316 and the infrared contrast agent indocyanine green (ICG)-near-infrared (NIR) technique13 have found that the generation of lymphatic vessels and the drainage function of lymphatic vessels play an important role in slowing down the progression of OA. In addition to this, in the course of studying lymphatic vessels, researchers have focused on specific genes that are highly correlated with the pathogenesis of lymphedema, and based on these genes, targeted therapies have been investigated.10
Although the lymphatic system plays a key role in organismal immunity and homeostasis maintenance, its specific mechanisms in MSDs are not clear. There is currently no systematic review that comprehensively summarizes the role of the lymphatic system in MSDs, especially the specific differences in the different tissue environments of bone, joint, and muscle and their potential therapeutic strategies. Based on this, this review focuses on the development of: (1) The distribution characteristics of lymphatic vessels in different musculoskeletal tissues (bone, muscle, and joints); (2) The mechanisms of the lymphatic system’s role in MSDs; (3) The existence of potential therapeutic strategies based on the functional modulation of lymphatic vessels.
The structure, physiology, and function of the lymphatic vessel network
The structure of the lymphatic vessel
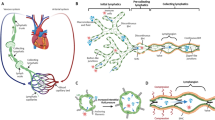
The structure of lymphatic vessels is well recognized (Fig. 1). Lymphatic vessels originate from the initial lymphatic vessels [immunostaining characterized as podoplanin-positive/α-smooth muscle actin-negative (PDPN+/α-SMA-)8,17,18,19], which are also known as terminal lymphatic vessels and lymphatic capillaries. From the initial lymphatic vessel, lymphatic fluid passes sequentially through intermediate lymphatic vessels, afferent lymphatic vessels, lymphatic nodes, efferent lymphatic vessels, and lymphatic trunks (e.g., thoracic duct, right lymphatic vessel, etc.) before finally converging into a vein.20,21,22,23 Among them, afferent and efferent lymphatic vessels are also collectively known as collecting lymphatic vessels [whose immunostaining characteristics show (PDPN+/α-SMA+)8,17,18,19].3 The morphology of initial lymphatic vessels varies greatly between species and tissues. Initial lymphatic vessels are present in the vicinity of the microcirculation24 and consist of a single layer of LECs and a discontinuous basement, with an intraluminal valve (composed mainly of endothelial cells and connective tissue) serving as a demarcation from the next level of lymphatic vessels.3 The initial lymphatic vessels are often blind-ended, a structural feature that allows coverage of more tissue surface.3,8,25 The cells of the wall overlap to form “primary lymphatic valves” that function as one-way valves and on which the intercellular fluid depends to enter the lumen and form lymphatic fluid.26 The wall cells are connected by “button-like” junctions composed of adhesion proteins (VE-calmodulin) and tight junction proteins [Occludin, Claudin-5, Zonula occludens-1 (ZO-1), Endothelial cell-Selective adhesion molecule (ESAM), Junctional adhesion molecule A (JAM-A), etc.].3,8,24 In addition, the anchor wires are connected to the surrounding interstitial space to anchor the initial lymphatic vessels, and it is still speculative whether they are related to the formation of lymphatic fluid.3,27,28 There are unidirectional valves in the intermediate lymphatic vessels, which act only as conduits for lymph flow and are not involved in lymph formation.3 Collecting lymphatic vessels have a continuous layer of basement membranes and lymphatic muscle cells (LMCs). In the collecting lymphatic vessels, there are corresponding “secondary lymphatic valves” called intraluminal valves, each of which consists of a double layer of endothelial cells, which are more numerous and thicker than the “primary lymphatic valves” and which function as a tandem lymphatic vessel.3,26 Immunostaining markers used in the study of lymphatic vessel distribution include LECs and LMCs. Among them, LYVE1,29,30 PDPN,31 prospero homeobox protein 1 (PROX1)32 and VEGFR333,34,35 were used for LECs immunostaining, and α-SMA was used for LMCs immunostaining.8,14,36 Factors such as α-SMA, PDPN, PROX1, and VEGFR3 were found to be positively expressed in pooled lymphatic vessels in immunostaining. Collecting lymphatic vessels pass through afferent lymphatic vessels, where specific immune-associated cells converge into the lymphatic fluid in the lymphatic sinus and eventually return to the bloodstream by the subclavian vein. These ducts form a three-dimensional lymphatic network with a branching structure, which is connected to the veins and accompanied by a network of small arteries, distributed throughout the body.3
The structure of the lymphatic vessel. a The initial lymphatic vessel consists of a single layer of lymphatic endothelial cells (LECs, green) that overlap to form a “primary lymphatic valve” with univalve function, and the intercellular fluid depends on this structure to enter the lumen to form lymphatic fluid. The intraluminal valve prevents backflow of lymphatic fluid. The anchor wires (black filaments) are connected to the LECs, and when they are pressurized by the interstitial fluid (fluid, lipids, macromolecules, and cells), they create gaps between adjacent LECs, whereby fluid enters the lymphatic vessels and participates in the formation of lymphatic fluid. However, this process is still in the speculative stage. Lymphatic muscle cells (pink) are spiral contractile elements regulating fluid propulsion. b There are B cells, macrophages, monocytes, dendritic cells, and T cells in the lymphatic nodes. B cells are mainly concentrated in the cortical area of the lymphatic nodes. T cells are mainly located in the paracortical area, and both T and B cells are found in the medullary area. With biofiltration, all monocytes, macrophages and dendritic cells in the lymph fluid remain in the lymphatic nodes
The physiology of the lymphatic vessel network
There are important physiological functions behind the formation of lymphatic fluid and the flow of lymphatic fluid through the lymphatic vessels. The “button-like” connections of primary LECs and the structure of anchor wires allow the interstitial fluid around the initial lymphatic vessels, which contains macromolecules or small molecules produced by cellular metabolism, to infiltrate into the lymphatic vessels to form lymphatic fluid.37 It has been hypothesized that when the pressure of the interstitial fluid increases, the anchor wires are compressed, so that the interstitial fluid enters lymphatic vessels through the gaps created between LECs and neighboring LECs that are connected to the anchor wires. But the mechanism by which anchor wires play a role in this process lacks scientific evidence.8,24
Lymphatic fluid flows through all levels of lymphatic vessels after its formation, and the direction of flow and the flow dynamics are two important factors of lymphatic flow. The structure of the lymphatic valves at all levels in the lymphatic vessels allows lymphatic fluid to flow in a fixed direction and plays a key role in preventing lymphatic reflux.3,8,21,37,38,39 LMCs act as endogenous pumps in driving the flow of lymphatic fluid. Tissue contraction and diastole in the heart, skeletal muscle, chest, and intestinal wall exert an exogenous pump.40,41,42 The endogenous and exogenous pumps push the lymphatic fluid in the lymphatic vessels continuously down to the next level of lymphatic vessels to accomplish the transportation of lymphatic fluid.37 These physiologic structures allow for the normal flow of lymphatic fluid through the lymphatic vessels.
The function of the lymphatic vessel network
In normal tissues, the lymphatic system transports some of the nutrients required by cells and removes excreted wastes, thereby participating in the regulation of body homeostasis.13,37 Beyond passive transport, there exists a multi-layered active regulatory relationship between lymphatic vessels and immune cells: LECs actively recruit immune cells such as dendritic cells and T cells by expressing chemokines (e.g., CCL21) and adhesion molecules, and increase permeability to accelerate immune cell migration under inflammatory conditions.43,44,45
For the circulatory system, the lymphatic system can eliminate pathogenic microorganisms from the bloodstream through immunosurveillance and immune responses using immunologically active substances in the lymph fluid and cells involved in the immune response.46,47,48,49,50,51,52,53,54,55 LECs are directly involved in immune regulation: they influence T cell differentiation and tolerance through antigen presentation;56, and they secrete cytokines to coordinate the balance between pro-inflammatory and anti-inflammatory responses.57,58 This process is mainly realized in the lymphatic nodes,59 where all monocytes, macrophages and dendritic cells in the lymph fluid remain in the lymphatic nodes through biofiltration, at which time T cells, B cells and antigen-presenting cells in the lymphatic nodes sample antigens in the lymph fluid that passes through the lymphatic nodes and function as immune surveillance and immune response. At the same time, the lymphatic nodes can exclude proteins from the lymphatic nodes through mechanical filtration and continue with the lymphatic fluid to the next level of lymphatic vessels.3,60,61,62
The lymphatic system uses lymphatic nodes to remove inflammatory factors and deliver immune cells through the functions of lymphatic drainage and reflux, which is important for maintaining the homeostasis of the internal environment, slowing down the progression of the disease, and recovering from the disease.13,16 Impairment of the above functions of the lymphatic system is closely related to the disruption of the homeostatic balance of the organism and to the processes of disease in the organism. This also explains to us the reason why lymphatic hyperplasia is often observed in diseased tissues in many cases. In tissues and organs such as bone, where the presence of lymphatic vessels in normal conditions is controversial, it has also been determined that new lymphatic vessels are generated in bone in pathological conditions such as OA.3
The hierarchical structure and function of the lymphatic system in the musculoskeletal system
The hierarchical structure and function of the lymphatic system in skeletal muscles
With respect to the distribution of lymphatic vessels in skeletal muscle, they initially originate from small veins63 in the skeletal muscle and are accompanied by arterioles.39 The accompanying arterioles around the initial lymphatics are responsible for providing pulse pressure to supplement the lack of endogenous pumping due to the absence of an initial lymphatic smooth muscle layer, while forming a homogeneous three-dimensional lymphatic network system in skeletal muscle that does not contain lymphatic sinuses (Fig. 2). Blood circulation in the body is accelerated after exercise, and there is a significant increase in blood flow in the small arteries that accompany lymphatic vessels in skeletal muscle. Due to the aforementioned properties of lymphatic vessels in skeletal muscle, the flow of lymph in skeletal muscle increases in response to the increase in blood flow in the arteries.3,64 In addition to these properties, the lymphatic vessels in skeletal muscle follow the general distribution of lymphatic vessels, with multiple levels of lymphatic vessels constituting the lymphatic system, with characteristic structures at each level.13 In addition to their usual function of transporting substances, lymphatic vessels in skeletal muscle play a role in the development of disease and immunity. When inflammation occurs in bones, joints, or surrounding skeletal muscle, the reflux function of the collecting lymphatic vessels is usually impeded, preventing the lymphatic system from functioning properly in immune surveillance and immune response. Therefore, improving lymphatic return drainage in MSDs can effectively slow down the progression of the diseases.13 Skeletal muscle undergoes morphological changes during exercise, and this change affects the lymphatic vessels distributed in it, driving and facilitating the flow of lymphatic fluid.65
The hierarchical structure and function of lymphatic system in skeletal muscles. a Aortas and veins can be connected to each other by a network of capillaries. From the aortas, the veins are connected sequentially by large to medium-sized arteries, small-sized arteries, arterioles, capillaries, and venules. Arterioles and venules in the skeletal muscle shown in the figure are connected by intermediate capillaries. b Lymphatic vessels in skeletal muscle are generally accompanied by arterioles. c The blind end of a lymphatic vessel (cecum) in skeletal muscle begins at the end of a venule. d Arterial blood flow increases during skeletal muscle contraction, and deformation of skeletal muscle and arteries promotes lymphatic fluid flow
The hierarchical structure and function of the lymphatic system in the joint
The synovium is an important part of the joint that connects the bones at both ends of the joint, and the lymphatic vessels at the joint are also distributed in the synovium and play an important physiological function (Fig. 3). The synovium is divided into two layers, containing a variety of macrophages and fibroblasts, and the distribution of lymphatic vessels varies greatly depends on the type and number of cells in the inner and outer layers of the synovium.66 Using markers such as 5‘-nucleotidase, cluster of differentiation (CD) 31, CD34, pathologic autopsy leiden endothelium (PAL-E) and multiple immunostaining labeling techniques, researchers have found that the outer layer of synovium, which covers the inner articular cavity, has scattered lymphatics and that there is no presence of lymphatics in the inner layer of the synovium or in the articular cartilage.16 LECs at the joints also express specific factors, and lymphatic vessels in the synovium can be recognized by positive expression of LYVE1, PDPN, PROX1, and VEGFR-3.8 Lymphatic vessels in the synovium are dependent on pre-existing lymphatic vessels induced through the VEGF-C/VEGFR-3 pathway, which regulates lymphangiogenesis by promoting cell proliferation and inhibiting apoptosis. VEGF-C can be derived from synovial macrophages, so the lymphatic system in the joints is mainly concentrated in the synovial membrane of the joints and is structurally similar to the lymphatic system of the general tissues. The lymphatic system in the synovium consists of lymphatic capillaries and lymphatic vessels. The “button-like” connections of LECs and the endogenous pumping action of LMCs allow the lymphatic vessels in the joints to collect fluids in the joint cavity, drain metabolites and inflammatory factors from the cells in the joints, and then flow with the lymphatic fluid to the lymphatic nodes. The flow of lymphatic fluid removes inflammatory factors in the lymphatic nodes and elsewhere.16,67,68 The drainage function of lymphatic vessels influences joint homeostasis. When lymphatic drainage is impaired, it causes infiltration of inflammatory cells in the joints and an increase in the expression of inflammatory factors, leading to inflammation and lymphatic hyperplasia, which in turn leads to the development of diseases such as OA.17,69 The specific mechanism of action of this process has not yet been clarified.70
The hierarchical structure and function of the lymphatic system in joint. a The initial lymphatic vessels at the joints consist of a single layer of lymphatic endothelial cells (LECs). b The collecting lymphatic vessels at the joints consist of LECs in the inner layer and an outermost layer of lymphatic muscle cells (LMCs). c Synovial fluid at the joint is formed by exudation of blood from synovial vessels and lymphatic fluid from lymphatic vessels and consists mainly of water, proteoglycans, collagen, and hyaluronic acid. d Immunostaining characteristics of initial lymphatic vessels: PDPN positive, α-smooth muscle actin (α-SMA) negative, lymphatic vascular endothelial hyaluronan receptor (LYVE) positive, prospero homeobox protein 1 (PROX1) positive, vascular endothelial growth factor receptor 3 (VEGFR3) positive. e Immunostaining characteristics of collecting lymphatic vessels: PDPN positive, α-SMA positive, LYVE positive, PROX1 positive, VEGFR3 positive
The hierarchical structure and function of the lymphatic system in the bone
Bone and skeletal muscle are closely related, and skeletal muscle is dependent on bone in the human body. Lymphatic vessels in skeletal muscle are more well established than in skeletal muscle, but lymphatic vessels in bone are more difficult to study. In studies on the distribution of lymphatic vessels in bone and skeletal muscle, it is clear that lymphatic vessels proliferate when the musculoskeletal system is in a state of inflammation or disease. For the normal musculoskeletal system, researchers used the lymphatic endothelial cell-specific expression factors LYVE1 and PDPN as evidence for the presence of lymphatic vessels in tests using rats and mice. The results showed that the presence of lymphatic vessels was not detected in normal bone, but the tissues surrounding the bone, such as periosteal fibrous tissue, fat, and skeletal muscle, had a distribution of lymphatic vessels. In some of the existing studies, it is very difficult to detect lymphatic vessels in bones due to the limited technical conditions before,71,72,73 so it is questionable whether there is a distribution of lymphatic vessels in normal bones or it is believed that lymphatic vessels do not exist in normal healthy bones, and that lymphatic vessel proliferation is only induced when OA or other MSDs occur that causes a distribution of lymphatic vessels in the bone.2
The existence of lymphatic vessels in bone has been controversial.74,75,76,77,78,79,80,81,82,83 And in recent years, the latest research results have broken the previous situation and created a new understanding.14 Using advanced light-sheet imaging technology, researchers identified LYVE1-positive lymphatic vessels predominantly in cortical bone and to a lesser extent in the bone marrow cavity of long bones, while excluding interference from mesenchymal cells and macrophages. The presence was further confirmed through multiple complementary techniques, including Evans blue staining, immunostaining for PDPN and PROX1, as well as flow cytometry analysis.14 However, a contrasting study using Prox1-tdTomato transgenic mice and polyethylene glycol-associated solvent system (PEGASOS) tissue clearing technology demonstrated that lymphatics are absent in both embryonic and adult bones under physiological conditions, though they form a rich network surrounding the bone.84 This study provides conclusive evidence overcoming previous contradictory reports about lymphatic vessels in bone, demonstrating their existence in both cortical and trabecular bone regions through the detection of specific LECs markers. These bone-associated lymphatic vessels (BALVs) form an intricate network that is interdigitated with blood vessels throughout the musculoskeletal system.85 Further experimental results revealed that genotoxic stress from radiotherapy and chemotherapy stimulates the proliferation of LECs, leading to lymphatic vessel expansion.14 This expansion occurs primarily in cortical bone and promotes myosin heavy chain 11 (Myh11) positive pericyte proliferation, thereby enhancing bone regeneration and hematopoiesis while mitigating radiation and chemotherapy toxicity. 14 However, it is worth noting that aging-associated dysfunction of collecting lymphatic vessels impairs their physiological roles, including lymphatic drainage, immune surveillance, and inflammatory regulation. This dysfunction can lead to impaired immune cell trafficking, chronic low-grade inflammation, and tissue degeneration—processes implicated in OA pathogenesis.70 Similar to synovial lymphangiogenesis, skeletal lymphangiogenesis is primarily regulated by VEGF-C/VEGFR3 signaling. Beyond this pathway, interleukin 6 (IL6) has been identified as another critical mediator of lymphatic vessel formation in bone, as demonstrated through functional experiments.14
The hierarchical structure and function of the lymphatic system in the intervertebral disc
The study of lymphatic vessels in the intervertebral disc has been an area of high potential in recent years. While conventional wisdom held that normal IVDs lack lymphatic vessels—with none detected in the nucleus pulposus or annulus fibrosus (AF), though present in surrounding ligaments86,87 —recent breakthroughs have overturned this paradigm.15 By demonstrating that lymphatic vessels regulate immune cell recycling and inflammation in IVDs—and that their decline correlates with degeneration—this work not only redefines the pathophysiology of disc degeneration but also positions lymphatic modulation as a novel therapeutic target, echoing its emerging role in bone homeostasis and disease.15 These findings align with observations that lymphatic vessels appear in degenerated discs (e.g., herniation) when fibrous tissue invasion occurs upon contact with surrounding soft tissues.86 While Khadanovich et al.88 anatomically mapped the thoracic duct’s consistent right-sided course and cisterna chyli’s left-sided predominance (70% prevalence) at lumbar levels. These complementary findings redefine IVDs' vascularity across scales. Crucially, the anatomical study revealed that these microscopic changes occur in a specific spatial context: the thoracic duct overlaps with the right diaphragmatic crus at L2-L3 vertebrae, while the cisterna chyli overlaps with the left crus at L3—creating high-risk zones for iatrogenic injury during spinal procedures.88 Their presence enables critical lymphatic functions: drainage and immune regulation, which may mitigate disease progression.89 Five lymphatic vessel-associated genes (LAGs), mesenchymal-epithelial transition factor (MET), hedgehog-interacting protein (HHIP), sprouty receptor tyrosine kinase signaling antagonist 1 (SPRY1), colony-stimulating factor 1 (CSF1), and thymocyte selection-associated high mobility group box (TOX), were utilized in the study to identify and diagnose patients with intervertebral disc degeneration (IVDD). There are 50 potential drugs targeting MET and 2 potential drugs targeting CSF1, which offer the possibility of targeted therapy.90
Roles of lymphatic vessels in musculoskeletal system diseases
Roles of lymphatic vessels in RA
The structural and functional alterations in synovial lymphatic vessels are fundamentally interconnected with the pathological progression of RA, a systemic autoimmune disorder characterized by chronic synovitis and progressive joint destruction.8,91 Recent studies using lymph nodes (LNs) biopsies reveal that neutrophils, typically absent in healthy LNs, significantly accumulate in RA patients’ lymph nodes through lymphatic transport, suggesting active neutrophil trafficking via lymphatic vessels during inflammation.92 During the initial stages of RA, before overt lymphatic contraction dysfunction becomes apparent, lymphangiogenesis is upregulated via the VEGF-C/VEGFR3 signaling pathway, primarily driven by pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) secreted by synovial fibroblasts and infiltrating macrophages.93,94,95 Concurrently, a distinct population of CD23+/CD21hi B cells (B-in cells) accumulates in draining lymph nodes (DLNs).96,97,98 These adaptive changes facilitate the clearance of metabolic byproducts and inflammatory mediators from the joints, temporarily mitigating inflammatory progression (Fig. 4).
Roles of lymphatic vessels in rheumatoid arthritis (RA). a Dilated and hyperplastic lymphatic vessels with B-in cells in the lymphatic nodes in the initial stage of RA. b With the aggravation of RA, the lymphatic nodes first show a tendency to increase in size. c With further aggravation, the lymphatic nodes become smaller and enter the collapsed stage, where B-in cells obstruct the lymphatic nodes. d In the initial stage of RA, inflammatory factors and macrophage inducible nitric oxide synthase (iNOS) induced production of nitric oxide (NO) appear in the lymphatic vessels. e Inflammatory factors and NO flowing in the lymphatic vessels damage lymphatic endothelial cells (LECs) and lymphatic muscle cells (LMCs). f Damage to LECs and LMCs impairs lymphatic drainage
As RA progresses to chronic stages, the sustained inflammatory microenvironment exerts dual damaging effects on lymphatic vasculature: (1) inflammatory mediators within lymph fluid directly impair both LECs and LMCs,68,69,99, and (2) macrophage-derived iNOS induction leads to excessive NO production, which disrupts normal lymphatic contractility through cGMP-dependent mechanisms.93,100 The discovery of neutrophil-specific protease recombinant cathepsin G (CTSG) overexpression in LN biopsies further supports that lymphatic vessels serve as conduits for inflammatory cell trafficking, potentially exacerbating LN dysfunction.92 This lymphatic dysfunction creates a vicious cycle where impaired drainage capacity results in progressive accumulation of inflammatory factors, further exacerbating synovitis and tissue damage. The VEGF-C/VEGFR3 signaling pathway continues to influence disease progression, as demonstrated in TNF-Tg mouse models, where lymphatic expansion phase features increased lymphatic density through VEGF-C/VEGFR3 upregulation, temporarily ameliorating inflammation through combined effects with B-in cell accumulation in DLNs.93,101
The subsequent lymphatic contractile phase marks a critical transition in RA pathogenesis, characterized by structural damage to collecting lymphatic vessels from persistent inflammatory insults, manifested by vessel wall abnormalities and luminal narrowing.69,99 This leads to the characteristic biphasic LNs dynamics - initial enlargement followed by progressive collapse - resulting from mechanical obstruction by accumulated B-in cells and impaired lymphatic flow.96,97 The eventual collapse phase represents end-stage lymphatic failure, featuring complete drainage dysfunction, lymph stasis, and uncontrolled inflammation amplification, which correlates with radiographic joint destruction and clinical disease progression.8,68 Notably, CD11b+ macrophages play dual roles in this process by both modulating lymphangiogenesis and differentiating into osteoclast precursors, thereby directly contributing to periarticular bone erosion.98,102,103
Roles of lymphatic vessels in OA
The development and progression of OA is intimately associated with dynamic alterations in synovial lymphatic structure and function, representing a critical but understudied aspect of joint homeostasis dysregulation (Fig. 5).16,68 Unlike the primarily immune-driven pathology of RA, OA involves a complex interplay between mechanical stress, low-grade inflammation, and metabolic factors that collectively influence lymphatic behavior (Table 1).8,104,105 In the early stages of OA, inflammatory factors (TNF-α, IL-1β) drive lymphatic vessel proliferation through the VEGF-C/VEGFR-3 signaling pathway, enhancing the clearance capacity of inflammatory mediators.8,106,107 Lymphatic vessels augment their drainage function through compensatory dilation, alleviating intra-articular pressure,106,108 during which time the draining LNs are often enlarged.99,100 Recent evidence suggests that lymphatic vessels and draining LNs function as an integrated unit in OA pathogenesis. While lymphatic vessels mediate initial inflammatory cell transport, subsequent T-cell retention in LNs—as demonstrated in load-induced OA models—may exacerbate joint damage through two mechanisms: (1) impaired immune surveillance due to reduced T-cell egress, and (2) sustained pro-inflammatory cytokine production (e.g., TNF-α/IL-6) that circulates back to the joint via lymphatic-venous anastomoses. This explains why sphingosine-1-phosphate (S1P) receptor inhibitors (which restore T-cell migration) attenuate cartilage degeneration despite not directly targeting lymphatic vessels.109
Roles of lymphatic vessels in osteoarthritis (OA). a Lymphatic vessels in normal joints include b capillary lymphatic vessels with arterioles accompanying, and c mature lymphatic vessels that perform drainage functions. d The number of capillary lymphatic vessels is increased in mild OA. e Decreased number of mature lymphatic vessels in mild OA. f Decreased number of capillary lymphatic vessels in severe OA. g A significant decrease in the number of mature lymphatic vessels in severe OA. h It is the mature lymphatic vessels that have the function of drainage. i Fluid accumulation in the joint at the onset of OA interferes with the lymphatic drainage function. j Pro-inflammatory macrophages in the diseased site of OA stimulate the proliferation of collecting lymphatic vessels. k Pro-inflammatory macrophages in the diseased site of OA reduce the drainage of the collecting lymphatic vessels
Current research faces significant challenges in elucidating OA lymphatic pathophysiology, primarily due to technical limitations in distinguishing lymphatic subtypes within synovial tissues where they coexist with blood vessels and are obscured by effusion and hyperplasia.17,18 Advanced whole-slide imaging systems have revealed spatial heterogeneity, demonstrating that mild OA correlates with increased capillary lymphatics, while progressive disease leads to a marked reduction in mature collecting vessels.17,18 As OA progresses, accumulated inflammatory factors (e.g., TNF-α) and collagen fragments disrupt LECs function: (1) TNF-α inhibits lymphatic contraction via nuclear factor-κB (NF-κB)-iNOS signaling93,110; (2) Oxidative stress and impaired VEGFR-3 signaling induce lymphatic atrophy107,111,112, and (3) age-related decline in VEGF-C disrupts VEGFR3 maintenance signaling.107 Postmortem analyses confirm decreased synovial lymphatic density in advanced OA, though this may reflect impaired lymphangiogenesis rather than absolute vessel loss, given the competing effects of synovial expansion.12,17,104
The functional consequences of lymphatic impairment create a self-perpetuating cycle of joint degeneration. Mature lymphatics normally mediate macromolecular drainage, but their dysfunction leads to the accumulation of cartilage-degrading enzymes (matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)) and pro-inflammatory cytokines.106,113,114,115,116 While the molecular mechanisms driving the reduction in mature lymphatic vessels remain incompletely understood, current evidence suggests involvement of inflammatory cytokine-mediated endothelial dysfunction and impaired VEGF-C/VEGFR3 signaling.17,117,118 Lymphatic dysfunction potentially affects bone metabolism through indirect pathways: retained pro-inflammatory cytokines (e.g., TNF-α) can promote osteoclastogenesis via receptor activator of nuclear factor kappa-B ligand (RANKL) signaling, while IL-6 exhibits dual roles—both promoting osteoclast differentiation and suppressing osteoblast activity in a context-dependent manner.119,120 For the collecting lymphatic vessels, on the one hand, pro-inflammatory macrophages at the site of inflammation reduce the drainage effect of the surrounding collecting lymphatic vessels. On the other hand, anti-inflammatory macrophages stimulate the proliferation of the collecting lymphatic vessels, which leads to an increase in the number of lymphatics. However, ultimately, the draining effect of lymphatic vessels was still significantly disrupted. Some specific influencing factors, such as TNF-α, IL-1, and IL-6, made it possible to study the specific therapeutic mechanism.8
Roles of lymphatic vessels in other bone diseases
The potential roles of lymphatic vessels in bone diseases remain an emerging area of research. While current evidence primarily comes from acute bone injury models, fractures, and tooth extractions, with a notable lack of studies on chronic bone diseases like osteoporosis, these findings provide preliminary insights into lymphatic-bone crosstalk. Recent studies demonstrate that Vegfc-expressing mesenchymal progenitor cells (MPCs) not only stimulate lymphangiogenesis at musculoskeletal injury sites but also directly differentiate into heterotopic bone, revealing a dual role in both lymphatic network expansion and pathological ossification.121
The skeletal lymphatic system serves as a dynamic regulator of bone homeostasis, with its strategic positioning along fracture calluses and marrow sinuses being particularly critical for bone repair.85 Interestingly, while lymphatic hypoplasia in Vegfr3wt/Chy mice does not impair fracture healing, complete lymphatic absence in Vegfr3Chy/Chy embryos similarly shows no developmental bone defects, indicating compensatory mechanisms may exist when lymphatics are deficient.84 In contrast to physiological conditions, traumatic musculoskeletal injury triggers robust lymphangiogenesis that parallels but does not invade heterotopic ossification (HO) lesions, suggesting spatial regulation of lymphatic invasion.121 In fracture healing models, lymphangiogenesis around bone implants coincides with improved bone regeneration.122 However, lymphatic dysfunction can exacerbate pathological changes: lymph platelet thrombi (LPT) frequently obstruct the drainage of subcapsular sinuses and collecting ducts after fractures.85 Conversely, impaired lymphatic drainage exacerbates fracture healing delays, as demonstrated by VEGFR3 inhibition (SAR131675), which reduces lymphatic clearance and popliteal lymph node (PLN) volume, leading to edema, pain, and poor callus formation. Anti-podoplanin (PDPN) neutralizing antibodies mitigate LPT, enhance drainage, and improve bone repair by unblocking lymphatic vessels and reducing inflammation.123 The dual capacity of this system in regenerative expansion and pathological obstruction underscores its significant role in bone physiology and pathology.
Beyond injury repair, lymphatic vessels also regulate inflammatory bone loss. In periprosthetic osteolysis, lymphatic activation via VEGF-C/VEGFR3 signaling counteracts osteoclast differentiation and bone resorption induced by titanium particles or inflammatory cytokines (e.g., TNF-α, LPS).124 However, aging impairs this protective mechanism due to senescence-associated secretory phenotype (SASP) from adipogenically differentiated mesenchymal stem cells (MSCs), which suppress LECs responsiveness to VEGF-C. JAK inhibitors (e.g., ruxolitinib) can restore lymphatic vessels function in aged mice, offering a therapeutic strategy for osteolysis prevention.124
Moreover, in bisphosphonate (BP)-related osteonecrosis of the jaw (BRONJ) models,125 impaired lymphatic drainage due to LECs apoptosis correlates with disease exacerbation. Similarly, in tooth extraction models, parathyroid hormone (PTH) stimulates lymphangiogenesis, aligning with its known anabolic effects on bone.126 While acute injury models (fracture/tooth extraction) differ from chronic osteoporosis, they collectively imply that lymphatic modulation could be a therapeutic target. We emphasize that current evidence supports lymphatic involvement in bone homeostasis generally, while osteoporosis-specific mechanisms remain hypothetical. This gap highlights an important research frontier where our review aims to stimulate investigation. Future studies should employ established osteoporosis models to clarify whether lymphatic dysfunction contributes to osteoporosis pathogenesis.
Roles of lymphatic vessels in CLA
The development of CLA is associated with abnormalities of the lymphatic vessels, and each of the disorders in CLA has its own characteristics. Gorham-Stout Disease (GSD), General Lymphatic Anomaly (GLA), Kaposiform Lymphangiomatosis (KLA), and Central Conducting Lymphatic Anomaly (CCLA) are collectively referred to as CLA, which is a serious physical and mental health risk for patients, with a low overall number of patients for whom there is no effective cure. Recent genomic analysis of GSD lesions identified a somatic Kirsten Ratsarcoma Viral Oncogene Homolog (KRAS) c.182 A > G (p.Q61R) mutation at 1% allele frequency in affected bone tissue, representing the first reported pathogenic variant in GSD that activates RAS/ mitogen-activated protein kinase (MAPK) signaling - a known oncogenic driver in vascular anomalies.127 Recent studies have identified somatic activating mutations in PIK3CA (including Glu542Lys, Gln546Lys, His1047Arg, and His1047Leu variants) as causative factors in GLA, with these mutations leading to hyperactivation of the PI3K-AKT-mTOR pathway and subsequent lymphatic hyperplasia.128 Histopathological correlation showed this KRAS-mutant GSD case exhibited characteristic LECs proliferation, osteoclast activation, and M1/M2 macrophage infiltration, suggesting mutant cells may drive disease through both direct lymphatic hyperplasia and paracrine cytokine signaling.127 Case studies have been conducted at the genetic level. For a long time, lymphatic vessels were thought to be absent from the bone, but in recent studies, they have been confirmed,14 and during the development of GSD, GLA, and KLA, the normal bone of the patients is resorbed by the invading lymphatic vessels.129,130,131,132,133 The proliferation of lymphatic vessels in GSD and KLA is associated with hyperactivation of Rat sarcomavirus and Mitogen-activated kinase (RASMEK) pathway in VEGF-C/VEGFR3 signaling induced by somatic mutations. Recent research demonstrates that hyperactive KRAS/MAPK signaling disrupts lymphatic vessel architecture and function, leading to lymphatic hyperplasia and impaired valve formation, while MEK1/2 inhibition (e.g., trametinib) partially reverses these defects by suppressing extracellular regulated protein kinases (ERK)1/2 phosphorylation and restoring lymphatic maturation gene expression.134 Additionally, lineage-tracing studies reveal that bone lymphatic endothelial cells (bLECs) originate from pre-existing Prox1-positive LECs, with osteoclasts playing a critical role in facilitating lymphatic invasion into bone through cortical resorption.135 Preclinical evidence shows that rapamycin effectively prevents lymphatic hyperplasia and dysfunction in mouse models expressing active PIK3CA mutations, while clinical observations demonstrate its ability to reduce pain in GLA patients. 128 Rapamycin, an mTOR inhibitor, effectively suppresses this pathological lymphangiogenesis in both GSD and GLA mouse models.135 New preclinical studies demonstrate that the MEK1/2 inhibitor trametinib selectively blocks VEGF-C-induced ERK1/2 activation in LECs, preventing lymphatic invasion of bone and cortical bone loss in animal models, though its efficacy is limited to early disease stages and shows minimal reversal of established bone lesions—suggesting a potential therapeutic window for early intervention in GSD patients with KRAS mutations.136 CCLA is often characterized by lymphatic malformations causing lymphedema.137,138 Mutations in the corresponding somatic cells in each disease do not directly lead to bone loss. They cause lymphangiectasia through overactivation of the cell signaling pathway, such as phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) pathway and RAS-MEK, and the lymphangiectasia contacting the skeleton progressively lyses the bone and leads to bone loss. However, the process of lymphatic vessel proliferation and lymphatic vessel osteolysis has not yet been elucidated in the molecular mechanism of the specific influencing factors and their mechanism of action.9
Roles of lymphatic vessels in muscular diseases
Lymphatic vessels are critical in muscular diseases, particularly in regulating inflammation and tissue homeostasis (Fig. 6). In muscle ischemia, impaired venous return and lymphatic drainage disrupt blood circulation and inflammatory factor clearance, exacerbating inflammation. VEGF-C, produced by integrin alpha M (CD11b) cells, activates the VEGFR-3 pathway to induce lymphangiogenesis, and intramuscular VEGF-C supplementation enhances lymphatic remodeling, mitigating ischemic damage.10,139 Muscle atrophy, resulting from malnutrition, disuse, or genetic abnormalities, is associated with lymphangiogenesis, where pre-existing lymphatic vessels develop buds and proliferate via the VEGF-C/VEGFR-3 axis, though the precise mechanisms remain unclear.10,11 In muscular dystrophy, myocyte damage leads to albumin release, while monocyte adhesion to peripheral lymphatic nodes with altered extracellular matrix ligand receptor expression contributes to lymphatic dysfunction, LNs atrophy, and impaired drainage, promoting inflammation.10
Roles of lymphatic vessels in muscular diseases. a In muscle atrophy, the morphology and number of myofibers in skeletal muscle change, with myofibers becoming thin and fewer in number. Myofibers release albumin when damaged. b Lymphatic vessels in skeletal muscle appear as new lymphatic buds during muscle atrophy, and these lymphatic buds further generate new lymphatic vessels. c Mononuclear cells adhere to lymphatic nodes in muscle atrophy, affecting the drainage function of the lymphatic nodes. d Impaired circulation of blood and lymphatic fluids leads to muscle ischemia, and the accumulation of inflammatory factors in the tissues causes inflammation in ischemic muscles. Vascular endothelial growth factor C (VEGF-C) factor produced by integrin alpha M (CD11b) cells can ameliorate muscle ischemia by inducing neolymphangiogenesis
Targeting the lymphatic vessels as potential treatment strategy in musculoskeletal system
The “button” and anchor wire structures in the initial lymphatic vessels, as well as the intraluminal valve structures and LMCs at all levels of lymphatic vessels, provide the structural basis for the drainage function of the lymphatic system. Only through normal drainage can the lymphatic vessels realize the functions of material transportation, immune surveillance, and immune reaction, and maintain the homeostasis of the organism. Various diseases of the musculoskeletal system show obvious impaired lymphatic drainage in the pathogenesis, accompanied by inflammation. Unclogging lymphatic vessels and increasing the number of lymphatic vessels are both beneficial in restoring their drainage function to slow down the progression of inflammation and MSDs. Currently, different therapeutic programs have been formulated for different MSDs from different perspectives to achieve the goals of increasing the number of lymphatic vessels and preventing lymphatic vessel blockage to enhance the drainage function from different levels and stages of disease development (Table 2).
Targeting the lymphatic vessels as a potential treatment strategy in joint
There are similarities in the clinical treatment of RA and OA, but at the same time, there are also different treatment directions for each. There are many approaches to the treatment of RA, and some drugs have shown good therapeutic effects in clinical use, while others are still in the stage of animal testing or have yet to be experimentally verified after theoretical proof.8 Recent studies demonstrate that in situ size amplification of nanoparticles (e.g., PD5NPs) through myeloperoxidase (MPO)-responsive aggregation can effectively reduce lymphatic clearance in arthritic joints, prolonging drug retention by 5-fold compared to conventional nanoparticles.140 The size of lymphatic vessels and lymphatic nodes shows significant changes during the development of RA, and these changes can be easily detected, suggesting the feasibility of targeting lymphatic vessels for treatment.68 One of the more mature aspects of the pathogenesis of OA is the influence of effusion in the disease process. Compared to the findings in RA, OA still has many undefined factors in terms of pathogenesis and therapeutic mechanisms. There are a variety of treatment options available for patients with mild OA, but the mechanisms behind some of the physical therapy options are unclear. The treatment options for patients with severe OA are relatively homogeneous, with total joint replacement being the only effective treatment option. There are many potentially effective therapeutic agents for the treatment of OA, and the clinical outcomes need to be further validated.8
TNF therapy: a therapeutic option for RA. Some of the TNF drugs in clinical use restore lymphatic drainage by increasing lymphatic constriction, a process in which there is macrophage apoptosis. This approach contrasts with novel MPO-responsive systems that achieve 3-fold greater reduction in inflammatory cytokines (TNF-α/IL-1β) through lymphatic transport inhibition rather than drainage enhancement.140 Targeted local administration is superior to systemically administered therapy.
B-cell depletion therapy: In the development of RA, although its characteristic B-in cells can play a role in slowing down inflammation in the early stage of RA, a large number of proliferating Bin cells will block lymphatic nodes and affect the function of lymphatic drainage with the development of RA. Drugs such as anti-CD20 mAb and rituximab can restore lymphatic drainage by removing the obstruction formed by B-in cells, and the experiments were conducted mainly in animals (mice), and the experiments still showed good results when the drugs were administered locally. Inspired by the fact that the obstruction of lymphatic nodes by B-in cells in RA affects the lymphatic drainage function, the obstruction of lymphatic drainage function in the pathogenesis of OA may also be related to the obstruction caused by B-cells. This therapy is still in the stage of theoretical feasibility in the treatment of OA without any experiments to support it, but it still has some research potential based on conjecture.
VEGF-C/VEGFR3 therapy: Lymphangiectasia in the early stages of RA is dependent on the VEGF-C/VEGFR3 signaling pathway, and lymphangiectasia reduces inflammation in the joints and slows down the progression of RA. VEGF-C adeno-associated viral topical therapy promotes lymphangiectasia to achieve therapeutic effects on RA, and this treatment regimen has already been shown to be effective in RA-modeling mice. Reliable results have been obtained in RA modeling mice. In contrast, in the pathogenesis of OA, although lymphangiogenesis is associated with the VEGF-C/VEGFR3 signaling pathway, the number of mature lymphatic vessels in the synovium surrounding OA appears to decrease at all stages of the disease. The therapy remains controversial and problematic, and the safety of the treatment cannot be guaranteed at this time.
Inhibitors of iNOS: Although lymphatic hyperplasia can slow down the inflammatory process in the early stage of RA, inflammatory factors and iNOS-induced production of NO can damage the surrounding lymphatic vessels to exacerbate the inflammation. Based on this, animal experiments used the iNOS inhibitor L-N(6)-(1-iminoethyl)lysine 5-tetrazole-amide to cause the lymphatic vessels constrict to increase lymphatic drainage and alleviate the inflammation. But at present, some key time points for the use of iNOS inhibitors in the course of the disease treatment are not clear. And in the therapeutic neighborhood of OA, there are many potentially good effects of this approach, but no clinical trial evidence has been seen.
Bortezomib therapy: a therapeutic option for OA. Whether diminished lymphatic drainage accelerates the progression of OA is in speculative stage. As a proteasome inhibitor, the bortezomib drug is still in the stage of animal testing, and its effect of reducing inflammation and improving lymphatic drainage through the NF-κB signaling pathway is currently showing a bright future, which needs to be supported by more experimental and theoretical evidence in the future.8
Targeting the lymphatic vessels as a potential treatment strategy in skeletal muscles
The VEGF-C/VEGFR3 pathway is an important target for targeting lymphatic vessels in skeletal muscle. Lymphatic vessel growth in normal tissues and proliferation in disease states are regulated by the VEGFR3 pathway mediated by VEGF-C. Activation of the VEGF-C/VEGFR3 signaling pathway promotes lymphatic vessel proliferation. In the treatment of skeletal muscle ischemia, injection of VEGF-C into the ischemic muscles of experimental mice can effectively enhance lymphatic drainage and venous return, eliminate muscle tissue inflammation, and improve blood supply conditions. Some researchers envision a combination of vascular endothelial cells and lymphatic vessel targeting agents for the treatment of skeletal muscle ischemia. Animal experiments with human vascular endothelial growth factor (VEGF) family members, vascular endothelial growth factor C/D (VEGF-C/-D), and VEGFR3-specific mutants (VEGF-C156S) and collagen- and calcium-binding transduction of the epidermal growth factor structural domain 1 (CCBE1) promoted lymphatic vascularization, which provides a possible approach for targeting lymphatic vessels for the treatment of skeletal muscle diseases. It is a possible approach that can be used as a reference for studying the site of action of targeted drugs for drug development in the future.8,10
Targeting the lymphatic vessels as a potential treatment strategy in bone
Therapeutic approaches targeting lymphatic vessels are currently not widely used in skeletal diseases, but some of the potential targeting sites identified in the study could serve as a new direction for future research into therapeutic approaches for skeletal diseases.
GSD is a type of CLA in which patients typically develop lymphatic reflux and bones gradually dissolve with the presence of ectopic lymphatic vessels in the bone, and as the disease progresses, celiac disease and other severe bone diseases may develop. The use of trametinib in GSD mice effectively prevents overactive KRAS signaling in LECs from damaging lymphatic valves and has a therapeutic effect of stopping lymphatic reflux. Mouse experiments and case studies have demonstrated that trametinib is an effective GSD therapeutic targeting lymphatic vessel drug, but the specific mechanism of action has not been elucidated.141
Acquired HO may occur after orthopedic surgery or be caused by severe trauma, burns, surgery, or central nervous system injury. When mice with acquired HO are given postoperative topical treatment with fibroblast growth factor 9 (FGF9), fibroblast growth factor receptor 3 (FGFR3) signaling, which regulates LECs proliferation, is activated, promoting lymphatic vessel formation and inhibiting HO formation. There is currently no effective treatment for acquired HO, but mouse experiments suggest that FGFR3 is an important target for future treatment of acquired HO by targeting lymphatic vessels.142
Current Challenges and Future Directions
Our understanding of lymphatic vessels in the musculoskeletal system remains incomplete, particularly regarding their structural and functional roles in both health and disease. While the VEGF-C/VEGFR3 signaling pathway has been extensively studied in musculoskeletal disorders,8,9,10,11,139 it remains unclear whether other molecular pathways contribute similarly to lymphatic regulation. Furthermore, existing research has primarily focused on morphological changes and drainage functions of lymphatic vessels in bones, joints, and skeletal muscles,,65,143,144,145,146,147, leaving critical gaps in our knowledge of exercise-induced adaptations and aging-related functional decline. Notably, comparative studies between different muscle groups (e.g., fast vs. slow muscles) are lacking, making it difficult to determine whether lymphatic structure and function vary across musculoskeletal tissues.145
The clinical implications of these knowledge gaps are particularly evident in conditions like CLA (congenital lymphatic abnormalities), where therapeutic development has been hampered by an incomplete understanding of pathogenesis.9 While research has focused on molecular mechanisms, treatment options remain limited, highlighting the urgent need to translate theoretical knowledge of lymphatic-targeted therapies into experimental validation.
The relationship between lymphatic circulation and immune function in musculoskeletal diseases warrants deeper investigation. While historically believed to be absent from bone tissue,2 lymphatic vessels are now recognized as important players in bone homeostasis and repair. Recent advances demonstrate their crucial role in vascularization and bone regeneration following injury,14 though this regenerative capacity appears to diminish with age. However, existing detection techniques (e.g., immunofluorescent labeling, single-cell sequencing, and spatial transcriptomics etc.) may still have limitations that affect the accurate knowledge of lymphatic vessels in tissue,s and future technological developments may further optimize the research methods, highlighting the need for improved imaging and analytical techniques.
Emerging evidence suggests complex interactions between bone and lymphatic systems,148 with potential implications for both musculoskeletal and neurological disorders. For example, cranial bone manipulation (CBM) has been shown to stimulate meningeal lymphangiogenesis and enhance drainage capacity, potentially reducing amyloid deposition in Alzheimer’s disease. This finding reveals a previously underappreciated mechanism whereby mechanical stimulation of bone can modulate lymphatic function through cytokine release (e.g., VEGF-C) and subsequent endothelial cell activation. Importantly, these effects appear persistent, suggesting long-term structural adaptations in both bone and lymphatic networks. Such discoveries open new avenues for exploring similar bone-lymphatic interactions in musculoskeletal disorders.
The gradual increase in musculoskeletal lymphatic research reflects an evolving understanding of their functional diversity - transitioning from being regarded primarily as passive drainage channels to being recognized as active contributors to tissue homeostasis, repair, and disease modulation.46,149,150 This conceptual shift, supported by key discoveries (e.g., the identification of intraosseous lymphatic vessels) and methodological advances (e.g., near-infrared fluorescence imaging with ICG labeling),85,151 has enabled preliminary visualization and functional assessment of musculoskeletal lymphatic networks. While these developments have identified potential therapeutic targets for inflammatory joint disorders and trauma repair, substantial work remains to fully characterize these systems. Current methodological progress suggests this field is poised for measured growth in the coming years.
Future research should focus on elucidating lymphatic-immune crosstalk in inflammatory joint diseases and bone disorders while developing more sophisticated approaches to study musculoskeletal-lymphatic interaction networks. While current evidence has not yet established a direct correlation between bone marrow lymphatic vessels and bone metabolism, substantial research has demonstrated the critical role of bone marrow microenvironment and its cellular components in regulating bone homeostasis and osteoporosis pathogenesis, such as cellular senescence and the inflammatory microenvironment.152,153,154 Considering the specialized functions of lymphatic vessels in immune surveillance and cell trafficking - distinct from those of blood vessels155,156 - it is plausible that future investigations may uncover novel relationships between bone marrow lymphatic networks and osteoporosis development. This potential connection warrants further exploration, particularly in light of emerging evidence suggesting lymphatic-immune interactions within the skeletal system.8,14,85 In summary, first, the study of the musculoskeletal system can draw on the mechanisms of the lymphatic vascular system in neurodegenerative diseases to explore the potential function of the musculoskeletal system in neuroinflammation and immunomodulation. Second, studies of the lymphatic vascular system can further explore its role in diseases of the musculoskeletal system, particularly the mechanisms of lymphatic drainage and immune cell migration in inflammatory joint diseases and bone disease. In addition, the combination of multi-omics techniques and advanced imaging methods can reveal the interaction network between the musculoskeletal system and lymphatic vascular system and provide a theoretical basis for the development of combined therapeutic strategies targeting disorders of the musculoskeletal system and the lymphatic vascular system. These research directions not only help to deepen the understanding of the physiological and pathological mechanisms of the two major systems, but also provide new perspectives for the comprehensive treatment of cross-system diseases (Fig. 7).
Current Challenges and Future Directions in Musculoskeletal-Lymphatic Research. This conceptual framework summarizes key challenges and emerging opportunities in musculoskeletal-lymphatic research. VEGF-C/VEGFR3 vascular endothelial growth factor C/vascular endothelial growth factor receptor 3, VEGF-C vascular endothelial growth factor C
Conclusion
The lymphatic system has been studied for a long time, and disorders of the lymphatic system have been closely associated with a wide range of diseases, directly or indirectly affecting the homeostasis of the organism, but at the same time providing a possible direction for the cure of diseases. The structure of the lymphatic system at all levels, as well as the functions associated with the structure, are now well defined and clearly understood. However, the initial formation of lymphatic vessels and lymphatic fluid has not yet been clarified, and the role played by anchor wires in the formation of lymphatic fluid is in the conjectural stage. Based on the knowledge of the structure and function of the lymphatic system, a large number of experiments have been conducted to study the distribution of lymphatic vessels in skeletal muscles, joints, and bones. The results of the research on lymphatic vessels in skeletal muscle and joints are more mature and are of great significance in guiding the treatment of related diseases. The discovery of lymphatic vessels in bones breaks through traditional concepts, proves the wide distribution of the lymphatic system in the musculoskeletal system, and provides new ideas for the study of bone diseases (e.g., fracture healing disorders, etc.). In the future, the specific role of the skeletal lymphatic system in bone remodeling and inflammatory response can be further explored using lymphatic tracer technology. Diseases related to the musculoskeletal system, such as RA, muscle ischemia, and muscular dystrophy, have been investigated in terms of their specific pathogenesis and therapeutic methods from the direction of lymphatic vessels. The research results on the pathogenesis and therapeutic methods of OA and CLA in the direction of lymphatic vessels are relatively few, but with a certain theoretical basis, future research has a bright prospect. Lymphatic vascular targeted therapy has shown potential clinical application, but further experiments are needed to verify its efficacy and safety. Future studies should focus on the mechanism of action, the scope of applicable diseases, and the long-term therapeutic effects of lymphatic vessel-targeted drugs, in order to promote the translation of this strategy from the laboratory to the clinic.
References
Rauniyar, K., Jha, S. K. & Jeltsch, M. Biology of Vascular Endothelial Growth Factor C in the morphogenesis of lymphatic vessels. Front Bioeng. Biotechnol. 6, 7 (2018).
Edwards, J. R. et al. Lymphatics and bone. Hum. Pathol. 39, 49–55 (2008).
Breslin, J. W. et al. Lymphatic vessel network structure and physiology. Compr. Physiol. 9, 207–299 (2018).
Iizuka, K., Machida, T. & Hirafuji, M. Skeletal muscle is an endocrine organ. J. Pharm. Sci. 125, 125–131 (2014).
Gomez-Galan, M., Perez-Alonso, J., Callejon-Ferre, A. J. & Lopez-Martinez, J. Musculoskeletal disorders: OWAS review. Ind. Health 55, 314–337 (2017).
Lidgren, L. The Bone and Joint Decade and the global economic and healthcare burden of musculoskeletal disease. J. Rheumatol. Suppl. 67, 4–5 (2003).
Cao, F. et al. Trends and cross-country inequalities in the global burden of osteoarthritis, 1990-2019: A population-based study. Ageing Res. Rev. 99, 102382 (2024).
Zhou, S. et al. Lymphatic vessels: roles and potential therapeutic intervention in rheumatoid arthritis and osteoarthritis. Theranostics 14, 265–282 (2024).
Solorzano, E. et al. Osteopathy in Complex Lymphatic Anomalies. Int. J. Mol. Sci 23, 8258 (2022).
Ji, R. C. Recent advances and new insights into muscular lymphangiogenesis in health and disease. Life Sci. 211, 261–269 (2018).
Ji, R. C., Eshita, Y., Xing, L. & Miura, M. Multiple expressions of lymphatic markers and morphological evolution of newly formed lymphatics in lymphangioma and lymph node lymphangiogenesis. Microvasc. Res. 80, 195–201 (2010).
Xu, H. et al. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann. Rheum. Dis. 62, 1227–1229 (2003).
Liang, Q. Q., Shi, Q., Wood, R. W., Xing, L. P. & Wang, Y. J. Peri-articular lymphatic system and “Bi” theory of Chinese medicine in the pathogenesis and treatment of arthritis. Chin. J. Integr. Med. 21, 648–655 (2015).
Biswas, L. et al. Lymphatic vessels in bone support regeneration after injury. Cell 186, 382–397.e324 (2023).
Zou, F. et al. Revealing the presence of lymphatic vessels within intervertebral discs: Novel insights into disc degeneration. Innovation 6, 100865 (2025).
Cao, M., Ong, M. T. Y., Yung, P. S. H., Tuan, R. S. & Jiang, Y. Role of synovial lymphatic function in osteoarthritis. Osteoarthr. Cartil. 30, 1186–1197 (2022).
Shi, J. et al. Distribution and alteration of lymphatic vessels in knee joints of normal and osteoarthritic mice. Arthritis Rheumatol. 66, 657–666 (2014).
Shi, J. X. et al. Use of a whole-slide imaging system to assess the presence and alteration of lymphatic vessels in joint sections of arthritic mice. Biotech. Histochem 88, 428–439 (2013).
Hou, T. et al. Ginsenoside Rg1 promotes lymphatic drainage and improves chronic inflammatory arthritis. J. Musculoskelet. Neuronal Interact. 20, 526–534 (2020).
Skandalakis, J. E., Skandalakis, L. J. & Skandalakis, P. N. Anatomy of the lymphatics. Surg. Oncol. Clin. N. Am. 16, 1–16 (2007).
Yang, Y. & Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Invest 124, 888–897 (2014).
Srinivasan, R. S. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422–2432 (2007).
Riquet, M., Le Pimpec Barthes, F., Souilamas, R. & Hidden, G. Thoracic duct tributaries from intrathoracic organs. Ann. Thorac. Surg. 73, 892–898 (2002). discussion 898–899.
Rovenska, E. & Rovensky, J. Lymphatic vessels: structure and function. Isr. Med Assoc. J. 13, 762–768 (2011).
Fischer, M. et al. Flow velocity of single lymphatic capillaries in human skin. Am. J. Physiol. 270, H358–H363 (1996).
Castenholz, A. Morphological characteristics of initial lymphatics in the tongue as shown by scanning electron microscopy. Scan Electron. Microsc. 1343–1352 (1984).
Leak, L. V. & Burke, J. F. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am. J. Anat. 118, 785–809 (1966).
Leak, L. V. & Burke, J. F. Ultrastructural studies on the lymphatic anchoring filaments. J. Cell Biol. 36, 129–149 (1968).
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Noda, Y., Amano, I., Hata, M., Kojima, H. & Sawa, Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem Cytochem 43, 61–68 (2010).
Breiteneder-Geleff, S. et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 (1997).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Jussila, L. et al. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 58, 1599–1604 (1998).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 92, 3566–3570 (1995).
Kenney, H. M. et al. The enigmas of lymphatic muscle cells: where do they come from, how are they maintained, and can they regenerate?. Curr. Rheumatol. Rev. 19, 246–259 (2023).
Zawieja, D. C. Contractile physiology of lymphatics. Lymphat Res. Biol. 7, 87–96 (2009).
Schulte-Merker, S., Sabine, A. & Petrova, T. V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Schmid-Schonbein, G. W. Microlymphatics and lymph flow. Physiol. Rev. 70, 987–1028 (1990).
Zawieja, D. Lymphatic biology and the microcirculation: past, present and future. Microcirculation 12, 141–150 (2005).
von der Weid, P. Y. & Zawieja, D. C. Lymphatic smooth muscle: the motor unit of lymph drainage. Int. J. Biochem Cell Biol. 36, 1147–1153 (2004).
Causey, L., Cowin, S. C. & Weinbaum, S. Quantitative model for predicting lymph formation and muscle compressibility in skeletal muscle during contraction and stretch. Proc. Natl. Acad. Sci. USA 109, 9185–9190 (2012).
Permanyer, M., Bošnjak, B. & Förster, R. Dendritic cells, T cells and lymphatics: dialogues in migration and beyond. Curr. Opin. Immunol. 53, 173–179 (2018).
Lucas, E. D. & Tamburini, B. A. J. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Front. Immunol. 10, 36 (2019).
Olate-Briones, A. et al. The meningeal lymphatic vasculature in neuroinflammation. Faseb j. 36, e22276 (2022).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science 369, eaax4063 (2020).
Stritt, S., Koltowska, K. & Makinen, T. Homeostatic maintenance of the lymphatic vasculature. Trends Mol. Med. 27, 955–970 (2021).
Ma, W. et al. Mitochondrial respiration controls the Prox1-Vegfr3 feedback loop during lymphatic endothelial cell fate specification and maintenance. Sci. Adv 7, eabe7359 (2021).
Petrova, T. V. & Koh, G. Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 215, 35–49 (2018).
Oliver, G., Kipnis, J., Randolph, G. J. & Harvey, N. L. The lymphatic vasculature in the 21(st) century: novel functional roles in homeostasis and disease. Cell 182, 270–296 (2020).
Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 17, 1371–1380 (2011).
Bovay, E. et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J. Exp. Med. 215, 2760–2777 (2018).
Srinivasan, R. S. et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 28, 2175–2187 (2014).
Wang, Y. & Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 24, 2115–2126 (2010).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Ulvmar, M. H. & Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res 111, 310–321 (2016).
Magold, A. I. & Swartz, M. A. Pathogenic exploitation of lymphatic vessels. Cells 11, 979 (2022).
Betterman, K. L. & Harvey, N. L. The lymphatic vasculature: development and role in shaping immunity. Immunol. Rev. 271, 276–292 (2016).
Padera, T. P., Meijer, E. F. & Munn, L. L. The lymphatic system in disease processes and cancer progression. Annu. Rev. Biomed. Eng. 18, 125–158 (2016).
Adair, T. H. & Guyton, A. C. Modification of lymph by lymph nodes. II. Effect of increased lymph node venous blood pressure. Am. J. Physiol. 245, H616–H622 (1983).
Adair, T. H., Moffatt, D. S., Paulsen, A. W. & Guyton, A. C. Quantitation of changes in lymph protein concentration during lymph node transit. Am. J. Physiol. 243, H351–H359 (1982).
Hay, J. B. & Andrade, W. N. Lymphocyte recirculation, exercise, and immune responses. Can. J. Physiol. Pharm. 76, 490–496 (1998).
Stingl, J. & Stembera, O. Distribution and ultrastructure of the initial lymphatics of some skeletal muscles in the rat. Lymphology 7, 160–168 (1974).
Skalak, T. C., Schmid-Schonbein, G. W. & Zweifach, B. W. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc. Res 28, 95–112 (1984).
Havas, E. et al. Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J. Physiol. 504, 233–239 (1997).
Pan, X. et al. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int Immunopharmacol. 75, 105742 (2019).
Doan, T. N., Bernard, F. C., McKinney, J. M., Dixon, J. B. & Willett, N. J. Endothelin-1 inhibits size dependent lymphatic clearance of PEG-based conjugates after intra-articular injection into the rat knee. Acta Biomater. 93, 270–281 (2019).
Bouta, E. M. et al. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat. Rev. Rheumatol. 14, 94–106 (2018).
Liang, Q. et al. Lymphatic muscle cells contribute to dysfunction of the synovial lymphatic system in inflammatory arthritis in mice. Arthritis Res. Ther. 23, 58 (2021).
Tong, L. et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 10, 60 (2022).
Fuller, K. M. & Munro, R. R. Lymphatic drainage of bone marrow. Aust. N. Z. J. Surg. 34, 11–14 (1964).
Greenbaum, A. et al. Bone CLARITY: Clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci. Transl. Med 9, eaah6518 (2017).
Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803–818 (2018).
Smith, R. J., Sage, H. H., Miyazaki, M. & Kizilay, D. The relationship of lymphatics to bone. Bull. Hosp. Jt. Dis. 21, 25–41 (1960).
Griffie, R. A. & Ecoiffier, J. Demonstration of the lymphatics by intra-osseous injections. C. R. Hebd. Seances Acad. Sci. 246, 635–636 (1958).
Olszewski, W. L. & Sawicki, Z. Radiological evidence of lymphatic drainage of bone marrow cavity in long bones. Lymphology 10, 1–4 (1977).
Vittas, D. & Hainau, B. Lymphatic capillaries of the periosteum: do they exist?. Lymphology 22, 173–177 (1989).
Lorenz, M. & Plenk, H. Jr. A perfusion method of incubation to demonstrate horseradish peroxidase in bone. Histochemistry 53, 257–263 (1977).
Dillaman, R. M. Movement of ferritin in the 2-day-old chick femur. Anat. Rec. 209, 445–453 (1984).
Seliger, W. G. Tissue fluid movement in compact bone. Anat. Rec. 166, 247–255 (1970).
Montgomery, R. J., Sutker, B. D., Bronk, J. T., Smith, S. R. & Kelly, P. J. Interstitial fluid flow in cortical bone. Microvasc. Res. 35, 295–307 (1988).
Bazantova, I. The blood and lymph bed in Haversian bone. Folia Morphol. 37, 213–215 (1989).
Dillaman, R. M., Roer, R. D. & Gay, D. M. Fluid movement in bone: theoretical and empirical. J. Biomech. 24, 163–177 (1991).
McCarter, A. L. et al. Bone development and fracture healing is normal in mice that have a defect in the development of the lymphatic system. Lymphology 53, 162–171 (2020).
Zheng, Y. et al. Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs. Nat. Commun. 16, 829 (2025).
Kliskey, K. et al. The presence and absence of lymphatic vessels in the adult human intervertebral disc: relation to disc pathology. Skelet. Radio. 38, 1169–1173 (2009).
Rudert, M. & Tillmann, B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop. Scand. 64, 37–40 (1993).
Khadanovich, A. et al. Anatomy of the diaphragmatic crura and other paraspinal structures relevant to en-bloc spondylectomy for lumbar spine tumours. Eur. Spine J 34, 3532–3539 (2025).
Fu, Y. et al. The paravertebral lymphatic system is involved in the resorption of the herniated nucleus pulposus and the regression of inflammation associated with disc herniation. Osteoarthr. Cartil. 32, 1566–1578 (2024).
Lin, M. et al. Machine learning-based diagnostic model of lymphatics-associated genes for new therapeutic target analysis in intervertebral disc degeneration. Front. Immunol. 15, 1441028 (2024).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 18001 (2018).
O’Byrne, A. M. et al. Divergent gene signatures and neutrophil enrichment in lymph nodes of inflammatory arthritis patients. Arthritis Res. Ther. 27, 94 (2025).
Chen, Y. et al. The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway. Microcirculation 24, 10.1111 (2017).
Shibuya, M. & Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 312, 549–560 (2006).
Wauke, K., Nagashima, M., Ishiwata, T., Asano, G. & Yoshino, S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J. Rheumatol. 29, 34–38 (2002).
Zhou, Q., Wood, R., Schwarz, E. M., Wang, Y. J. & Xing, L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis Rheum. 62, 1881–1889 (2010).
Li, J. et al. Expanded CD23+/CD21hi B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. J. Immunol. 184, 6142–6150 (2010).
Zhang, Q. et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res. Ther. 9, R118 (2007).
Scallan, J. P. et al. Ex vivo demonstration of functional deficiencies in popliteal lymphatic vessels from TNF-transgenic mice with inflammatory arthritis. Front Physiol. 12, 745096 (2021).
Bell, R. D. et al. iNOS dependent and independent phases of lymph node expansion in mice with TNF-induced inflammatory-erosive arthritis. Arthritis Res. Ther. 21, 240 (2019).
Guo, R. et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 60, 2666–2676 (2009).
Li, P. et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 50, 265–276 (2004).
Watari, K. et al. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem. Biophys. Res. Commun. 377, 826–831 (2008).
Walsh, D. A. et al. Lymphatic vessels in osteoarthritic human knees. Osteoarthr. Cartil. 20, 405–412 (2012).
Scanzello, C. R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 29, 79–85 (2017).
Michalaki, E. et al. Effect of human synovial fluid from osteoarthritis patients and healthy individuals on lymphatic contractile activity. J. Biomech. Eng 144, 071012 (2022).
Lin, X. et al. Targeting synovial lymphatic function as a novel therapeutic intervention for age-related osteoarthritis in mice. Arthritis Rheumatol. 75, 923–936 (2023).
Zhou, C., Sun, T., Dong, Z., Lu, F. & Li, B. The interplay between lymphatic vessels and macrophages in inflammation response. FASEB J. 38, e23879 (2024).
Wheeler, T. A. et al. Mechanical loading of joint modulates T cells in lymph nodes to regulate osteoarthritis. Osteoarthr. Cartil. 32, 287–298 (2024).
de Jesus, F. N. & von der Weid, P. Y. Increased contractile activity and dilation of popliteal lymphatic vessels in the TNF-α-overexpressing TNF(ΔARE/+) arthritic mouse. Life Sci. 335, 122247 (2023).
Pal, S., Meininger, C. J. & Gashev, A. A. Aged Lymphatic Vessels and Mast Cells in Perilymphatic Tissues. Int. J. Mol. Sci 18, 965 (2017).
Kenney, H. M. et al. Persistent popliteal lymphatic muscle cell coverage defects despite amelioration of arthritis and recovery of popliteal lymphatic vessel function in TNF-Tg mice following anti-TNF therapy. Sci. Rep. 12, 12751 (2022).
Song, X. et al. An injectable thermosensitive hydrogel delivering M2 macrophage-derived exosomes alleviates osteoarthritis by promoting synovial lymphangiogenesis. Acta Biomater. 189, 130–142 (2024).
Wang, W. et al. Attenuated joint tissue damage associated with improved synovial lymphatic function following treatment with Bortezomib in a mouse model of experimental posttraumatic Osteoarthritis. Arthritis Rheumatol. 71, 244–257 (2019).
Grillet, B. et al. Matrix metalloproteinases in arthritis: towards precision medicine. Nat. Rev. Rheumatol. 19, 363–377 (2023).
Mobasheri, A. et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13, 302–311 (2017).
Kormano, M. & Makela, P. Lymphatics filled at knee arthrography. Acta Radio. Diagn. 19, 853–858 (1978).
Wallis, W. J., Simkin, P. A., Nelp, W. B. & Foster, D. M. Intraarticular volume and clearance in human synovial effusions. Arthritis Rheum. 28, 441–449 (1985).
Li, Z. & Tian, Y. Role of NEL‑like molecule‑1 in osteogenesis/chondrogenesis (Review). Int. J. Mol. Med 55, 5 (2025).
Bi, J. et al. Age-related bone diseases: Role of inflammaging. J. Autoimmun. 143, 103169 (2024).
Vishlaghi, N. et al. Vegfc-expressing cells form heterotopic bone after musculoskeletal injury. Cell Rep. 43, 114049 (2024).
Lips, K. S. et al. Podoplanin immunopositive lymphatic vessels at the implant interface in a rat model of osteoporotic fractures. PLoS One 8, e77259 (2013).
Zhao, C. et al. Preventing periprosthetic osteolysis in aging populations through lymphatic activation and stem cell-associated secretory phenotype inhibition. Commun. Biol. 7, 962 (2024).
Zheng, Y. et al. A novel therapy for fracture healing by increasing lymphatic drainage. J. Orthop. Transl. 45, 66–74 (2024).
Qin, Z. et al. Targeting endoplasmic reticulum stress-induced lymphatic dysfunction for mitigating bisphosphonate-related osteonecrosis. Clin. Transl. Med 14, e70082 (2024).
Tanoue, R., Koi, K. & Yamashita, J. Effect of Alendronate on bone formation during tooth extraction wound healing. J. Dent. Res 94, 1251–1258 (2015).
Nozawa, A. et al. A somatic activating KRAS variant identified in an affected lesion of a patient with Gorham-Stout disease. J. Hum. Genet. 65, 995–1001 (2020).
Rodriguez-Laguna, L. et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J. Exp. Med. 216, 407–418 (2019).
Manevitz-Mendelson, E. et al. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 21, 287–298 (2018).
Liu, Y. et al. Gorham-Stout disease: radiological, histological, and clinical features of 12 cases and review of literature. Clin. Rheumatol. 35, 813–823 (2016).
Gorham, L. W. & Stout, A. P. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J. Bone Jt. Surg. Am. 37-A, 985–1004 (1955).
Kato, H., Ozeki, M., Fukao, T. & Matsuo, M. MR imaging findings of vertebral involvement in Gorham-Stout disease, generalized lymphatic anomaly, and kaposiform lymphangiomatosis. Jpn J. Radio. 35, 606–612 (2017).
Ricci, K. W. & Iacobas, I. How we approach the diagnosis and management of complex lymphatic anomalies. Pediatr. Blood Cancer 69, e28985 (2022).
Fernandes, L. M. et al. Hyperactive KRAS/MAPK signaling disrupts normal lymphatic vessel architecture and function. Front. Cell Dev. Biol. 11, 1276333 (2023).
Monroy, M., McCarter, A. L., Hominick, D., Cassidy, N. & Dellinger, M. T. Lymphatics in bone arise from pre-existing lymphatics. Development 147, dev184291 (2020).
McCarter, A. L. & Dellinger, M. T. Trametinib inhibits lymphatic vessel invasion of bone in a mouse model of Gorham-Stout disease. J. Vasc. Anom 4, e070 (2023).
Ricci, K. W. et al. Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham-Stout disease. Pediatr. Blood Cancer 66, e27614 (2019).
Trenor, C. C. 3rd & Chaudry, G. Complex lymphatic anomalies. Semin Pediatr. Surg. 23, 186–190 (2014).
Kuwahara, G., Nishinakamura, H., Kojima, D., Tashiro, T. & Kodama, S. Vascular endothelial growth factor-C derived from CD11b+ cells induces therapeutic improvements in a murine model of hind limb ischemia. J. Vasc. Surg. 57, 1090–1099 (2013).
Qin, X. et al. In situ size amplification strategy reduces lymphatic clearance for enhanced arthritis therapy. J. Nanobiotechnol. 22, 755 (2024).
Homayun-Sepehr, N. et al. KRAS-driven model of Gorham-Stout disease effectively treated with trametinib. JCI Insight 6, e149831 (2021).
Zhang, D. et al. Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway. Nat. Commun. 12, 4391 (2021).
Moriondo, A., Solari, E., Marcozzi, C. & Negrini, D. Diaphragmatic lymphatic vessel behavior during local skeletal muscle contraction. Am. J. Physiol. Heart Circ. Physiol. 308, H193–H205 (2015).
Mazzoni, M. C., Skalak, T. C. & Schmid-Schonbein, G. W. Effects of skeletal muscle fiber deformation on lymphatic volumes. Am. J. Physiol. 259, H1860–H1868 (1990).
Taketa, Y. et al. Lymphatic capillarization in different fiber types of rat skeletal muscles with growth and age. Microcirculation 31, e12879 (2024).
Cocks, M. & Wagenmakers, A. J. The effect of different training modes on skeletal muscle microvascular density and endothelial enzymes controlling NO availability. J. Physiol. 594, 2245–2257 (2016).
Coates, G., O’Brodovich, H. & Goeree, G. Hindlimb and lung lymph flows during prolonged exercise. J. Appl Physiol. (1985) 75, 633–638 (1993).
Lu, X. et al. Cranial bone maneuver ameliorates Alzheimer’s disease pathology via enhancing meningeal lymphatic drainage function. Alzheimers Dement 21, e14518 (2025).
Yeo, K. P., Lim, H. Y. & Angeli, V. Leukocyte Trafficking via Lymphatic Vessels in Atherosclerosis. Cells 10, 1344 (2021).
González-Loyola, A. & Petrova, T. V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 169, 63–78 (2021).
Duan, M. et al. Preparation of the biodegradable lymphatic targeting imaging agent based on the indocyanine green mesoporous silicon system. Front Chem. 10, 847929 (2022).
Huo, S. et al. Epigenetic regulations of cellular senescence in osteoporosis. Ageing Res. Rev. 99, 102235 (2024).
Zhao, J. et al. Bmi-1 epigenetically orchestrates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells to delay bone aging. Adv. Sci. 11, e2404518 (2024).
Boueya, I. L., Sandhow, L., Albuquerque, J. R. P., Znaidi, R. & Passaro, D. Endothelial heterogeneity in bone marrow: insights across development, adult life and leukemia. Leukemia 39, 8–24 (2025).
Davis, M. J., Zawieja, S. D. & King, P. D. Transport and immune functions of the lymphatic system. Annu Rev. Physiol. 87, 151–172 (2025).
Bano, F. et al. Structure and unusual binding mechanism of the hyaluronan receptor LYVE-1 mediating leucocyte entry to lymphatics. Nat. Commun. 16, 2754 (2025).
Sanchez-Lopez, E., Coras, R., Torres, A., Lane, N. E. & Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 18, 258–275 (2022).
Dainese, P. et al. Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthr. Cartil. 30, 516–534 (2022).
Robinson, W. H. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 580–592 (2016).
Schuster, R., Rockel, J. S., Kapoor, M. & Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 302, 126–146 (2021).
Kakei, Y., Akashi, M., Shigeta, T., Hasegawa, T. & Komori, T. Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphat. Res. Biol. 12, 136–143 (2014).
Huh, Y. M. et al. The role of popliteal lymph nodes in differentiating rheumatoid arthritis from osteoarthritis by using CE 3D FSPGR MR imaging: relationship of the inflamed synovial volume. Korean J. Radio. 6, 117–124 (2005).
Manferdini, C. et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthr. Cartil. 25, 1161–1171 (2017).
Zhang, Q. et al. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J. Biol. Chem. 283, 13491–13499 (2008).
Bouta, E. M. et al. Brief Report: Treatment of tumor necrosis factor-transgenic mice with anti-tumor necrosis factor restores lymphatic contractions, repairs lymphatic vessels, and may increase monocyte/macrophage Egress. Arthritis Rheumatol. 69, 1187–1193 (2017).
Aldrich, M. B. et al. Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis. Arthritis Res. Ther. 19, 116 (2017).
Polomska, A. K. & Proulx, S. T. Imaging technology of the lymphatic system. Adv. Drug Deliv. Rev. 170, 294–311 (2021).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Kuang, L. et al. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann. Rheum. Dis. 79, 112–122 (2020).
Klaourakis, K., Vieira, J. M. & Riley, P. R. The evolving cardiac lymphatic vasculature in development, repair and regeneration. Nat. Rev. Cardiol. 18, 368–379 (2021).
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2023YFB4606705, No. 2023YFC3603400), National Natural Science Foundation of China (No. 82272611, 82072506, 92268115, 82472522), Hunan Provincial Science Fund for Distinguished Young Scholars (No. 2024JJ2089), Science and Technology Innovation Program of Hunan Province (No. 2023SK2024, 2021RC3025), National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No.2021KFJJ02, 2021LNJJ05), Natural Science Foundation of Hunan Province (No.2023JJ30949), the Program of Health Commission of Hunan Province (202204074879). The study sponsors had no role in the study design, collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Yan Luo and Jie Song were the major contributors to literature reviewing, manuscript writing, and descriptive figures creating; Yan Luo, Jie Song, Shengyuan Zheng, Jianfeng Sun, Gaoming Liu and Zirui Xiao were the major contributors to screen the literature; Yusheng Li and Wenfeng Xiao were the major contributors to design the study; Djandan Tadum Arthur Vithran and Jiaxue Zhou assisted in the literature review and tables editing; Yan Luo and Jie Song were major contributors to manuscript revision; Djandan Tadum Arthur Vithran was responsible for the content flow and language editing; The final manuscript was read and approved by all authors. Figures 1–7 in the review are created in https://BioRender.com.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Y., Song, J., Zheng, S. et al. The role of the lymphatic system in musculoskeletal system health and disease: research progress and future directions. Bone Res 14, 8 (2026). https://doi.org/10.1038/s41413-025-00479-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41413-025-00479-0