Abstract
Bone regeneration is initiated after a bone injury, such as a bone fracture or tooth extraction. It is a highly complex biological process involving multiple cell types, signaling molecules, and molecular pathways. The hypoxic microenvironment in the early stage of bone regeneration poses challenges to cell status and the final outcome of bone regeneration. During this phase, two key regulators—HIF-1α (the critical mediator of hypoxia response) and BMAL1 (the central component of the circadian rhythm)—orchestrate the activities of bone-regenerating cells, ensuring proper cellular function and orderly progression of bone repair. Existing studies have shown that there is a close crosstalk between HIF-1α and BMAL1, including regulation of gene expression, protein interaction, and regulation of downstream pathways. In this review, we discuss the respective regulatory roles of HIF-1α and BMAL1 in bone regeneration and further summarize their interactions within cells. Additionally, we extend the discussion to their interactions in other bone-related diseases, and summarize the existing research directions and deficiencies, providing new insights for in-depth studies of the hypoxia response and circadian rhythm systems.
Similar content being viewed by others
Introduction
Bone is a metabolically active organ, wherein numerous diverse cell types actively participate in intricate bone metabolic processes. This enables the maintenance of structural and functional stability of the bone, as well as its capacity for self-repair and regeneration when injured.1,2 Bone regeneration is commonly observed in the wound following bone fracture or tooth extraction.3,4 The structure, continuity, and function of the local bone tissue in both conditions are disrupted, necessitating initiation of the repair process, execution of bone remodeling, and completion of bone regeneration. Investigating the mechanisms influencing bone regeneration to further regulate and promote this process represents a current research focus.
The process of bone regeneration is a highly intricate biological process, encompassing various cellular types, signaling molecules, and molecular pathways. It follows a precise temporal and spatial sequence to accomplish the induction and conduction of bone formation, ultimately leading to the complete repair of bone structure and restoration of functionality.5,6,7 Bone regeneration involves a series of metabolic processes and can be described by three overlapping phases: the inflammatory phase, the proliferative phase, and the modeling phase.3,8 Among them, the response during the inflammatory phase significantly determines the ultimate outcome of bone healing.9 Hypoxia resulting from vascular disruption activates specific transcription factors essential to the repair process, thereby inducing cellular metabolic reprogramming.10,11,12 The intercellular communication between immune cells and bone mesenchymal stem cells (BMSCs) plays a pivotal role in regulating this process.8,13 Therefore, the identification of key molecules that regulate inflammatory responses and cellular biological behavior has emerged as a promising target for the treatment of diseases associated with bone remodeling disorders.
At the initial stage of bone regeneration, the disruption of blood vessels during bone trauma leads to the formation of a hematoma, which isolates the site of injury from adequate blood perfusion, thereby exacerbating local hypoxia.14 Hypoxia-inducible factor-1 (HIF-1) serves as a pivotal transcriptional regulator in cellular response to hypoxia.15 HIF-1 consists of HIF-1α and HIF-1β subunits, which interact with the hypoxia-responsive element (HRE) to further modulate the expression of target genes. Under normoxic conditions, HIF-1α is degraded via the ubiquitination pathway mediated by proteins such as prolyl hydroxylase domain (PHD) enzymes and the von Hippel–Lindau (VHL) tumor suppressor protein.16,17,18 However, hypoxia inhibits the hydroxylation of HIF-1α, causing its accumulation in the nucleus where it binds to HIF-1β and initiates transcription of hypoxia-related genes such as vascular endothelial growth factor (VEGF).19,20,21,22 Therefore, the hypoxic microenvironment during bone regeneration plays a critical role in regulating HIF-1α signaling. HIF-1α is involved in the regulation of bone regeneration, which is primarily mediated through two distinct mechanisms: (1) Facilitating the synergistic interaction between angiogenesis and osteoblast activity to enhance bone formation; and (2) orchestrating metabolic adaptation in osteoblasts by upregulating anaerobic glycolysis pathways, thereby sustaining cellular bioenergetics in hypoxic microenvironments and potentiating bone repair processes.23
In addition to the hypoxia-mediated HIF-1α pathway, circadian rhythm also plays an important role in bone regeneration. The circadian rhythm is regulated by a group of central clock genes, among which Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (Bmal1) is a frequently mentioned gene in regulating bone metabolism and bone homeostasis.24 These genes form negative feedback loops that regulate the circadian rhythm of biological metabolism through transcription and translation processes. BMAL1 and Circadian Locomotor Output Cycles Kaput (CLOCK) can form a heterodimer that binds to intragenic E-boxes, stimulating the transcription and translation of genes.25,26 BMAL1 is the core of the circadian rhythm. Knocking out the Bmal1 gene in mice results in the immediate and complete loss of circadian rhythms in continuous darkness, providing direct evidence for its indispensable role in the generation of circadian rhythms.27 A significant body of research has demonstrated a close relationship between circadian rhythm and the physiological activities of bone. The circadian rhythm plays a crucial role in endochondral ossification, facilitating rapid DNA replication in cells during the day, cell mitosis, and matrix synthesis in the evening.28 Through its effects on key factors like osteoblast-mediated bone formation, osteoclast-driven resorption, and communication between these two cell types, circadian rhythms can modulate the balance between bone formation and resorption during bone remodeling.28,29,30,31
Intriguingly, many studies have shown that hypoxia and circadian rhythms interact and mutually regulate each other.32 The circadian rhythm influences cellular responses to hypoxia,33 while hypoxia can disrupt the balance of the biological clock.34 BMAL1 has also been identified in various cell types as a regulator of HIF-1 activity under low oxygen conditions.35 Current studies indicate a certain relationship between BMAL1 and HIF-1α in osteogenesis,36 where BMAL1 shares some structural similarities with HIF-1α.37,38 Under specific hypoxic conditions, BMAL1 forms a heterodimer with HIF-1α to regulate physiological processes.35,39,40,41 Above evidence indicates the crosstalk between BMAL1 and the hypoxic microenvironment in modulating bone regeneration, though the specific regulatory mechanism remains unclear. This manuscript reviews the role of HIF-1α and BMAL1 in regulating bone regeneration, discusses the crosstalk between hypoxia response and circadian rhythm in bone regeneration, and summarizes their possible role in oral and systemic bone metabolic diseases.
HIF-1α-mediated cellular response during the inflammatory phase
Progression of the inflammatory phase
The inflammatory phase can be further divided into two components: the formation of blood clots and the migration of inflammatory cells. For example, tooth extraction leads to rupture of blood vessels in and around the socket, resulting in immediate bleeding and local hematoma formation.8 Damaged blood vessels and cells release proteins that initiate the coagulation cascade, leading to clot formation.8 This can lead to blockage of blood supply in the wound area, resulting in hypoxia, exacerbating inflammation, acidity, and lower temperature, and is rich in calcium and lactic acid.42,43 Hypoxia affects various biological processes such as cell proliferation, differentiation, migration, chemotaxis, phagocytosis, and apoptosis.
Blood clots contain numerous growth factors that induce and facilitate the migration of neutrophils, monocytes/macrophages, etc., to the site of injury.43 According to the measurement of bone aspirates, the oxygen level reaching the bone tissue is approximately 6.6%–8.6%,44 while the rupture of blood vessels at the fracture site will cause further local hypoxia, with the pO2 dropping to 0.8%–3%.45 Following the release of inflammatory signals from local tissues at the defect site during the inflammatory stage, neutrophils are typically the first and most abundant immune cells to arrive.46 They subsequently orchestrate the recruitment of other immune cell types and BMSCs by releasing cytokines, thereby establishing an appropriate immune microenvironment that initiates the bone regeneration process.47 Simultaneously, these cells undergo differentiation and heightened activity, thereby initiating an inflammatory response aimed at removing dead cells and tissue debris from the area.48 Within 2–3 days following tooth extraction, a significant influx of inflammatory cells migrates to the defect site, where they merge with vascular buds and immature fibroblasts to form granulation tissue.3 Vascular endothelial cells also undergo extensive proliferation, giving rise to numerous new blood vessels within the matrix that facilitate oxygen and nutrient supply for an increasing number of cells.49,50 Then, bone marrow mesenchymal stem cells (BMSCs) accumulate, undergo proliferation, and deposit extracellular matrix within the defect area, gradually replacing blood clots.51 Subsequently, BMSCs surround these newly formed blood vessels and proceed to proliferate and differentiate into osteoblasts in order to directly generate woven bone.52 The formation of woven bone can be observed within a time frame as short as 2 weeks following tooth extraction in humans.3,53
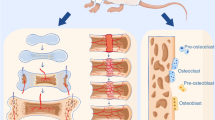
As the blood supply is established, the oxygen pressure gradually returns to normal. And the recovery of oxygen pressure is associated with the sequence in which cells appear. During bone regeneration, the level gradually decreases until it starts to rise again after about 2–3 days, and it takes 2–3 weeks to return to the highest level.13 Neutrophils arrive at the fracture site within hours, accompanying hematoma formation. Subsequently, by approximately 3 days, endothelial cells and immune cells such as macrophages appear. Mesenchymal stem cells are recruited by approximately 5 days, followed by the emergence of osteoblasts around 7 days. By 2 weeks, osteoblasts and osteoclasts couple to initiate the bone remodeling process.2,3,54 It is worth noting that, although blood perfusion has returned, these cells are still working in a hypoxic environment, and the degree of hypoxia is gradually changing. Under such challenging hypoxic conditions, each healing process initiates with a phylogenetically conserved inflammatory response. However, its spatial and temporal intensity must be strictly regulated.9 Pro-inflammatory signaling constitutes a critical event for launching the healing cascade and facilitates the recruitment of diverse cell types. Yet the timing of this process is paramount—if pro-inflammatory signals persist abnormally, healing becomes delayed or even arrested.55 Consequently, successful bone regeneration demands precise signal control to navigate the cellular challenges posed by shifting oxygen environments: the transition from normoxia (before bone defect) to hypoxia (during bone regeneration) and then back to normoxia (after bone regeneration). This regulatory mechanism may be mediated by the integration of hypoxia response and circadian rhythm. Concurrent with the restoration of oxygen tension sensed by PHDs, HIF-1α undergoes progressive degradation, leading to a declining role in cellular regulation.16,17,18,56 Meanwhile, the improved oxygen availability alleviates the hypoxic suppression of BMAL1, thereby enabling its transcriptional activity and promoting a pro-regenerative outcome in bone regeneration.57 A schematic of the temporal changes in blood perfusion, HIF-1α, BMAL1, and the sequence of cell recruitment is presented in Fig. 1.
Temporal changes during bone regeneration. The blood flow in the bone defect area initially decreased due to vascular rupture and contraction. Subsequently, the blood supply gradually recovered as new blood vessels formed. The activities of HIF-1α and BMAL1 are altered. HIF-1α is activated by initial hypoxia, while BMAL1 is suppressed; both subsequently change as oxygen levels recover. The relative regulatory weight is represented as the change in the dominant role of the two in cellular regulation. The sequential appearance of different cell types is shown at the bottom
HIF-1α-mediated cellular response
Neutrophils
Neutrophils play both positive and negative roles in bone regeneration.58 Herath et al. demonstrated that local application of autologous neutrophils significantly enhanced bone regeneration in a rabbit calvarial defect model. The positive effect was particularly evident at the early healing stage (4 weeks), and repetitive application yielded superior outcomes compared to a single application.59 However, an excessive number of neutrophils will lead to disorders in bone regeneration.60,61 Uncontrolled inflammatory factors attract excessive neutrophils, which inhibit the osteogenic differentiation of BMSCs and subsequent mineralization.62 The formation of neutrophil extracellular traps (NETs) inhibits the migration and differentiation of MSCs, resulting in cell death and compromised bone formation.63 Consequently, the proper functioning of neutrophils critically influences the outcome of bone regeneration.
The initial stage of the inflammatory period of bone regeneration is an acute hypoxic microenvironment. The response of neutrophils to local oxygen tension is regulated by HIF-1α.64 Neutrophil degranulation is an important form in which it exerts its functions.65 Research has shown that hypoxia triggers a rapid degranulation of neutrophils within a short period of time (2 h),66,67 but this process does not seem to rely on HIF-1α at this stage.68 However, the subsequent degranulation reaction (lasting for 24 h) was HIF-1α-dependent, manifested by an increase in the expression of the MMP-8 gene in the promoter region that contains HREs.67 Furthermore, HIF-1α plays a direct role in the regulation of neutrophil apoptosis. The neutrophils isolated from the bone marrow of myeloid-targeted HIF-1α knockout mice showed a significant decrease in survival rate under hypoxic conditions (Fig. 2b).69 In summary, neutrophils play a balanced role in bone regeneration, where their beneficial effects are concentration-dependent. The hypoxic fracture microenvironment critically regulates their sustained functions, such as degranulation and survival, primarily through HIF-1α signaling. Thus, HIF-1α emerges as a key modulator of neutrophil activity, ensuring a controlled inflammatory response that supports effective bone healing.
Cellular response under hypoxic conditions. a Six cell types—neutrophils, macrophages, endothelial cells, mesenchymal stem cells, osteoblasts, and osteoclasts—participate in bone regeneration, all of which are influenced by the hypoxic microenvironment during the inflammatory phase. b Under hypoxic conditions, HIF-1α binds to the HRE of MMP-8 to promote its expression, thereby facilitating the degranulation reaction of neutrophils. Knocking out HIF-1α will weaken the anti-apoptotic ability of neutrophils. c Under hypoxic conditions, the crosstalk between HIF-1α and NFκB pathways activates macrophage inflammatory responses and promotes M1 polarization. d Hypoxia-induced HIF-1α activation upregulates VEGF expression and enhances glycolysis in endothelial cells, thereby promoting angiogenesis. e Under hypoxic conditions, the intracellular oxidative stress level in BMSCs is significantly elevated, and their osteogenic differentiation potential is inhibited. f Hypoxic condition activates the TNF-α pathway within osteoblasts, inhibit the PI3K/Akt pathway, aggravate oxidative stress response, promote osteoblast apoptosis, and inhibit osteogenic ability. g Under hypoxic conditions, osteoclasts exhibit elevated oxidative stress levels and receive signaling molecules (ATP, RANKL) from osteoblasts, which collectively enhance intracellular glycolysis and ultimately lead to increased bone resorption activity
Macrophages
During the inflammatory phase, macrophages not only phagocytose necrotic cells and tissue debris at the fracture site but also recruit mesenchymal stem cells and vascular progenitor cells from the periosteum, bone marrow, and blood circulation.70 Depending on their environment, macrophages can polarize their phenotype into either a pro-inflammatory (M1) or anti-inflammatory (M2) category.71 Under hypoxic conditions, macrophages exhibited marked upregulation in the expression of inflammatory cytokines (interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α)) and NLRP3 inflammasome components (NLRP3, ASC, pro-caspase-1), demonstrating a pronounced inflammatory response.72 During the inflammatory phase of bone regeneration, acute hypoxia activates macrophage nuclear factor kappa-B (NF-κB) signaling, amplifying inflammation. This hypoxia-inflammation interplay involves HIF-1α (hypoxia regulator) and NF-κB (inflammation regulator) interaction.73 HIF-1α promotes IL-1β production and drives inflammation by shifting activated macrophage metabolism from oxidative phosphorylation to glycolysis, with the resulting lactate serving as a key mediator of macrophage phenotype through epigenetic and chromatin-mediated gene regulation.74 Ultimately, the glycolytic pathway may influence macrophage polarization toward an M1 phenotype.71 Consequently, within the hypoxic microenvironment during the inflammatory phase of bone regeneration, macrophages manifest potent pro-inflammatory effects mediated by HIF-1α (Fig. 2c).
While studies specifically examining the effects of myeloid HIF-1α deletion on macrophages in the context of bone regeneration are currently lacking, insights from other pathological conditions highlight its essential role in regulating macrophage survival and function. Following LPS stimulation in myeloid-specific HIF-1α knockout mice (LysM-Cre, Hif-1αflox/flox), Karshovska et al. observed that the absence of HIF-1α resulted in the upregulation of anti-inflammatory markers, including Mrc1 and Ym1, in macrophages.75 Furthermore, their investigation revealed that Hif-1α deletion enhanced oxidative phosphorylation, elevated ATP levels, and upregulated the expression of genes related to mitochondrial proteins. This metabolic shift consequently led to a reduction in reactive oxygen species production and necroptosis. Scheerer et al.76 reported that myeloid HIF-1α deficiency(LysM-Cre, Hif-1αflox/flox) resulted in a critical defect in clearing necrotic cell debris and regenerating endothelial networks following soft tissue injury. This defect was attributed to the delayed invasion of macrophages into the damaged site. In summary, HIF-1α is a pivotal driver of the potent pro-inflammatory response in macrophages within the hypoxic fracture microenvironment, primarily by promoting a glycolytic metabolic reprogramming. However, the specific impact of disrupting this pathway via myeloid-specific HIF-1α deletion on macrophage function and the overall bone regeneration process remains an open and critical question.
Endothelial cells
The development and maintenance of the functional circulatory system rely on the proliferation, migration, differentiation, and apoptosis of endothelial cells (ECs).77 Following the bone defect, there is a substantial increase in the proliferation of vascular endothelial cells, which promotes neovascularization and supplies oxygen and nutrients to the damaged area.49,50 Hypoxic microenvironment promotes intracellular VEGF expression, thereby enhancing angiogenic capacity.78 This occurs because hypoxia induces HIF-1α accumulation and nuclear translocation, enabling its binding to the Vegf gene promoter and subsequent transcriptional activation.79 VEGF plays a crucial role in angiogenesis by activating genes related to blood vessel formation. The expression of VEGF can be promoted by hypoxic environments, inflammation-related cytokines, and hormones.80 Endothelial cell-specific knockout of HIF-1α (Tie-2-Cre, HIF-1αloxP/loxP) disrupts a hypoxia-driven VEGF autocrine loop, leading to impaired proliferation, migration, and tube formation of endothelial cells.81
ECs also respond to hypoxia through metabolic alterations. Hypoxia induces metabolic reprogramming in ECs, characterized by enhanced glycolysis and pentose phosphate pathway (PPP) activity alongside reduced tricarboxylic acid (TCA) cycle flux and mitochondrial respiration. Notably, inhibition of either glycolysis or PPP (or conversely, promotion of mitochondrial respiration) reverses hypoxia’s capacity to maintain cellular stemness.82 Under hypoxic conditions, the forward TCA cycle decreases, while the reverse TCA cycle increases. Pharmacological inhibition of the reverse TCA cycle attenuates Hif-1α and Vegf expression, ultimately impairing ECs proliferation and angiogenic potential (Fig. 2d).83 Specific knockout of endothelial HIF-1α (Tie-2-Cre, HIF-1αloxP/loxP) downregulated Glucose transporter-1 (Glut1) expression and consequently impaired tissue glucose utilization.84 Together, these findings establish HIF-1α as a central hub in ECs, integrating hypoxic signaling, gene expression, and metabolic adaptation to support vascularized bone repair.
BMSCs
The efficacy of bone regeneration is largely determined by the cellular state of BMSCs, with varying degrees of hypoxia exerting differential effects. Several studies have investigated the impact of different oxygen concentrations on BMSCs. When cultured under moderate hypoxia (5% O₂), BMSCs exhibit suppressed senescence and enhanced osteogenic differentiation efficiency.85,86,87,88 However, more severe hypoxia may inhibit osteoblast growth and differentiation, ultimately impairing bone formation.89 Under extreme hypoxia (1%–2% O₂), the Notch1 and extracellular signal-regulated kinase 1/2 (ERK1/2)/p38 mitogen-activated protein kinase (MAPK) signaling pathways are activated in BMSCs, leading to suppressed osteogenic differentiation.90,91,92 Hypoxia reduces both the expression and activity of Runt-related transcription factor 2 (RUNX2), and the differentiation of mesenchymal cells into osteoblasts is reduced.93 Furthermore, hypoxia downregulates Runx2 expression in pre-osteoblasts while promoting receptor activator of NF-κB ligand (RANKL) synthesis. Subsequent hypoxia and/or RANKL stimulation enhances tartrate-resistant acid phosphatase (TRAP) production in macrophages.94
The hypoxic microenvironment reduces osteogenic efficacy during bone regeneration, not only by directly reducing cellular activity but also by promoting the accumulation of pro-inflammatory mediators such as reactive oxygen species (ROS).95 Elevated ROS levels can induce apoptosis in both osteoblast precursor cells and mature osteoblasts, while simultaneously suppressing the expression of osteogenic markers including alkaline phosphatase (ALP), osteocalcin (OCN), and RUNX2, thereby impairing BMSC osteogenic differentiation (Fig. 2e).96,97,98 However, hypoxia-activated HIF-1α signaling may exert protective effects on BMSC function. The HIF-1α pathway enhances BMSC proliferation and migration. Pharmacological stabilization of HIF-1α using IOX2 (a prolyl hydroxylase domain inhibitor) increases intracellular Ca²⁺ while reducing ROS levels, significantly improving cellular proliferation/migration, and facilitating fracture repair.99 Similar pro-regenerative effects were observed with another HIF-1α-stabilizing agent, which likewise elevated BMSC proliferative and migratory capacity.100 HIF-1α overexpression promoted the repair of rat calvarial defects in vivo by enhancing vascularization, which was achieved through the significant upregulation of key angiogenic factors (VEGF, SDF-1, bFGF, PLGF, ANGPT1, and SCF) at both the mRNA and protein levels in BMSCs.101 Furthermore, overexpression of HIF-1α can also significantly enhance the osteogenic differentiation ability of BMSCs.102 Nevertheless, the net outcome of hypoxia on BMSCs appears to depend on the balance between its inhibitory effects—mediated partly by oxidative stress and inflammatory pathways—and the protective, pro-regenerative responses orchestrated by HIF-1α signaling. While in vitro models have limitations in fully recapitulating the in vivo hypoxic condition, current evidence suggests that strategies aimed at stabilizing HIF-1α or reducing oxidative stress can enhance BMSC function and support bone regeneration.
Osteoblasts
Hypoxic microenvironments delay osteoblasts’ growth and differentiation, significantly impairing bone formation capacity.89 This occurs through hypoxia-mediated suppression of matrix mineralization-related enzymes, as well as reduced expression and activity of alkaline phosphatase.89 Furthermore, hypoxia inhibits the PI3K/Akt pathway, interfering with the anti-apoptotic ability of osteoblasts.103 The concomitant inflammatory microenvironment under hypoxia leads to progressive accumulation of TNF-α, which induces osteoblast pyroptosis and suppresses osteogenic differentiation via activation of the NLRP3-GSDMD/GSDME pathway.104 This may be associated with the aggravation of inflammatory responses when cells around osteoblasts, such as macrophages, are stimulated by hypoxia. Furthermore, hypoxia stimulates osteoblasts to release adenosine triphosphate (ATP), which promotes osteoclast activation and subsequent bone resorption.105 Hypoxia also intensifies intracellular oxidative stress in osteoblasts, increasing ROS production and activating mitochondrial apoptotic pathways that ultimately lead to cell death.106,107 These ROS inhibit Runx2 and osterix (Osx) expression, further diminishing osteogenic activity (Fig. 2f).108
However, hypoxia does not always exert detrimental effects on osteoblasts. Chronic intermittent hypobaric hypoxia, a treatment with moderate hypoxia simulating high altitude, interrupted by normoxia, may activate HIF-1α and osteogenesis-related genes to enhance fracture healing, while increasing bone mineral density and mechanical strength.109 Hypoxic stimulation upregulates VEGF, establishing an angiogenesis-osteogenesis coupling mechanism that concurrently promotes bone vascularization and potentiates bone formation.56,110 HIF-1α overexpression enhanced osteogenic differentiation, as evidenced by increased osteoblast proliferation, elevated ALP activity, and promoted nodule mineralization, potentially by inducing autophagy.111 Another study has shown that the hypoxic environment upregulates the expression of HIF-1a, which activates the BMP4/SMAD signaling pathway, leading to an increase in ALP content and enhanced expression of osteogenic-related factors OCN and OPN, thereby promoting the osteogenic differentiation of BMSCs.112 Osteoblast-specific knockout of HIF-1α (OC-Cre, HIF-1αf/f) severely impairs both neoangiogenesis and bone regeneration essential for skeletal repair.113 In summary, the hypoxic microenvironment exerts a dualistic effect on osteoblast function and bone regeneration. Acute or severe hypoxia impairs osteogenic differentiation and promotes apoptosis by inducing oxidative stress, suppressing key osteogenic pathways, and exacerbating inflammatory responses. In contrast, moderate or intermittent hypoxia can enhance osteogenic activity by activating HIF-1α, which upregulates VEGF to couple angiogenesis with osteogenesis or stimulates signaling pathways such as BMP4/SMAD. The indispensable role of HIF-1α is evident as its deficiency severely compromises bone repair, highlighting its pivotal function as a central regulator of hypoxic adaptation and a determinant of regenerative outcomes.
Osteoclasts
During the inflammatory phase of bone regeneration under hypoxic condition, both the number and activity of osteoclasts are significantly enhanced.114 Remarkably, osteoclast activity increased 21-fold in exposures to 2% oxygen.115 This is because the main energy driving form of osteoclasts is glycolysis.116 Glycolysis is the main energy source for bone resorption.117 Hypoxia regulates the intracellular glucose metabolism to be dominated by glycolysis, and the high glycolytic rate of osteoclasts may be further potentiated under low oxygen conditions.118 HIF-1α has been identified as a crucial metabolic switch that activates anaerobic respiration, rapidly boosting ATP production in osteoclasts.119 By upregulating the expression of pro-osteoclastic genes and enhancing glycolytic activity, HIF-1α directly stimulates bone resorption.120
The hypoxia-induced increase in osteoclast numbers may result from bidirectional interactions between osteoblasts and osteoclasts.105 On one hand, HIF-1α modulates osteoclastogenesis by regulating the RANKL/osteoprotegerin (OPG) ratio secreted by osteoblasts.121 On the other hand, hypoxia stimulates osteoblasts to secrete adenosine, which activates the P1 adenosine receptor A2B on osteoclast membranes. HIF stabilization enhances A2B transcription and subsequent signal transduction. This augmented A2B activation further stimulates HIF-1α through an intracellular feedback loop, ultimately amplifying glycolytic flux and mitochondrial reductase activity.122 HIF-1α is indispensable in osteoclasts. It can affect the absorption of calcified cartilage matrix by osteoclasts through the AMPK signaling pathway. Conditional knockout of HIF-1α in osteoclasts of mice (Ctsk-cre, HIF-1αflox/flox) will result in severe condylar deformity during condylar growth, with shortened condylar length and disordered fibrocartilage arrangement.123 Besides, HIF-1α enhances the osteoclast-mediated differentiation stimulation of BMSCs by secreting CT-1. Knocking out HIF-1α in osteoclasts inhibits bone resorption and the expression of CT-1.124Furthermore, the hypoxic microenvironment elevates intracellular oxidative stress in osteoclasts, leading to increased ROS production.119 Functioning as secondary messengers, ROS participate in RANKL signaling during monocyte-osteoclast differentiation by upregulating nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) expression.125 ROS induction enhances the expression of key osteoclast markers, including c-Fos, NFATc1, and TRAP (Fig. 2g).126 Collectively, these hypoxia-mediated mechanisms potentiate osteoclast activity, resulting in enhanced bone resorptive capacity during the inflammatory phase of bone regeneration.
Role of BMAL1 in the inflammatory phase of bone regeneration
Role of BMAL1 in neutrophils
Neutrophil activity is subject to circadian regulation. The kinetics of neutrophil recruitment to tissues in response to damage or endotoxin challenge in mice are influenced by the zeitgeber time of the insult and correlate with the rhythmic expression of endothelial adhesion molecules.127 Moreover, the demonstrated circadian oscillations in the expression of cell adhesion molecules and in neutrophil phagocytic ability provide further evidence for the circadian control of neutrophil recruitment and function.128,129 The aging process of neutrophils themselves is also influenced by the circadian clock, as the proportion and number of aged neutrophils in the blood fluctuate throughout the day.130
The chemokine C-X-C motif chemokine ligand 2 (CXCL2), also known as macrophage inflammatory protein-2-alpha (MIP-2α), influences neutrophil recruitment and activation via MAPK signaling by binding and activating its specific receptors, CXCR1 and CXCR2.131 Activation of CXCR2 has been shown to promote neutrophil aging. Research has revealed that the core clock gene BMAL1 controls neutrophil aging in a cell-autonomous manner by regulating the expression of CXCL2 (Fig. 3). Genetic deletion of Bmal1 or Cxcr2 in neutrophils impedes the manifestation of cellular aging, whereas knockout of Cxcr4, a negative regulator of CXCR2 signaling, accelerates it. Neutrophil aging disrupts cytoskeletal integrity, thereby impairing neutrophil migration.132 Furthermore, the expression level of CXCL5 itself exhibits robust circadian oscillation and can recruit neutrophils to sites of tissue damage in a time-dependent manner.133 However, it has also been suggested that BMAL1 accumulation in the nuclei of neutrophils is minimal, with only a low-phosphorylation form of the protein detectable. Given that the BMAL1 function is highly dependent on its nuclear localization and phosphorylation status, the molecular oscillator governed by Bmal1 may not operate effectively in mature human peripheral neutrophils.134 Since the initial inflammatory phase is crucial for bone repair, the circadian clock’s control over neutrophil activity and aging presents a novel temporal mechanism that could affect the efficiency of bone regeneration.
Role of Bmal1 in macrophages, endothelial cells, and BMSCs. a Overexpression of Bmal1 in macrophages downregulates M1-type markers (TNF-α, IL-1β) and upregulates M2-type markers (IL-10, ARG-1), while suppressing glycolytic activity to promote macrophage M2 polarization. Conversely, inhibition of Bmal1 enhances M1 polarization of macrophages. b In endothelial cells, BMAL1 binds to the E-box element of VEGF to promote its expression, while simultaneously activating the Transforming Growth Factor-β (TGF-β)/Small Mother Against Decapentaplegic (SMAD3) pathway, enabling endothelial cells to modulate bone tissue metabolism. Conversely, Bmal1 inhibition suppresses endothelial cell proliferation and promotes apoptosis. c The osteogenic differentiation capacity of BMSCs is regulated by BMAL1 expression. BMAL1 inhibits glycogen synthase kinase-3β (GSK-3β) to activate the Wnt pathway, and its overexpression upregulates osteogenic markers (ALP, OCN, RUNX2) to promote differentiation. Conversely, BMAL1 knockdown suppresses RUNX2 and p53 expression, impairing osteogenesis
Role of BMAL1 in macrophages
The activity of macrophages exhibits a distinct circadian rhythm, and approximately 8%-15% of genes in macrophages display circadian oscillations.135,136,137 The transcription levels of Bmal1 in M1 macrophages show reduced amplitude and rhythmicity, but do not significantly affect the period of oscillation. On the other hand, the circadian oscillation cycle of M2 macrophages is extended without causing any changes in amplitude or rhythmicity.138 In Zhou et al.‘s study,139 overexpression of Bmal1 suppressed the expression of M1 macrophage markers (TNFα, IL-1β) while increasing the expression of M2 markers (IL-10, arginase-1 (ARG-1)) (Fig. 3a). They found that blocking the glycolysis pathway could significantly inhibit the M1 polarization, and after overexpression of Bmal1, the glycolysis level was effectively suppressed.139 This is also consistent with the results of Hong et al.140
Bmal1 regulates the inflammatory response of macrophages. Knocking out Bmal1 in macrophages can disrupt the NF-κB pathway and increase the expression of p65, thereby enhancing the expression of inflammatory factors.141 The NLRP3 inflammasome is the core of the inflammatory response in macrophages. It amplifies inflammation by processing and releasing inflammatory cytokines, such as IL-1β.142 The NLRP3 inflammasome is controlled by the circadian rhythm throughout the initiation and activation stages, and the absence of Bmal1 enhances the activity of the NLRP3 inflammasome.143 This regulation occurs through mitochondrial membrane potential (ΔΨm)-dependent mechanisms, as NLRP3 inflammasome activation requires ΔΨm oscillations that are intrinsically linked to circadian rhythms. Disruption of these rhythmic ΔΨm fluctuations potentiates inflammasome-triggered pyroptosis and IL-1β secretion (Fig. 3a).143 Besides, Bmal1 regulates time-dependent inflammatory responses following Toll-like receptor 4 (TLR4) activation by modulating enhancer activity, establishing circadian control over macrophage inflammatory output.143 In summary, during the process of bone regeneration, BMAL1 may influence the polarization state of macrophages and the inflammatory response, thereby affecting the outcome of bone regeneration.
Role of BMAL1 in endothelial cells
The endothelial cell-participated bone regeneration process is regulated by various signaling and metabolic pathway networks. Among them, BMAL1 plays a crucial role. Astone et al.144 examined the expression levels of all circadian genes in ECs and found that the expression of these genes exhibited significant rhythmic oscillations within 24 h. Knockdown of BMAL1 affected the vitality of ECs and significantly inhibited cell proliferation. The cell cycle progression was blocked at the G0/G1 phase, and the percentage of apoptotic cells slightly increased, while the sensitivity to apoptotic stimuli was enhanced. This impaired the wound healing ability and the budding ability of ECs.144 Notably, ECs exhibit osteogenic regulatory functions. ECs lacking BMAL1 led to a significant decrease in BMSC proliferation, reduced osteogenic activity of BMSCs, a slight increase in osteoclasts in bone tissue, and ultimately, age-related bone loss.145 When ECs maintain robust circadian BMAL1 expression, SMAD3 phosphorylation and downstream TGF-β/SMAD3 signaling components display synchronized circadian oscillations, thereby preserving rhythmic bone metabolism. Conversely, BMAL1 ablation induces TGF-β/SMAD3 pathway activation continuously, leading to chronic suppression of BMSC proliferation and eventual stem cell exhaustion (Fig. 3b).145,146,147
Currently, it is widely believed that BMAL1 exerts its influence on angiogenesis through the regulation of VEGF. In the zebrafish model, it was discovered that BMAL1 can bind to crucial E-box elements in the VEGF promoter and activate its activity (Fig. 3b).148 Suppression of BMAL1 function inhibits Notch-dependent vascular sprouting.148 Mice with disrupted circadian rhythms exhibit reduced levels of VEGF in ischemic tissues and plasma, resulting in a diminished angiogenic response and impaired ability to restore blood supply.149 However, Astone et al. did not observe a significant downregulation of VEGF-α expression in ECs following BMAL1 knockdown; instead, they found a notable decrease in the expression of cell cycle regulators such as Cyclin A1 (CCNA1) and CDK1. Therefore, they suggested that BMAL1 may play a more pivotal role in regulating endothelial cells through cell cycle interaction. It has been observed that BMAL1 may upregulate the expression of VEGF and Angiopoietin 2 (ANG2) via the HIF-1α pathway.36 The knockout of the Bmal1 gene also leads to impaired angiogenesis in mice.150 Reduced Bmal1 expression enhances ox-LDL uptake by endothelial cells while impairing proliferation, migration, and angiogenesis.150 Therefore, BMAL1 plays a significant role in regulating the formation of blood vessels during the bone regeneration process by influencing endothelial cells.
Role of BMAL1 in BMSCs
Bone formation initiates with the migration of mesenchymal stem cells into the site of the bone defect, followed by their subsequent proliferation and differentiation. Weger et al.151 provided evidence for the existence of a negative feedback regulatory mechanism controlling rhythm genes in BMSCs. BMAL1 can enhance the differentiation of BMSCs and augment the population of functional osteoblasts through activation of the classical Wnt signaling pathway, thereby exerting a positive regulatory effect on osteogenesis.152,153,154 The knockout of Bmal1 in mice by Samsa et al.155 demonstrated a diminished capacity of BMSCs isolated from Bmal1−/− mice to undergo osteoblastic differentiation in vitro. Concurrently, this study revealed that the absence of BMAL1 resulted in a mouse phenotype characterized by reduced bone mass, indicating the regulatory role of BMAL1 in the expression and function of genes involved in bone formation during bone development. Bmal1 is capable of suppressing the expression of GSK-3β, consequently activating the Wnt signaling pathway.156 Overexpression of Bmal1 can facilitate the expression of osteogenic markers such as ALP, OCN, and RUNX2 transcription factors, ultimately restoring the osteogenic differentiation potential of BMSCs (Fig. 3c).157 GSK-3β functions as an inhibitor within the Wnt signaling pathway and phosphorylates BMAL1 to maintain normal cell cycle progression. The expression levels of GSK-3β exhibit a negative correlation with those of BMAL1, suggesting that BMAL1 may serve as a key regulator within Wnt signaling pathways.157
In addition, the p53/p21 signaling pathway regulates the senescence and apoptosis of BMSCs. The decreased expression of Bmal1 disrupts the balance of the sympathetic clock axis, thereby inhibiting p53 expression.158 The expression of Smad, a downstream signal transduction protein in the TGF-β superfamily, exhibits rhythmicity in BMSCs. The BMAL1:CLOCK heterodimer promotes Smad3 expression, suggesting that circadian genes may regulate BMSCs' osteogenic differentiation through modulation of the Smad family.159 Furthermore, BMAL1 is involved in BMSCs development and ossification by regulating Runx2 and Osx.160 However, knockout of Bmal1 leads to disordered Runx2 expression and loss of rhythm (Fig. 3c).161 Nevertheless, some studies have proposed a negative role for BMAL1 in regulating BMSC osteogenic differentiation; however, further investigation is required to elucidate the specific mechanism. These inconsistent results may be attributed to variations in mouse age selection during experiments or differences in BMSC extraction processes, or research methodologies employed.162
Role of BMAL1 in osteoblasts
BMAL1 also plays a crucial role in the regulation of osteoblast proliferation and function. In vitro experiments have demonstrated the existence of the circadian system in osteoblasts.155 The absence of Bmal1 leads to a reduction in osteoblast numbers and impairs terminal differentiation, resulting in morphological changes in bone trabecular and cortical structures.155 Clock genes have been shown to regulate bone formation in osteoblasts through leptin-dependent sympathetic signaling.163 Leptin-dependent sympathetic signaling promotes osteoblast proliferation by activating protein 1 (AP-1) expression. Conversely, β-adrenoceptors negatively regulate bone mass and osteoblast proliferation via Bmal1 and Per.164 Additionally, BMAL1 may mediate cytoplasmic protein synthesis in osteoblasts through the rapamycin (mTOR) signaling pathway.165,166 Signals transmitted by the mTOR pathway promote mRNA translation. The promotion of mTOR effector protein kinase ribosomal S6 protein kinase 1 (S6K1) serves as an important regulator of translation, with mTOR stimulating translation through phosphorylation of S6K1/2. Furthermore, S6K1 rhythmically phosphorylates BMAL1. The phosphorylation of BMAL1 at S42 by S6K1 is essential for its interaction with the translational machinery and stimulation of protein synthesis, thereby placing BMAL1 in the context of other translation-related substrates of S6K1.166,167 These findings establish a direct molecular connection between BMAL1 and the mTOR pathway.
After Bmal1 knockout, Li et al.168 observed impaired osteoblast differentiation and mineralization ability. They also found decreased expression of osteogenic markers and alkaline phosphatase activity, along with increased apoptosis and inflammation. Furthermore, Bmal1 knockout promoted the phosphorylation of ERK and JNK, as well as mTOR activity, while inhibiting GSK-3β/β-catenin signaling and reducing β-catenin expression and GSK-3β phosphorylation.168 Consequently, upregulation of Bmal1 expression may lead to elevated bone morphogenetic proteins (Bmp2) transcription through increased Inhibitor of DNA binding 1 (Id1), RUNX2, and Ocn expression, effectively promoting osteoblast differentiation.169 However, Qian et al.170 demonstrated that knocking out Bmal1 in osteoblasts resulted in an increase in trabecular bone volume but a significant decrease in cortical thickness within the femur of mice. Their data indicate that the absence of Bmal1 can activate the BMP2/SMAD1 signaling pathway, thereby upregulating the BMP signaling cascade, to enhance the osteoblast activity within the bone trabeculae. This is because BMAL1 can exert transcriptional inhibitory effects by directly binding to the Bmp2 promoter. Additionally, knockout of Bmal1 in osteoblasts led to enhanced osteoclast formation, resulting in increased bone resorption and ultimately leading to low bone mass.171 Therefore, it is evident that circadian rhythm plays a crucial role in regulating osteoblasts’ function and bone formation; however, further research is needed to elucidate the underlying mechanisms.
Role of BMAL1 in osteoclasts
In vivo studies have demonstrated that genes associated with osteoclasts, such as cathepsin K (CTSK) and NFATc1, exhibit circadian rhythm expression in the cancellous bone of the femur,172 with Bmal1 also confirmed to display rhythmic expression in osteoclasts.173 BMAL1 can regulate osteoclast differentiation and function through direct or indirect mechanisms. For instance, deactivating BMAL1 in RAW264.7 cells effectively promotes osteoclast differentiation, leading to osteoporosis by upregulating matrix metalloprotease 13 (MMP13) levels [149]. Knockdown of Bmal1 in BMSCs or MC3T3-E1 cells, followed by co-culture with RAW264.7 cells, reduces OPG levels while enhancing osteoclast differentiation.174 Overexpression of Bmal1 inhibits NF-κB signaling pathway activity, thereby suppressing osteoclast differentiation.175
BMAL1 is also involved in the communication between osteoblasts and osteoclasts. Clock genes in osteoblasts can regulate bone resorption. Expression of BMSCs after restoration of BMAL1 activity restrained the NF-κB pathway, restored the osteogenic ability of BMSCs, inhibited their ability to induce osteoclast formation, and improved bone metabolism and function.175 Takarada et al. proposed that Bmal1 is rhythmically expressed in osteoblasts and regulates RANKL expression by inducing 1,25(OH)2D3-dependent formation of osteoblast-dependent osteoclast.171 Osteoclasts derived from bone marrow macrophages remove old or broken bones under the stimulation of osteoblasts while releasing growth factors into the bone matrix, thereby recruiting and migrating BMSCs to repair areas where they differentiate into osteoblasts and contribute to new bone formation within specific regions during the process of bone regeneration.176,177 Thus, the circadian gene BMAL1 is a key regulator of bone remodeling during regeneration, as it directly inhibits osteoclast differentiation and function, and modulates the critical osteoblast-osteoclast crosstalk that determines the balance between bone resorption and formation.
Crosstalk between hypoxia response and circadian rhythm
Hypoxia can lead to disruption of the biological clock and physiological imbalance.178 Patients with obstructive sleep apnea syndrome (OSA) or nocturnal hypoxemia (NH), both of which are characterized by recurrent hypoxia, tend to develop circadian rhythm disorders due to enhanced HIF-1α gene expression mediated by hypoxia in OSA.179,180 Under the combined influence of OSA-induced hypoxia and rhythm disorders, the homeostasis of bone metabolism is disrupted, resulting in a microenvironment that is unfavorable for bone maintenance and repair.181,182While research has demonstrated that the transcriptional response to hypoxia in tissues exhibits remarkable time-of-day dependency, which is governed by the endogenous circadian clock within the tissue.183 The liver-specific Bmal1-knockout mouse model (Alb-Cre, Bmal1fl/fl) confirmed that this time-dependent hypoxic response strictly relies on a functionally intact tissue-intrinsic clock, rather than being solely regulated by systemic signals.183 It can be seen that the hypoxic response is closely related to the circadian rhythm. The core regulators of each, HIF-1α and BMAL1, are functionally interconnected in the liver, enabling the integration of hypoxic signaling into the circadian framework and ensuring a time-of-day-dependent physiological response to hypoxia.184 These insights inspire the hypothesis that a similar regulatory axis may operate within the hypoxic microenvironment of bone regeneration, potentially influencing the progression of osteogenesis. In bone regeneration processes, such as fracture healing, the regenerative niche is often characterized by fluctuating oxygen tension and strong circadian rhythm of cellular activities. It is possible that the interplay between HIF-1α and BMAL1 helps coordinate the timing of critical events—including angiogenesis, osteoblast differentiation, and matrix mineralization—in a time-dependent manner. Disruption of this coupling could underlie suboptimal healing outcomes in conditions accompanied by circadian rhythm disorder or pathological hypoxia. In order to further clarify the potential interaction between the hypoxic response and the circadian rhythm during bone regeneration, we have further summarized the known and inferred interactions between HIF-1α and BMAL1 as follows.
Heterodimerization of HIF-1α and BMAL1
Currently, BMAL1 has been identified in various cell types as a regulator of HIF-1 activity under low oxygen conditions.35,185 This can be attributed to that BMAL1, CLOCK, HIF-1α, and HIF-1β belong to the basic helix-loop-helix-PAS (bHLH-PAS) transcription factor family, sharing structural similarities in their bHLH-PAS domains.37,38 The basic structures of BMAL1, CLOCK, HIF-1α, and HIF-1β all consist of: the bHLH domain that binds to DNA, the PAS domain that performs dimerization function, and the transactivation domains (TADs), and the corresponding domains between each molecule are similar (Fig. 4a).38,186,187,188,189 Structurally, the bHLH domain is defined by a short loop that links two α-helices (as H1 and H2), together forming a functional unit of roughly 50 amino acids.190 The bHLH domain is responsible for recognizing and binding to the E-box (CACGTG) DNA sequence.188 A PAS domain with the 260 ~ 310 amino acids is subdivided into two well-conserved PAS-A and PAS-B domains, and a loop linker.191 The PAS-B domain is more crucial for the dimerization of BMAL1 and CLOCK.192 The TAD domain interacts with transcriptional co-activators such as p300 and CBP to promote the transcription of downstream genes.189,193 HIF-1α contains two TADs, which are respectively called N-TAD and C-TAD. The N-TAD is associated with the degradation of HIF-1α, while the C-TAD binds transcriptional co-activators to regulate gene transcription. Additionally, HIF-1α has an oxygen-dependent degradation domain (ODDD) that overlaps with the N-TAD. This ODDD plays a crucial role in mediating the O2-regulated stability of HIF-1α.193
Structure of the bHLH-PAS family members and the heterodimer of BMAL1 and HIF-1α under hypoxic conditions. a As members of the bHLH-PAS family, BMAL1, CLOCK, HIF-1α, and HIF-1β have similar structures. b In normoxic conditions, BMAL1 associates with CLOCK to form a complex that translocates into the nucleus for regulating circadian-controlled genes (CCGs) transcription. In the presence of oxygen, HIF-1α is subjected to ubiquitin-mediated degradation. During hypoxia, BMAL1 has the potential to dimerize with HIF-1α and bind to E-BOX or HREs in order to initiate transcription
The bHLH-PAS protein family is divided into two classes based on dimerization specificity. Class I members, exemplified by the aryl hydrocarbon receptor (AhR), hypoxia-inducible factors (HIF-1α, HIF-2α, HIF-3α), and single-minded proteins (SIM1, SIM2), are unable to form homodimers or interact with other Class I partners. To become transcriptionally active, they must heterodimerize with a Class II bHLH-PAS factor. In contrast, Class II proteins readily form both homodimers and heterodimers. The archetypal Class II member is the universally expressed ARNT(HIF-1β), while other representatives include the tissue-restricted ARNT2 and the circadian regulators BMAL1 and BMAL2.194 In addition to the exploration of the conventional bHLH-PAS family members’ combinations, such as BMAL1/CLOCK and HIF-1α/HIF-1β, other combinations are increasingly attracting people’s interest. Among them, the combination of BMAL1 and HIF-1α is particularly worthy of study. The structure of BMAL1 shares many similarities with HIF-1β, and there are also numerous repetitions in the sequence. They exhibit sequence identities of 66% in the bHLH domain, 51% in the PAS-A domain, and 39% in the PAS-B domain.195 Therefore, there is a possibility of forming a heterodimer with HIF-1α. Hogenesch et al. demonstrated through yeast two-hybrid assays that HIF-1α can directly interact with BMAL1 at the protein level. Further gel shift and reporter gene assays confirmed that they form a heterodimeric complex in vitro, bind to a specific DNA response element, and drive transcription in cells while responding to hypoxic signals.40 This was also confirmed in the research conducted by Takahata et al.41 The co-immunoprecipitation experiment directly demonstrated that HIF-1α and BMAL1 have a direct physical interaction at the protein level.196 This indicates that they can at least form a complex or even dimerize to regulate gene transcription. The possible heterodimer formed by BMAL1 and HIF-1α can bind to the E-BOX or HRE elements of DNA chains, regulating the expression of genes such as the Circadian clock, oxidative metabolism, glycolysis, angiogenesis, etc., playing a role in integrating the hypoxic microenvironment and circadian rhythm, and may play an important role in bone regeneration (Fig. 4b).197
Reciprocal regulation between HIF-1α and BMAL1
In Suyama et al.‘s experiments,198 no direct interaction between BMAL1:CLOCK and HIF-1α was observed; however, knockout of global Bmal1 (Global Bmal1−/−) resulted in a decline in HRE activity. This suggests that BMAL1 may regulate the function of HIF-1α. They proposed that the interaction between BMAL1:CLOCK and E-box sequences in gene promoters could potentially modulate the recruitment and activity of HIF-1α at HRE sites located near or partially overlapping with E-box sequences, thereby effectively regulating the expression of its target genes.198 In the study conducted by Peek et al., it was similarly observed that knockdown of Bmal1 (ACTA-rtTA-TRE-Cre, Bmalfx/fx) in mouse skeletal muscle resulted in a reduction in HIF-1α accumulation and decreased sensitivity to the simulated hypoxic environment. The diminished functionality of HIF-1α may be attributed to impaired transcription of Hif-1α caused by Bmal1 gene knockout. This phenomenon can potentially be explained by a reduced interaction between HIF-1α and BMAL1, leading to decreased stability of monomeric HIF-1α, as seen in cells lacking HIF-1β.195 However, it is also possible that BMAL1 is involved in the transcriptional regulation of the HIF-1α gene. Studies have demonstrated that BMAL1 overexpression in primary glioma cells upregulates ANG2 and VEGF expression via the HIF-1α pathway, thereby enhancing angiogenesis.36 Conversely, BMAL1 knockout in chondrocytes (Col2α1-CreER, Bmal1flox/flox) significantly reduces VEGF and HIF-1α levels while increasing MMP13 and Runx2 expression, ultimately suppressing cell proliferation and promoting apoptosis.199
HIF-1α is also involved in regulating the expression of BMAL1. Transfecting the expression plasmid of HIF-1α into liver cancer cells, or treating with CoCl2 to increase the protein level of HIF-1α in the cells, both will lead to an increase in the mRNA expression levels of Clock and Bmal1.200 This result is also applicable in human colorectal cancer cell lines, and they considered that this cascade promoted glycolytic activity and reduced apoptosis in hypoxic conditions.201 When U2OS cells were treated with HIF-1α stabilizers such as DMOG, Ni2+, and Co2+, it was observed that the rhythmic cycle of the BMAL1:LUC reporter gene was significantly prolonged, and the amplitude was weakened.196 In summary, a reciprocal regulatory loop exists between BMAL1 and HIF-1α, wherein BMAL1 modulates both the activity and transcription of HIF-1α, while HIF-1α in turn regulates BMAL1 expression and circadian rhythm. Although similar studies have not yet been conducted in bone tissue or bone defect models, it is noteworthy that both circadian rhythms and hypoxia signaling play essential roles in bone regeneration—orchestrating angiogenesis, cell differentiation, and metabolic adaptation. This reciprocal regulatory interplay may provide a mechanistic basis for understanding the integration of hypoxic responses and circadian rhythm during bone regeneration.
HIF-1α and BMAL1 co-regulate intracellular oxidative stress levels under hypoxic conditions
Hypoxia can diminish the osteogenic effect during bone regeneration and proliferation due to its direct suppression of cell activity, as well as its promotion of pro-inflammatory mediators such as ROS.95 Elevated levels of ROS can induce apoptosis in osteoblast precursor cells and mature osteoblasts, while also interfering with the osteogenic differentiation of BMSCs by inhibiting the expression of key markers like ALP, OCN, and RUNX2.96,97,98 Therefore, ensuring a proper oxygen supply and effective removal of ROS during bone regeneration and proliferation is crucial for promoting successful bone tissue regeneration. The relationship between ROS and HIF-1α exhibits multifaceted complexity. On one hand, ROS can activate the HIF-1α signaling pathway, thereby inducing oxidative stress-related damage.202 Conversely, HIF-1α enhances ROS production through mechanisms such as elevating NADPH oxidase activity.203 Simultaneously, downstream proteins activated by HIF-1α mediate metabolic reprogramming from oxidative phosphorylation to aerobic glycolysis, while maintaining redox homeostasis. This transition reduces mitochondrial ROS generation by suppressing glucose oxidation in the TCA cycle. Furthermore, HIF-1α signaling promotes glutathione biosynthesis to bolster cellular antioxidant defense capacity, establishing a regulatory feedback loop between oxidative stress and hypoxic adaptation.204,205
As a pivotal circadian rhythm gene, Bmal1 plays a central role in counteracting oxidative stress.206 BMAL1 orchestrates the circadian oscillation of the antioxidant transcription factor Nuclear factor erythroid 2-related factor 2 (Nrf2) expression, which governs intracellular ROS homeostasis (Fig. 5).207 Macrophages deficient in BMAL1 (Lyz2Cre, Bmal1LoxP/LoxP) exhibited a significant elevation in oxidative stress response, accompanied by the accumulation of ROS and HIF-1α, as well as a reduction in NRF2 activity.208 These antioxidant response pathways inhibit HIF-1α, a pivotal regulator of glycolytic metabolism and inflammation, in a ROS-dependent manner. Recent studies have demonstrated that BMAL1’s role extends beyond regulating and oscillating core clock genes to include maintaining redox balance and enhancing cell viability under oxidative conditions.209,210 Sun et al.‘s experiment showed that a consistent supply of oxygen can significantly increase BMAL1 levels in hypoxic microenvironments for osteoblasts.57 Their study also found that sustained local release of oxygen promotes rapid blood vessel formation at bone defect sites, which is beneficial for osteogenesis. Furthermore, as mentioned earlier, under certain circumstances, low oxygen conditions lead to dimer formation between BMAL1 and HIF-1α, which then bind with HREs for transcriptional activation. Thus, BMAL1 acts as a rheostat, fine-tuning HIF-1α activity to maintain redox equilibrium. Further investigation into the crosstalk between BMAL1 and HIF-1α interaction under hypoxia is crucial. Understanding this relationship is key to elucidating the mechanisms of bone regeneration.
BMAL1 and HIF-1α cooperatively regulate intracellular ROS levels. BMAL1 suppresses ROS generation by upregulating the expression of NRF2, a core regulator of cellular antioxidant defense. HIF-1α can be activated by either hypoxic conditions or elevated ROS levels. HIF-1α exhibits dual regulation of ROS: (1) it increases ROS production via NADPH oxidase upregulation, and (2) it reduces ROS levels by enhancing glutathione synthesis
In summary, BMAL1 and HIF-1α form a critical regulatory axis that maintains intracellular redox homeostasis under hypoxia, a key determinant for successful bone regeneration. BMAL1 counteracts oxidative stress by orchestrating the circadian expression of the antioxidant master regulator NRF2. Simultaneously, it fine-tunes the dual role of HIF-1α, which can both induce ROS production and initiate protective metabolic reprogramming to limit mitochondrial ROS generation. This cooperative regulation ensures that the hypoxic response is precisely controlled, preventing the detrimental accumulation of ROS that impairs osteoblast survival and BMSC differentiation. Therefore, the BMAL1-HIF-1α interplay is fundamental for creating a redox-balanced microenvironment conducive to bone healing.
HIF-1α and BMAL1 co-regulate metabolic reprogramming under hypoxic conditions
With the progression of research, deeper mechanisms underlying oxidative stress regulation have been revealed, particularly the metabolic interplay orchestrated by BMAL1 and HIF-1α. These two factors jointly regulate cellular metabolic reprogramming under hypoxic conditions, which critically influences bone regeneration processes. The functional crosstalk between BMAL1 and HIF-1α fundamentally hinges on metabolic adaptation. BMAL1 and HIF-1α serve as pivotal molecular hubs integrating circadian rhythms and hypoxic adaptation to coordinate bone regeneration. Their functional interplay encompasses transcriptional regulation, oxidative stress modulation, and metabolic reprogramming, profoundly influencing cellular homeostasis and tissue repair. The BMAL1-HIF-1α axis critically governs redox balance during bone regeneration. BMAL1 maintains mitochondrial oxidative phosphorylation (OXPHOS) to sustain ATP production in human embryonic stem cell-derived cardiomyocytes, and using the CRISPR/Cas9 technology to knockout the Bmal1 gene leads to a significant weakening of mitochondrial OXPHOS and impaired cardiomyocyte function.211 Consequently, BMAL1 deficiency elevates ROS levels, exacerbates HIF-1α-driven inflammatory responses (e.g., IL-1β upregulation), and compromises mitochondrial function.208,212 In contrast, HIF-1α promotes glycolytic metabolism and reduces mitochondrial ROS generation by suppressing TCA cycle activity.204 This will enhance the tolerance of mitochondria to hypoxic injury, indicating that hypoxia regulates energy metabolism by adaptively inducing high expression of HIF-1α.213 However, HIF-1α-driven glycolytic activation promotes osteoclast-mediated bone resorption, ultimately hindering bone regeneration.203,214
Some key glycolysis-related enzymes are simultaneously regulated by BMAL1 and HIF-1α. Skeletal muscle BMAL1 modulates HIF-1α-regulated glycolytic enzymes (e.g., 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, PFKFB3), thereby influencing glycolytic flux.215 PFKFB3 is recognized as a critical glycolytic regulator. In Bmal1-knockout mice (HSA-rtTA;TRE-Cre, Bmal1fx/fx), expression of Pfkfb3 is downregulated, while subsequent VHL knockout stabilizes HIF-1α and restores Pfkfb3 transcription and protein expression.215 Pyruvate kinase M2 (PKM2) serves as a rate-limiting enzyme in glycolysis, converting phosphoenolpyruvic acid (PEP) to pyruvate. PKM2 responds to lipopolysaccharide (LPS) stimulation via HIF-1α and acts as a key determinant of macrophage metabolic reprogramming, with a positive feedback loop existing between PKM2 and HIF-1α.216 PKM2 functions both as an activating cofactor and a transcriptional target of HIF-1α.217 After nuclear translocation, PKM2 directly interacts with HIF-1α to mediate mitochondrial apoptosis, leading to excessive mitochondrial ROS generation, reduced ΔΨm (mitochondrial membrane potential), disrupted mitochondrial morphology, and mtDNA release.218 As a co-activator for HIF target genes, PKM2 plays a role in the transformation from oxidative metabolism to glycolytic metabolism.217 However, BMAL1 can inhibit the transcription of PKM2, and macrophages lacking Bmal1 exhibit higher levels of glycolytic metabolism.219 BMAL1 overexpression inhibits glycolytic activity to suppress M1 macrophage polarization.139 BMAL1 regulates the level and localization of glycolysis enzyme PKM2. The knockout of Bmal1 leads to an increase in the phosphorylation of signal transducer and activator of transcription 3 (STAT3), further driving the expression of IL-1β mRNA, which may be mediated by the elevated PKM2 level.220 Thus, BMAL1 and HIF-1α establish functional crosstalk through PKM2. Succinate, a TCA cycle metabolite, accumulates to promote SDH-derived mitochondrial ROS production, which inhibits HIF-1α prolyl hydroxylases and stabilizes HIF-1α.221 The succinate-HIF-1α axis constitutes a critical link between oxidative and glycolytic metabolism: succinate oxidation promotes HIF-1α stabilization and drives glycolytic switching.222 Notably, succinate levels increase in Bmal1-knockout macrophages.220 This reveals an additional metabolic linkage between BMAL1 and HIF-1α via succinate regulation. Hexokinase 1 (HK1) catalyzes the first step of glycolysis,223 while lactate dehydrogenase A (LDHA) converts pyruvate to lactate in the final step of anaerobic glycolysis.224 Both LDHA and HK1 are transcriptionally regulated by HIF-1α.225,226 BMAL1 overexpression significantly suppresses cellular glycolysis and lactate production by reducing HK1 and LDHA protein levels.227 We have mapped the regulatory network of BMAL1 and HIF-1α in glucose metabolism as Fig. 6. Additionally, other key metabolic enzymes or metabolites regulated by BMAL1 and HIF-1α have been compiled in Table 1. Collectively, these findings demonstrate the interplay between BMAL1 and HIF-1α in metabolic reprogramming.
Metabolic reprogramming under hypoxic conditions is coordinated by BMAL1 and HIF-1α. Under hypoxic conditions, the HIF-1α-mediated metabolic reprogramming (suppressing oxidative phosphorylation/promoting glycolysis) can be counterbalanced by BMAL1, which restores mitochondrial oxidative phosphorylation and suppresses key glycolytic enzymes (e.g., PFKFB3, PKM2, HK1, LDHA) to constrain glycolytic flux
In summary, BMAL1 and HIF-1α co-regulate metabolic reprogramming under hypoxia by targeting key glycolytic enzymes, constituting a fundamental mechanism for bone regeneration. BMAL1 promotes oxidative phosphorylation to maintain energy supply and mitochondrial integrity, while HIF-1α drives glycolytic flux. Their interplay is exemplified through shared targets: BMAL1 inhibits PKM2, HK1, and LDHA to restrain glycolysis, whereas HIF-1α activates them. This balance is crucial, as unchecked HIF-1α-driven glycolysis can promote bone resorption. Furthermore, metabolic intermediates like succinate link BMAL1 deficiency to HIF-1α stabilization. Thus, the BMAL1-HIF-1α axis ensures a metabolically balanced microenvironment essential for effective bone repair. We summarize the crosstalk between BMAL1 and HIF-1α at different levels and their effects on bone regeneration in Fig. 7.
The crosstalk between BMAL1 and HIF-1α at different levels. BMAL1 /CLOCK and HIF-1α/HIF-1β bind to specific E-box and HREs, respectively. In addition, there is an interaction between BMAL1 and HIF-1α proteins. These two proteins may combine to form a heterodimer, which further regulates the cellular functions under hypoxic conditions. They co-regulate a set of downstream target genes involved in different processes. This collaborative regulation influences critical cellular functions, including the regulation of ROS and metabolic reprogramming, thereby integrating circadian rhythm and hypoxia response signals to promote bone regeneration
HIF-2α and its interaction with BMAL1
To facilitate a more comprehensive discussion of the crosstalk between hypoxia and circadian rhythms, it is also essential to consider another well-studied key molecule in the hypoxic response: HIF-2α. HIF-2α is another isoform of HIF-α. It retains highly conserved sequences, shares similar domain structures with HIF-1α, and binds to the same HRE sequence, 5′-(A/G)CGTG-3′, in the promoters of specific target genes.228 While they share similar regulatory roles in bone regeneration, there are distinct differences between them. Holmquist-Mengelbier et al. found that HIF-1α is associated with the acute hypoxic response, while HIF-2α is linked to the chronic hypoxic response.229 In contrast to HIF-1α, which supports chondrocyte phenotype maintenance and metabolic adaptation to hypoxia, HIF-2α promotes the progression of osteoarthritis.230 Lee et al. found that HIF-1α and HIF-2α have similar but mechanistically distinct roles in bone remodeling: both inhibit osteoblast differentiation via the TWIST2-RUNX2-OCN axis, but HIF-1α indirectly promotes osteoclastogenesis by upregulating HIF-2α, whereas HIF-2α directly drives this process.231 HIF-1α and HIF-2α are involved in regulating angiogenesis (e.g., VEGF) to couple osteogenesis, but HIF-1α primarily influences early osteoblast differentiation and may inhibit load-induced bone formation, whereas HIF-2α has a more pronounced role in regulating bone mass under specific conditions (e.g., in mature osteoblasts) and may exhibit functional complementarity or specific target gene regulation compared to HIF-1α.45
Although there are few existing studies on the relationship between HIF-2α and BMAL1, we can still infer that there is a close connection between them. In contrast to normoxia, where HIF-2α mRNA expression is arrhythmic in zebrafish, hypoxia induces a circadian rhythm in its expression.232 Due to the high structural similarity between HIF-1α and HIF-2α, HIF-2α may also form a heterodimer with BMAL1. This specific dimeric complex (HIF-2α/BMAL1) may preferentially bind to gene regulatory regions containing E-box elements, thereby directly integrating hypoxic signaling into circadian rhythm regulation. A recent study has substantiated this perspective. Ruan et al. employed a combination of AlphaFold structural prediction and cryo-EM to elucidate, for the first time, the three-dimensional structure of the BMAL1/HIF2A/DNA complex. This heterodimer recognizes and binds to a non-canonical HRE sequence (CAGGTG), thereby cooperatively activating the rhythmic transcription of the downstream target gene amphiregulin (AREG) and mediating circadian-dependent cardioprotection. Knockout of Bmal1, Hif-2α, or Areg was sufficient to abolish the diurnal variation in myocardial infarction, establishing the central role of the BMAL1–HIF2A–AREG signaling axis.233 HIF-2α may also be involved in regulating Rev-erbα within the circadian rhythm system, thereby influencing BMAL1.234 Consequently, HIF-2α and BMAL1 are also intricately connected. However, the functional significance of this relationship in bone regeneration necessitates further elucidation.
The clinical significance of the crosstalk between hypoxia and circadian regulation
In the preceding part, we have summarized the roles of BMAL1 and HIF-1α in bone regeneration. Clinically, numerous bone-related pathologies exhibit microenvironments analogous to those in bone defects. Under such conditions, the crosstalk between BMAL1 and HIF-1α critically influences both bone regeneration and therapeutic outcomes. Herein, we present several representative scenarios:
Osteoporosis
Shift work-induced osteoporosis
A follow-up study of night-shift nurses revealed that prolonged shift work may lead to lower trabecular and cortical bone mineral density (BMD), thereby increasing the risk of hip and wrist fractures. These findings collectively indicate that night-shift work constitutes a risk factor for osteoporosis.235,236 Similar adverse effects on bone tissue were observed among steel workers, where night-shift workers exhibited a higher prevalence of abnormal BMD compared to day-shift workers. This prevalence increased with cumulative night-shift exposure.237 Night-shift work disrupts sleep patterns and impairs melatonin secretion, resulting in circadian rhythm disruption. Additionally, reduced sunlight exposure during night shifts may decrease endogenous vitamin D synthesis, further compromising skeletal health237,238
Circadian rhythm disruption also manifests through altered oxidative stress levels. Shift work disrupts the pro-/antioxidant balance, favoring oxidative stress and subsequent physiological dysfunction.239 Night-shift work can induce DNA oxidative damage, which remains elevated even after one month of routine shift work.240 These studies suggest that osteoporosis associated with circadian rhythm disruption may involve oxidative stress mechanisms. In conclusion, the disruption of circadian rhythms caused by shift work may disrupt the normal expression of BMAL1, thereby interfering with its synergistic interaction with HIF-1α. As mentioned earlier, BMAL1 is a key regulatory factor for maintaining the balance of intracellular oxidative stress. The loss of BMAL1 function leads to the accumulation of ROS and abnormal stability of HIF-1α protein, thereby triggering excessive inflammatory responses and damaging mitochondrial function. In the context of bone regeneration, this dysregulation of the BMAL1-HIF-1α axis will disrupt the microenvironment necessary for bone regeneration, ultimately hindering bone formation and repair processes, providing a potential mechanism explanation for osteoporosis related to shift work. Further investigation is warranted to elucidate these relationships and develop strategies for preserving bone health in shift workers.
Circadian rhythm disruption in diabetic osteoporosis
Diabetes mellitus (DM) is a widely prevalent chronic metabolic disease, with type 2 diabetes (T2DM) being the main type.241 A cohort study has shown that the prevalence of bone loss and osteoporosis in T2DM patients is 50.6% and 17.7% respectively.242 Hyperglycemia inhibits the maturation/differentiation of osteoblasts, accelerates the aging and apoptosis of bone cells, and exacerbates cellular oxidative stress. Concurrently, it stimulates osteoclasts, enhancing bone resorption and disrupting bone remodeling balance.243
Physiological glucose homeostasis is regulated by circadian rhythms. Core metabolic organs – including skeletal muscle, liver, white adipose tissue, and pancreatic islets – exhibit robust intrinsic circadian oscillations. Circadian rhythm disruption may induce insulin resistance, impair glucose homeostasis, and promote T2DM pathogenesis.244 Conversely, T2DM itself causes cellular circadian rhythm disruption. Research conducted by Li et al.245 indicates that type II diabetes in rats leads to reduced expression of the rhythm Bmal1 gene in BMSCs. Overexpression of Bmal1 rescues osteogenic differentiation potential of BMSCs under T2DM microenvironments. Animal studies demonstrate that eldecalcitol (ED-71), an active vitamin D analog, upregulates Bmal1, suppresses oxidative stress, promotes osteogenesis, and attenuates T2DM-mediated bone loss.246 These results strongly suggest that the downregulation of Bmal1 in the diabetic microenvironment is a crucial factor leading to bone regeneration disorders.
In diabetes, multiple tissues are in a state of hypoxia. However, HIF-1α activation within cells fails to reach maximal levels due to impaired signaling caused by hyperglycemia and elevated fatty acids. This suppresses HIF-1α stability and function, resulting in inadequate adaptive responses to hypoxia.247 Suppressed HIF-1 signaling predisposes individuals to complications, including impaired wound healing, diabetic nephropathy, cardiovascular disease, diabetic retinopathy, obesity, and β-cell dysfunction.247 Both circadian disruption and diabetes induce aberrant Bmal1 expression. This genetic disturbance impairs BMAL1-HIF-1α crosstalk, further compromising HIF-1α function.195 BMAL1 deficiency reduces glucose utilization in skeletal muscle, as BMAL1 and HIF-1α cooperatively regulate the key glycolytic enzyme PFKFB3. Notably, stabilizing HIF-1α improves impaired glucose tolerance in Bmal1-knockout mice.215 Therefore, in diabetic osteoporosis, factors such as high blood sugar jointly cause abnormal expression of BMAL1 and inhibition of HIF-1α function, disrupting the crucial BMAL1-HIF-1α communication. The failure of this axis directly damages multiple key processes essential for bone regeneration: the dysfunction of HIF-1α will weaken angiogenesis, while the coordinated regulation of metabolic reprogramming and the balance of oxidative stress by BMAL1-HIF-1α are also disrupted. Ultimately, the osteogenic differentiation ability of BMSCs is impaired, the balance between bone formation and bone resorption is disrupted, and bone regeneration fails. By regulating BMAL1 through drugs or genetic means to restore its normal interaction with HIF-1α, it is expected to become a new strategy for reversing diabetic bone loss.
Osteoarthritis
Osteoarthritis (OA) is a chronic degenerative joint disorder characterized by progressive cartilage degradation, synovial inflammation, and subchondral bone remodeling, representing one of the most prevalent joint diseases.248 Circadian rhythm disruption is one of the key risk factors for OA.249 Substantial evidence indicates altered circadian rhythms in OA chondrocytes, manifesting as dysregulated clock gene expression.250,251,252,253 Specifically, Bmal1 expression is downregulated. Bmal1 knockout reduces cartilage matrix synthesis, diminishes chondrocyte proliferation, and enhances chondrocyte hypertrophy and apoptosis in condylar cartilage, ultimately inducing temporomandibular joint OA.254 The pathogenic effects of Bmal1 deficiency likely involve dysregulation of TGF-β and NFAT signaling pathways, coupled with reduced expression of critical matrix-associated genes, including SRY-box transcription factor 9 (Sox9), aggrecan (Acan), and Col2a1. This disrupts metabolic signaling, exacerbates inflammation, and promotes progressive OA-like cartilage degeneration.255 Alternatively, Bmal1 loss may hyperactivate the mTORC1 pathway in OA cartilage, driving cartilage degradation and chondrocyte apoptosis.256
HIF-1α is essential for cartilage development and homeostasis. Due to joint microstructure, chondrocytes exist in a chronically hypoxic microenvironment.257 Under such conditions, HIF-1α activation sustains anaerobic glycolysis and promotes extracellular matrix synthesis.258 However, only stabilized HIF-1α exerts protective effects.259 Notably, HIF-1α levels in articular cartilage and synovial fluid positively correlate with disease severity in knee OA patients.260 And specific knockout of HIF-1α increased the expression of MMP13 and cartilage destruction in OA mice.261 Cartilage degeneration coincides with disrupted hypoxia and HIF-1α degradation. Maintaining intra-cartilaginous hypoxia and stabilizing chondrocytic HIF-1α may decelerate OA progression.262
Crucially, HIF-1α in cartilage is regulated by BMAL1. BMAL1 not only governs circadian gene expression in cartilage but also modulates physiological chondrogenesis through coupling with HIF-1α and VEGF. After knocking out Bmal1, growth plate chondrocytes exhibit reduced VEGF and HIF-1α alongside elevated Mmp13 and Runx2 expression.199 Knocking out Bmal1 in primary cultured chondrocytes also affects their anti-apoptotic ability, partly due to the inhibition of Hif-1α and Vegf expression, while HIF-1α can regulate pro-apoptotic and anti-apoptotic genes.230,263 While HIF-1α-mediated signaling couples with circadian rhythm in chondrocytes, the precise mechanisms underlying this interaction remain incompletely understood.264 Consequently, the downregulation of BMAL1 during OA disrupts the protective BMAL1-HIF-1α axis. This disruption not only leads to metabolic abnormalities, such as dysregulated glycolysis and exacerbated inflammation, but, more importantly, impairs the HIF-1α-mediated adaptive survival response of chondrocytes. This accelerates the degradation of the cartilage matrix and chondrocyte apoptosis, thereby driving OA progression. Targeting the restoration of the BMAL1-HIF-1α axis function in chondrocytes may present a novel therapeutic strategy for delaying cartilage degeneration.
Titanium implant
Titanium is widely used in dental and orthopedic surgeries due to its mechanical properties, corrosion resistance, and biocompatibility.265 Implant placement disrupts local bone architecture, initiating a bone repair process analogous to alveolar bone regeneration in extraction sockets, ultimately achieving osseointegration.266 Circadian rhythms also participate in peri-implant osseointegration.267 BMAL1 overexpression in BMSCs surrounding implants enhances osseointegration.268 Post-implantation, surgical trauma, and persistent titanium surface oxidation create a local hypoxic microenvironment.269 Both BMAL1 and HIF-1α contain bHLH-PAS domains enabling hypoxia sensing. BMAL1 may heterodimerize with HIF-1α under hypoxia, activating pathways that promote osteogenic differentiation.269 While moderate hypoxia facilitates HIF-1α activation and peri-implant angiogenesis,270 excessive hypoxia (e.g., high-altitude conditions) impedes osseointegration.271 Recent surface modification strategies incorporate Co2+/Mg2+ ions to activate HIF-1α signaling, enhancing angiogenesis and accelerating osseointegration.272 It is worth noting that the surface modification of the implant also affects the peripheral circadian rhythm of human BMSCs in vitro.273 Here, we propose a feasible but unexplored direction: modifying the implant surface to promote the activation of BMAL1, in order to utilize the communication and interaction characteristics between BMAL1 and HIF-1α to further promote bone integration. By specifically activating BMAL1 through implant surface modification, it is possible to reinforce the BMAL1-HIF-1α signaling pathway in the postoperative hypoxic microenvironment. This enhancement synergistically improves the osteogenic capability of BMSCs and promotes angiogenesis, which are critical for successful osseointegration that requires efficient bone regeneration involving BMSC recruitment, differentiation, and new vessel formation. Consequently, this approach can accelerate and enhance the osseointegration outcome.
Current research limitations and future research prospects
The research on the crosstalk between BMAL1 and HIF-1α has been ongoing for a long time. Many scholars have devoted their time and energy to this field and have achieved significant results. However, when summarizing the existing research, there are still some shortcomings. Currently, the communication between BMAL1 and HIF-1α is mostly explored using cell models, investigating the relationships between pathways or the regulatory relationships, with insufficient exploration in animal models. Therefore, we propose that the circadian rhythm disruption model (control of light-dark cycle),28 or circadian rhythm gene knockout274 combined with the bone defect model (mandibular defects,275 or tooth extraction276) can be used to explore the impact of circadian rhythm disorders on bone regeneration at the site of bone defect, and then study the interaction between BMAL1 and HIF-1α during the process of bone regeneration. In the previous part, we concluded that the current mainstream view holds that the crosstalk between BMAL1 and HIF-1α is manifested in three aspects: (1) BMAL1 and HIF-1α mutually regulate gene expression; (2) BMAL1 and HIF-1α bind to each other to form a heterodimer, regulating the expression of downstream genes; (3) There is overlap or mutual regulation between the downstream genes that each of BMAL1 and HIF-1α regulates, ultimately jointly regulating the physiological functions of the cells.
At present, there are still deficiencies in the research on each part. First, transcriptional changes in Bmal1 and Hif-1α may not reflect direct regulation due to confounding factors. Under hypoxic conditions, the inhibition of ceramide kinase (a key enzyme in multiple cellular processes) leads to the autophagic degradation of BMAL1.34 HIF-1α can also enhance the amplitude of the circadian rhythm oscillation by directly binding to the promoter of Per2 (a gene involved in inhibiting the expression of BMAL1 in the circadian rhythm).264 Similarly, the changes in BMAL1 may cause changes in HIF-1α that are not directly regulated. BMAL1 can fine-tune the activity of HIF-1α through its role in mitochondrial metabolism and by regulating oxidative stress.212 The CRY (a gene involved in inhibiting the expression of BMAL1 in the circadian rhythm) protein negatively regulates HIF-1α by reducing its half-life and decreasing its binding to the promoter of target genes.277,278 It is recommended that in the research, methods such as dual luciferase should be used to exclude the interference of other factors and accurately verify the regulatory relationship between BMAL1 and HIF-1α.195
Secondly, the true role of the heterodimer formed by the binding of BMAL1 and HIF-1α proteins is still controversial. Under normal physiological conditions, BMAL1 binds to CLOCK,25 and HIF-1α binds to HIF-1β to exert its function.15 These dimer forms are the main forms in which the two exert their main functions. In the real hypoxic condition, how much and what proportion of the BMAL1-HIF-1α heterodimer is formed, and whether it has a regulatory effect on cellular physiology? According to a previous study, the binding of HIF-1α and HIF-1β formed a strong band, much higher than the binding of HIF-1α and BMAL1.40 Additionally, with the development of emerging technologies, there are more means to verify the protein-level interaction between BMAL1 and HIF-1α, such as using molecular interaction instruments to verify the binding situation, or using software to predict the binding sites of the two proteins, and then modifying/mutating the sites to observe the binding situation.
While we have endeavored to synthesize the current understanding of HIF-1α and BMAL1 crosstalk in bone regeneration, our review also reveals several critical knowledge gaps that warrant further investigation. A primary limitation pertains to the spatiotemporal dynamics of hypoxic and circadian regulation across different cell types. Specifically, the current literature lacks detailed mechanistic studies that delineate how the specific roles of HIF-1α and BMAL1 are fine-tuned within different cells, each of which experiences a distinct hypoxic intensity and duration due to their sequential recruitment into the regeneration process. While we have provided a conceptual framework, direct experimental evidence linking the dynamically changing oxygen gradient to cell-type-specific HIF-1α/BMAL1 activity and downstream functional outputs remains scarce. Furthermore, the precise molecular impact of differential hypoxic sensitivity among these cells on the functional interplay between the HIF-1α and BMAL1 pathways is still largely unexplored. Future research employing cell-type-specific genetic models, real-time oxygen biosensing, and time-series transcriptomic analyses across the phases of healing will be essential to move from a macroscopic model to a precise, mechanistic understanding of this complex regulatory axis.
After summarizing a large number of studies, we also found that the expression or function of BMAL1 is tissue-specific. The results obtained from the verification in one tissue may be different or even opposite in another tissue. Based on the experiments classifying alterations in cellular metabolism according to gene regulation across different tissues, we have summarized the findings in Table 2 to provide a reference for tissue-specific research.
Conclusion
In this review, we summarize the key role of the inflammatory phase in bone regeneration and the significant roles of HIF-1α and BMAL1 in bone regeneration. During the inflammatory phase of bone regeneration, cells are in a hypoxic microenvironment, and how to carry out orderly life activities under such conditions is a challenge. On the one hand, the hypoxia response mediated by HIF-1α helps cells resist it, and on the other hand, BMAL1 promotes the resolution of inflammation and the progress of osteogenesis. Beyond their individual roles in hypoxia response and circadian rhythm, we emphasize their synergistic interaction. HIF-1α and BMAL1 exhibit close connections at multiple levels: genetic, protein, and downstream regulatory pathways. These connections collectively regulate intracellular expression changes and metabolic reprogramming to balance oxidative stress, glycolytic flux, and osteo-angiogenic coupling, ultimately ensuring the normal physiological activities of cells. Crucially, this interaction extends beyond physiological bone healing to pathological contexts. In osteoporosis (shift work- or diabetes-associated), osteoarthritis, and titanium implant osseointegration. Despite significant research progress, key challenges and limitations persist. In our view, the communication and interaction between HIF-1α and BMAL1 require more precise exploration. Future research should further investigate the spatiotemporal interplay between hypoxia response and circadian rhythm to unravel the underlying mechanisms of bone regeneration.
References
Datta, H. K. et al. The cell biology of bone metabolism. J. Clin. Pathol 61, 577–587 (2008).
Einhorn, T. A. & Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54 (2015).
Araújo, M. G., Silva, C. O., Misawa, M. & Sukekava, F. Alveolar socket healing: what can we learn? Periodontology 2000(68), 122–134 (2015).
Majidinia, M., Sadeghpour, A. & Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 233, 2937–2948 (2018).
Schlundt, C. et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 106, 78–89 (2018).
Pajarinen, J. et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 196, 80–89 (2019).
Dimitriou, R., Jones, E., McGonagle, D. & Giannoudis, P. V. Bone regeneration: current concepts and future directions. BMC Med. 9, 66 (2011).
Maruyama, M. et al. Modulation of the Inflammatory Response and Bone Healing. Front Endocrinol. (Lausanne) 11, 386 (2020).
Duda, G. N. et al. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 19, 78–95 (2023).
Gaber, T. et al. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann. Rheum. Dis. 64, 971–980 (2005).
Lang, A. et al. MIF does only marginally enhance the pro-regenerative capacities of DFO in a mouse-osteotomy-model of compromised bone healing conditions. Bone 154, 116247 (2022).
Loeffler, J., Duda, G. N., Sass, F. A. & Dienelt, A. The metabolic microenvironment steers bone tissue regeneration. Trends Endocrinol. Metab. 29, 99–110 (2018).
Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143 (2012).
Mangiavini, L. et al. Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev. Biol. 393, 124–136 (2014).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Ohh, M. et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2, 423–427 (2000).
Campochiaro, P. A. Ocular neovascularization. J. Mol. Med. 91, 311–321 (2013).
Park, S. Y. et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J. Pineal Res. 48, 178–184 (2010).
Semenza, G. L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 13, 167–171 (2001).
Wu, Y. C. et al. Hypoxia-induced retinal neovascularization in zebrafish embryos: a potential model of retinopathy of prematurity. PLoS One 10, e0126750 (2015).
Tao, J. et al. Spatiotemporal correlation between HIF-1α and bone regeneration. FASEB J 36, e22520 (2022).
Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 (2012).
Ripperger, J. A. & Schibler, U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat. Genet. 38, 369–374 (2006).
Yoshitane, H. et al. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol. Cell. Biol. 34, 1776–1787 (2014).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Yu, S. et al. Circadian rhythm modulates endochondral bone formation via MTR1/AMPKβ1/BMAL1 signaling axis. Cell Death Differ 29, 874–887 (2022).
Kikyo, N. Circadian regulation of bone remodeling. Int. J. Mol. Sci. 25, 4717 (2024).
Luo, B. et al. Circadian rhythms affect bone reconstruction by regulating bone energy metabolism. J. Transl. Med. 19, 410 (2021).
Wu, Q. Y. et al. Emerging role of circadian rhythm in bone remodeling. J. Mol. Med. 97, 19–24 (2019).
Nie, T., Nepovimova, E. & Wu, Q. Circadian rhythm, hypoxia, and cellular senescence: From molecular mechanisms to targeted strategies. Eur. J. Pharmacol. 990, 177290 (2025).
Li, Y. et al. Circadian clock gene clock is involved in the pathogenesis of preeclampsia through hypoxia. Life Sci. 247, 117441 (2020).
Tian, L. et al. Ceramide-1-phosphate alleviates high-altitude pulmonary edema by stabilizing circadian ARNTL-mediated mitochondrial dynamics. J. Adv. Res. 60, 75–92 (2024).
Ghorbel, M. T., Coulson, J. M. & Murphy, D. Cross-talk between hypoxic and circadian pathways: cooperative roles for hypoxia-inducible factor 1α and CLOCK in transcriptional activation of the vasopressin gene. Mol. Cell. Neurosci. 22, 396–404 (2003).
Wang, F. et al. BMAL1 may be involved in angiogenesis and peritumoral cerebral edema of human glioma by regulating VEGF and ANG2. Aging 13, 24675–24685 (2021).
Huang, N. et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science 337, 189–194 (2012).
Wu, D. et al. Structural integration in hypoxia-inducible factors. Nature 524, 303–308 (2015).
Bersten, D. C., Sullivan, A. E., Peet, D. J. & Whitelaw, M. L. bHLH-PAS proteins in cancer. Nat. Rev. Cancer 13, 827–841 (2013).
Hogenesch, J. B., Gu, Y. Z., Jain, S. & Bradfield, C. A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA 95, 5474–5479 (1998).
Takahata, S. et al. Transcriptionally active heterodimer formation of an Arnt-like PAS protein, Arnt3, with HIF-1a, HLF, and clock. Biochem. Biophys. Res. Commun. 248, 789–794 (1998).
Teh, S. W. et al. Hypoxia in bone and oxygen releasing biomaterials in fracture treatments using mesenchymal stem cell therapy: a review. Front. Cell Dev. Biol. 9, 634131 (2021).
Walters, G., Pountos, I. & Giannoudis, P. V. The cytokines and micro-environment of fracture haematoma: Current evidence. J. Tissue Eng. Regen. Med. 12, e1662–e1677 (2018).
Harrison, J. S., Rameshwar, P., Chang, V. & Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 99, 394–394 (2002).
Yellowley, C. E. & Genetos, D. C. Hypoxia signaling in the skeleton: implications for bone health. Curr. Osteoporos. Rep. 17, 26–35 (2019).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Cai, B. et al. N2-polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a missing piece of the bone regeneration puzzle. Adv. Sci. 8, e2100584 (2021).
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 (2016).
Ghiasi, M. S. et al. Bone fracture healing in mechanobiological modeling: a review of principles and methods. Bone Rep. 6, 87–100 (2017).
Hankenson, K. D., Gagne, K. & Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 94, 3–12 (2015).
Camal Ruggieri, I. N., Cícero, A. M., Issa, J. P. M. & Feldman, S. Bone fracture healing: perspectives according to molecular basis. J. Bone Min. Miner. 39, 311–331 (2021).
Kenkre, J. S. & Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 55, 308–327 (2018).
Trombelli, L. et al. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 35, 630–639 (2008).
Łuczak, J. W. et al. The future of bone repair: emerging technologies and biomaterials in bone regeneration. Int. J. Mol. Sci. 25, 12766 (2024).
Reinke, S. et al. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 5, 177ra36 (2013).
Wu, C. et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 29, 817–831 (2015).
Sun, H. et al. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 24, 477–496 (2023).
Lu, F. et al. Is there a role for N1-N2 neutrophil phenotypes in bone regeneration? A systematic review. Bone 181, 117021 (2024).
Herath, T. D. K. et al. In vivo efficacy of neutrophil-mediated bone regeneration using a rabbit calvarial defect model. Int. J. Mol. Sci. 22, 13016 (2021).
Bastian, O. W. et al. Neutrophils inhibit synthesis of mineralized extracellular matrix by human bone marrow-derived stromal cells in vitro. Front. Immunol. 9, 945 (2018).
Meesters, D. M. et al. Deficiency of inducible and endothelial nitric oxide synthase results in diminished bone formation and delayed union and nonunion development. Bone 83, 111–118 (2016).
Dishowitz, M. I. et al. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PLoS One 8, e68726 (2013).
Linnemann, C., Nussler, A. K., Histing, T. & Ehnert, S. Febrile-range hyperthermia can prevent toxic effects of neutrophil extracellular traps on mesenchymal stem cells. Int. J. Mol. Sci. 23, 16208 (2022).
Walmsley, S. R., McGovern, N. N., Whyte, M. K. & Chilvers, E. R. The HIF/VHL pathway: from oxygen sensing to innate immunity. Am. J. Respir. Cell Mol. Biol. 38, 251–255 (2008).
Cassatella, M. A., Östberg, N. K., Tamassia, N. & Soehnlein, O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 40, 648–664 (2019).
Hoenderdos, K. et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 71, 1030–1038 (2016).
Ong, C. W. M. et al. Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci. Rep. 8, 11475 (2018).
McGovern, N. N. et al. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J. Immunol. 186, 453–463 (2011).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J. Exp. Med. 201, 105–115 (2005).
Wu, A. C., Raggatt, L. J., Alexander, K. A. & Pettit, A. R. Unraveling macrophage contributions to bone repair. Bonekey Rep. 2, 373 (2013).
Wang, S. et al. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 452, 14–22 (2019).
Hu, J., Liu, X. & Tang, Y. HMGB1/Foxp1 regulates hypoxia-induced inflammatory response in macrophages. Cell Biol. Int. 46, 265–277 (2022).
Rius, J. et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811 (2008).
Ivashkiv, L. B. The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol. 20, 85–86 (2020).
Karshovska, E. et al. HIF-1α (hypoxia-inducible factor-1α) promotes macrophage necroptosis by regulating miR-210 and miR-383. Arterioscler Thromb. Vasc. Biol. 40, 583–596 (2020).
Scheerer, N. et al. Myeloid hypoxia-inducible factor-1α is essential for skeletal muscle regeneration in mice. J. Immunol. 191, 407–414 (2013).
Trimm, E. & Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 20, 197–210 (2023).
Nguyen, V. T. et al. Effect of chemically induced hypoxia on osteogenic and angiogenic differentiation of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells in direct coculture. Cells 9, 757 (2020).
Ahluwalia, A. & Tarnawski, S. A. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 19, 90–97 (2012).
Hoeben, A. et al. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56, 549–580 (2004).
Tang, N. et al. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6, 485–495 (2004).
Lin, D. et al. Hypoxia-induced reprogramming of glucose-dependent metabolic pathways maintains the stemness of human bone marrow-derived endothelial progenitor cells. Sci. Rep. 13, 8776 (2023).
He, Q. et al. Enhancement of de novo lipogenesis by the IDH1 and IDH2-dependent reverse TCA cycle maintains the growth and angiogenic capacity of bone marrow-derived endothelial progenitor cells under hypoxia. Free Radic. Biol. Med. 213, 327–342 (2024).
Huang, Y. et al. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1α-dependent function. Proc. Natl. Acad. Sci. USA 109, 17478–17483 (2012).
Dos Santos, F. et al. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol 223, 27–35 (2010).
Kwon, S. Y. et al. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng. Regen. Med. 14, 595–604 (2017).
Pattappa, G. et al. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part C. Methods 19, 68–79 (2013).
Weijers, E. M. et al. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng. Part A 17, 2675–2685 (2011).
Utting, J. C. et al. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell Res. 312, 1693–1702 (2006).
Antebi, B. et al. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 9, 265 (2018).
Yang, M. et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect. Tissue Res. 60, 583–596 (2019).
Zhang, P. et al. Hypoxia suppresses osteogenesis of bone mesenchymal stem cells via the extracellular signal regulated 1/2 and p38 mitogen activated protein kinase signaling pathways. Mol. Med. Rep. 16, 5515–5522 (2017).
Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 658, 43–49 (2010).
Hong, C. Y. et al. Metformin reduces bone resorption in apical periodontitis through regulation of osteoblast and osteoclast differentiation. J. Endod. 49, 1129–1137 (2023).
Li, J. et al. Targeting endogenous hydrogen peroxide at bone defects promotes bone repair. Adv. Funct. Mater. 32, 2111208 (2022).
Lee, D. H., Lim, B. S., Lee, Y. K. & Yang, H. C. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol. Toxicol. 22, 39–46 (2006).
Mody, N., Parhami, F., Sarafian, T. A. & Demer, L. L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 31, 509–519 (2001).
Okamoto, T. et al. Phosphate enhances reactive oxygen species production and suppresses osteoblastic differentiation. J. Bone Miner. Metab. 32, 393–399 (2014).
Chen, C., Yan, S., Geng, Z. D. & Wang, Z. Fracture repair by IOX2: regulation of the hypoxia inducible factor-1α signaling pathway and BMSCs. Eur. J. Pharmacol. 921, 174864 (2022).
Chen, C. et al. HIF/Ca2+/NO/ROS is critical in roxadustat treating bone fracture by stimulating the proliferation and migration of BMSCs. Life Sci. 264, 118684 (2021).
Zou, D. et al. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1α mediated BMSCs. Biomaterials 33, 2097–2108 (2012).
Zou, D. et al. In vitro study of enhanced osteogenesis induced by HIF-1α-transduced bone marrow stem cells. Cell Prolif. 44, 234–243 (2011).
Zou, W. et al. Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J. Bone Miner. Metab. 33, 615–624 (2015).
Zhang, W. et al. MicroRNA-18a regulates the pyroptosis, apoptosis, and necroptosis (PANoptosis) of osteoblasts induced by tumor necrosis factor-α via hypoxia-inducible factor-1α. Int. Immunopharmacol. 128, 111453 (2024).
Wang, G. et al. Short-term hypoxia accelerates bone loss in ovariectomized rats by suppressing osteoblastogenesis but enhancing osteoclastogenesis. Med. Sci. Monit. 22, 2962–2971 (2016).
Kok, S. H. et al. Sirtuin 6 modulates hypoxia-induced apoptosis in osteoblasts via inhibition of glycolysis: implication for pathogenesis of periapical lesions. J. Endod. 41, 1631–1637 (2015).
Lai, E. H. et al. Metformin ameliorates periapical lesions through suppression of hypoxia-induced apoptosis of osteoblasts. J. Endod. 44, 1817–1825 (2018).
Li, S. et al. Metallothionein 3 promotes osteoblast differentiation in C2C12 cells via reduction of oxidative stress. Int. J. Mol. Sci. 22, 4312 (2021).
Zhang, L. et al. Chronic intermittent hypobaric hypoxia enhances bone fracture healing. Front. Endocrinol. 11, 582670 (2020).
Peng, Y., Wu, S., Li, Y. & Crane, J. L. Type H blood vessels in bone modeling and remodeling. Theranostics 10, 426–436 (2020).
Qiao, J., Liu, A., Sun, C. & Liu, Q. HIF1A overexpression promotes osteoblast differentiation through activation of autophagy to alleviate osteoporosis. Sci. Rep. 15, 30370 (2025).
Chen, C. et al. Activation of BMP4/SMAD pathway by HIF-1α in hypoxic environment promotes osteogenic differentiation of BMSCs and leads to ectopic bone formation. Tissue Cell 88, 102376 (2024).
Wan, C. et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 105, 686–691 (2008).
Orriss, I. R. et al. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 220, 155–162 (2009).
Arnett, T. R. et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 196, 2–8 (2003).
Lemma, S. et al. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 79, 168–180 (2016).
Indo, Y. et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Min. Res. 28, 2392–2399 (2013).
Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016).
Morten, K. J., Badder, L. & Knowles, H. J. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 229, 755–764 (2013).
Hulley, P. A. et al. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J. Pathol. 242, 322–333 (2017).
Kang, H. et al. Osteoblast hypoxia-inducible factor-1α pathway activation restrains osteoclastogenesis via the interleukin-33-microRNA-34a-Notch1 pathway. Front. Immunol. 8, 1312 (2017).
Knowles, H. J. The adenosine A2B receptor drives osteoclast-mediated bone resorption in hypoxic microenvironments. Cells 8, 624 (2019).
Tang, Y. et al. HIF-1α mediates osteoclast-induced mandibular condyle growth via AMPK. signaling. J. Dent. Res. 99, 1377–1386 (2020).
Tian, Y. et al. HIF-1α regulates osteoclast activation and mediates osteogenesis during mandibular bone repair via CT-1. Oral. Dis. 28, 428–441 (2022).
Nakashima, T. & Takayanagi, H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y Acad. Sci. 1240, E13–E18 (2011).
Wang, N., Hao, Y. & Fu, L. Trimethylamine-N-oxide promotes osteoclast differentiation and bone loss via activating ROS-dependent NF-κB signaling pathway. Nutrients 14, 3955 (2022).
Scheiermann, C. et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 (2012).
Melchart, D. et al. Circadian variation of the phagocytic activity of polymorphonuclear leukocytes and of various other parameters in 13 healthy male adults. Chronobiol. Int. 9, 35–45 (1992).
Hriscu, M. L. Modulatory factors of circadian phagocytic activity. Ann. N. Y. Acad. Sci. 1057, 403–430 (2005).
Casanova-Acebes, M. et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035 (2013).
Rittner, H. L. et al. CXCR1/2 ligands induce p38 MAPK-dependent translocation and release of opioid peptides from primary granules in vitro and in vivo. Brain Behav. Immun. 21, 1021–1032 (2007).
Adrover, J. M. et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 50, 390–402.e10 (2019).
Gibbs, J. et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20, 919–926 (2014).
Ella, K., Csépányi-Kömi, R. & Káldi, K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 57, 209–221 (2016).
Geiger, S. S., Curtis, A. M., O’Neill, L. A. J. & Siegel, R. M. Daily variation in macrophage phagocytosis is clock-independent and dispensable for cytokine production. Immunology 157, 122–136 (2019).
Keller, M. et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 106, 21407–21412 (2009).
Oliva-Ramírez, J. et al. Crosstalk between circadian rhythmicity, mitochondrial dynamics and macrophage bactericidal activity. Immunology 143, 490–497 (2014).
Lellupitiyage Don, S. S. et al. Macrophage circadian rhythms are differentially affected based on stimuli. Integr. Biol. 14, 62–75 (2022).
Zhou, Y. et al. Bmal1 regulates macrophage polarize through glycolytic pathway in alcoholic liver disease. Front. Pharmacol. 12, 640521 (2021).
Hong, H. et al. REV-ERBα agonist SR9009 suppresses IL-1β production in macrophages through BMAL1-dependent inhibition of inflammasome. Biochem. Pharm. 192, 114701 (2021).
Tian, Y. et al. Bmal1 knockout aggravates Porphyromonas gingivalis-induced periodontitis by activating the NF-κB pathway. J. Appl. Oral. Sci. 33, e20240388 (2025).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
O’Siorain, J. R. et al. Time-of-day control of mitochondria regulates NLRP3 inflammasome activation in macrophages. FASEB J. 38, e70235 (2024).
Astone, M. et al. The circadian protein BMAL1 supports endothelial cell cycle during angiogenesis. Cardiovasc. Res. 119, 1952–1968 (2023).
Yin, Y. et al. Endothelial BMAL1 decline during aging leads to bone loss by destabilizing extracellular fibrillin-1. J. Clin. Invest. 134, e176660 (2024).
Grafe, I. et al. TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb. Perspect. Biol. 10, a022202 (2018).
Grafe, I. et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med. 20, 670–675 (2014).
Jensen, L. D. et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2, 231–241 (2012).
Tsuzuki, K. et al. Adverse effect of circadian rhythm disorder on reparative angiogenesis in hind limb ischemia. J. Am. Heart Assoc. 10, e020896 (2021).
Xu, L. et al. Bmal1 downregulation worsens critical limb ischemia by promoting inflammation and impairing angiogenesis. Front. Cardiovasc Med. 8, 712903 (2021).
Weger, M. et al. Stem cells and the circadian clock. Dev. Biol. 431, 111–123 (2017).
He, Y., Chen, Y., Zhao, Q. & Tan, Z. Roles of brain and muscle ARNT-like 1 and Wnt antagonist Dkk1 during osteogenesis of bone marrow stromal cells. Cell Prolif. 46, 644–653 (2013).
Zhang, H. et al. Adipocytes derived from human bone marrow mesenchymal stem cells exert inhibitory effects on osteoblastogenesis. Curr. Mol. Med. 11, 489–502 (2011).
Lin, F. et al. Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem. Funct. 31, 166–172 (2013).
Samsa, W. E., Vasanji, A., Midura, R. J. & Kondratov, R. V. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84, 194–203 (2016).
Guo, B. et al. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J 26, 3453–3463 (2012).
Sahar, S. et al. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One 5, e8561 (2010).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).
Sato, F. et al. Smad3 and Snail show circadian expression in human gingival fibroblasts, human mesenchymal stem cell, and in mouse liver. Biochem. Biophys. Res. Commun. 419, 441–446 (2012).
Chen, Y. et al. Age-related BMAL1 change affects mouse bone marrow stromal cell proliferation and osteo-differentiation potential. Arch. Med. Sci. 8, 30–38 (2012).
Reale, M. E. et al. The transcription factor Runx2 is under circadian control in the suprachiasmatic nucleus and functions in the control of rhythmic behavior. PLoS One 8, e54317 (2013).
Zhuo, H., Wang, Y. & Zhao, Q. The interaction between Bmal1 and Per2 in mouse BMSC osteogenic differentiation. Stem Cells Int. 2018, 3407821 (2018).
Fu, L. et al. The molecular clock mediates leptin-regulated bone formation. Cell 122, 803–815 (2005).
Hirai, T., Tanaka, K. & Togari, A. β-adrenergic receptor signaling regulates Ptgs2 by driving circadian gene expression in osteoblasts. J. Cell Sci. 127, 3711–3719 (2014).
Jouffe, C. et al. The circadian clock coordinates ribosome biogenesis. PLoS Biol 11, e1001455 (2013).
Lipton, Jonathan, O. et al. The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell 161, 1138–1151 (2015).
Patel, S. A., Velingkaar, N. S. & Kondratov, R. V. Transcriptional control of antioxidant defense by the circadian clock. Antioxid. Redox Signal 20, 2997–3006 (2014).
Li, H. et al. BMAL1 regulates osteoblast differentiation through mTOR/GSK3β/β-catenin pathway. J. Mol. Endocrinol. 70, e220181 (2023).
Xiaoguang, L., Xiao-long, G. & Bin, G. BMAL1 gene regulates the osteogenic differentiation of bone marrow mesenchymal stem cells. Hua Xi Kou Qiang Yi Xue Za Zhi 34, 312–316 (2016).
Qian, Z. et al. Postnatal conditional deletion of Bmal1 in osteoblasts enhances trabecular bone formation via increased BMP2 signals. J. Bone Miner. Res. 35, 1481–1493 (2020).
Takarada, T. et al. Bone resorption is regulated by circadian clock in osteoblasts. J. Bone Miner. Res. 32, 872–881 (2017).
Aoyama, S. & Shibata, S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front. Neurosci. 11, 63 (2017).
Xu, C. et al. Circadian clock regulates bone resorption in mice. J. Bone Miner. Res. 31, 1344–1355 (2016).
Zhou, X. et al. BMAL1 deficiency promotes skeletal mandibular hypoplasia via OPG downregulation. Cell Prolif. 51, e12470 (2018).
Li, X. et al. BMAL1 regulates balance of osteogenic-osteoclastic function of bone marrow mesenchymal stem cells in type 2 diabetes mellitus through the NF-κB pathway. Mol. Biol. Rep. 45, 1691–1704 (2018).
Liu, M., Sun, Y. & Zhang, Q. Emerging role of extracellular vesicles in bone remodeling. J. Dent. Res. 97, 859–868 (2018).
Zhou, X., Cao, H., Yuan, Y. & Wu, W. Biochemical signals mediate the crosstalk between cartilage and bone in osteoarthritis. Biomed. Res. Int. 2020, 5720360 (2020).
Koritala, B. S. C. et al. Intermittent hypoxia alters the circadian expression of clock genes in mouse brain and liver. Genes. 12, 1627 (2021).
Xie, T. et al. The relationship between HIF1α and clock gene expression in patients with obstructive sleep apnea. Nat. Sci. Sleep. 14, 381–392 (2022).
Gabryelska, A. et al. Disruption of circadian rhythm genes in obstructive sleep apnea patients—possible mechanisms involved and clinical implication. Int. J. Mol. Sci. 23, 709 (2022).
Swanson, C. M. et al. Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J. Bone Miner. Res. 30, 199–211 (2015).
Sadaf, S. et al. Effect of obstructive sleep apnea on bone mineral density. Turk. Thorac. J 22, 301–310 (2021).
Manella, G. et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl. Acad. Sci. USA 117, 779–786 (2020).
Dandavate, V. et al. Hepatic BMAL1 and HIF1α regulate a time-dependent hypoxic response and prevent hepatopulmonary-like syndrome. Cell Metab. 36, 2038–2053.e5 (2024).
Kim, E.-J. et al. Transcriptional activation of HIF-1 by RORα and its role in hypoxia signaling. Arterioscler. Thromb. Vasc. Biol. 28, 1796–1802 (2008).
Diao, X. et al. Structural basis for the ligand-dependent activation of heterodimeric AHR-ARNT complex. Nat. Commun. 16, 1282 (2025).
Michael, A. K. et al. Cooperation between bHLH transcription factors and histones for DNA access. Nature 619, 385–393 (2023).
Xue, T. et al. Investigations of the CLOCK and BMAL1 proteins binding to DNA: a molecular dynamics simulation study. PLoS One 11, e0155105 (2016).
Zhang, J. et al. Systematic and comprehensive insights into HIF-1 stabilization under normoxic conditions: implications for cellular adaptation and therapeutic strategies in cancer. Cell Mol. Biol. Lett. 30, 2 (2025).
Atchley, W. R. & Fitch, W. M. A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. USA 94, 5172–5176 (1997).
Crews, S. T. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 12, 607–620 (1998).
Lee, Y. et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J. Cell Sci. 123, 3547–3557 (2010).
Li, J. et al. Advances in inhibition of protein-protein interactions targeting hypoxia-inducible factor-1 for cancer therapy. Bioorg. Med. Chem. 27, 1145–1158 (2019).
Kewley, R. J., Whitelaw, M. L. & Chapman-Smith, A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 36, 189–204 (2004).
Peek, C. B. et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92 (2017).
Wu, Y. et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 25, 73–85 (2017).
Peek, C. B. Metabolic implications of circadian–HIF crosstalk. Trends Endocrinol. Metab. 31, 459–468 (2020).
Suyama, K. et al. Circadian factors BMAL1 and RORα control HIF-1α transcriptional activity in nucleus pulposus cells: implications in maintenance of intervertebral disc health. Oncotarget. 7, 23056–23071 (2016).
Ma, Z. et al. Deletion of clock gene Bmal1 impaired the chondrocyte function due to disruption of the HIF1α-VEGF signaling pathway. Cell Cycle 18, 1473–1489 (2019).
Yu, C. et al. Hypoxia disrupts the expression levels of circadian rhythm genes in hepatocellular carcinoma. Mol. Med. Rep. 11, 4002–4008 (2015).
Ran, J. et al. Hypoxia regulates glycolysis through the HIF-1α/BMAL1/ALDOC axis to reduce oxaliplatin sensitivity in colorectal cancer. J. Cancer 16, 2503–2515 (2025).
Iacobini, C. et al. Mutual regulation between redox and hypoxia-inducible factors in cardiovascular and renal complications of diabetes. Antioxidants 11, 2183 (2022).
Zhang, C. et al. Oxidative stress: a common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 163, 114834 (2023).
Samanta, D. & Semenza, G. L. Maintenance of redox homeostasis by hypoxia-inducible factors. Redox Biol. 13, 331–335 (2017).
Stegen, S. et al. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 23, 265–279 (2016).
Pekovic-Vaughan, V. et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 28, 548–560 (2014).
Mezhnina, V., Ebeigbe, O. P., Poe, A. & Kondratov, R. V. Circadian control of mitochondria in reactive oxygen species homeostasis. Antioxid. Redox Signal. 37, 647–663 (2022).
Early, J. O. et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 115, E8460–E8468 (2018).
Kondratov, R. V., Vykhovanets, O., Kondratova, A. A. & Antoch, M. P. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 1, 979–987 (2009).
Stangherlin, A. & Reddy, A. B. Regulation of circadian clocks by redox homeostasis. J. Biol. Chem. 288, 26505–26511 (2013).
Li, E. et al. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell 11, 661–679 (2020).
Alexander, R. K. et al. Bmal1 integrates mitochondrial metabolism and macrophage activation. eLife 9, e54090 (2020).
Wang, X. et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J. Ethnopharmacol. 241, 111801 (2019).
Meng, X., Wielockx, B., Rauner, M. & Bozec, A. Hypoxia-inducible factors regulate osteoclasts in health and disease. Front Cell Dev. Biol. 9, 658893 (2021).
Chaikin, C. A. et al. Control of circadian muscle glucose metabolism through the BMAL1-HIF axis in obesity. Proc. Natl. Acad. Sci. USA 122, e2424046122 (2025).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Dengler, V. L., Galbraith, M. & Espinosa, J. M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 49, 1–15 (2014).
Miao, Z. et al. Role of Selenoprotein W in participating in the progression of non-alcoholic fatty liver disease. Redox Biol. 71, 103114 (2024).
Deng, W. et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 24, 366–378 (2018).
Timmons, G. A. et al. The circadian clock protein BMAL1 acts as a metabolic sensor in macrophages to control the production of Pro IL-1β. Front. Immunol. 12, 700431 (2021).
Mills, E. & O’Neill, L. A. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24, 313–320 (2014).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e13 (2016).
De Jesus, A. et al. Hexokinase 1 cellular localization regulates the metabolic fate of glucose. Mol. Cell 82, 1261–1277.e9 (2022).
Yao, S. et al. Astrocytic lactate dehydrogenase A regulates neuronal excitability and depressive-like behaviors through lactate homeostasis in mice. Nat. Commun. 14, 729 (2023).
Liu, B. et al. Hypoxia affects HIF-1/LDH-A signaling pathway by methylation modification and transcriptional regulation in Japanese Flounder (Paralichthys olivaceus). Biology 11, 1233 (2022).
Masoud, G. N. & Li, W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 5, 378–389 (2015).
Yoo, I. D. et al. Elevated CLOCK and BMAL1 contribute to the impairment of aerobic glycolysis from astrocytes in Alzheimer’s disease. Int. J. Mol. Sci. 21, 7862 (2020).
Biddlestone, J., Bandarra, D. & Rocha, S. The role of hypoxia in inflammatory disease (review). Int. J. Mol. Med. 35, 859–869 (2015).
Holmquist-Mengelbier, L. et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10, 413–423 (2006).
Zhang, F. J., Luo, W. & Lei, G. H. Role of HIF-1α and HIF-2α in osteoarthritis. Jt. Bone Spine 82, 144–147 (2015).
Lee, S. Y. et al. Differential but complementary roles of HIF-1α and HIF-2α in the regulation of bone homeostasis. Commun. Biol. 7, 892 (2024).
Pelster, B. & Egg, M. Multiplicity of hypoxia-inducible transcription factors and their connection to the circadian clock in the zebrafish. Physiol. Biochem. Zool. 88, 146–157 (2015).
Ruan, W. et al. BMAL1-HIF2A heterodimer modulates circadian variations of myocardial injury. Nature 641, 1017–1028 (2025).
Ramakrishnan, S. K. et al. Loss of von Hippel-Lindau protein (VHL) increases systemic cholesterol levels through targeting hypoxia-inducible factor 2α and regulation of bile acid homeostasis. Mol. Cell. Biol. 34, 1208–1220 (2014).
Feskanich, D., Hankinson, S. E. & Schernhammer, E. S. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos. Int. 20, 537–542 (2009).
Quevedo, I. & Zuniga, A. M. Low bone mineral density in rotating-shift workers. J. Clin. Densitom. 13, 467–469 (2010).
Zhang, H. et al. Correlation between occupational hazard exposure and abnormal bone mineral density in steelworkers. BMC Public Health 25, 1431 (2025).
Kohl, I. S. et al. Association between shift work and vitamin D levels in Brazilian female workers. Nutrients 17, 1201 (2025).
Buyukhatipoglu, H. et al. Oxidative stress increased in healthcare workers working 24-hour on-call shifts. Am. J. Med. Sci. 340, 462–467 (2010).
Zou, Y. et al. Nightshift work can induce oxidative DNA damage: a pilot study. BMC Public Health 23, 891 (2023).
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98 (2018).
Zhang, W. et al. Associations of metabolic dysfunction-associated fatty liver disease and hepatic fibrosis with bone mineral density and risk of osteopenia/osteoporosis in T2DM patients. Front. Endocrinol. 14, 1278505 (2023).
Sheu, A., White, C. P. & Center, J. R. Bone metabolism in diabetes: a clinician’s guide to understanding the bone-glucose interplay. Diabetologia 67, 1493–1506 (2024).
Javeed, N. & Matveyenko, A. V. Circadian etiology of type 2 diabetes mellitus. Physiology 33, 138–150 (2018).
Li, X. et al. Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 cooperates with glycogen synthase kinase-3β to regulate osteogenesis of bone-marrow mesenchymal stem cells in type 2 diabetes. Mol. Cell. Endocrinol. 440, 93–105 (2017).
Liu, T. et al. ED-71 ameliorates bone loss in type 2 diabetes mellitus by enhancing osteogenesis through upregulation of the circadian rhythm coregulator BMAL1. Drug Des. Dev. Ther. 18, 3903–3919 (2024).
Catrina, S. B. & Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 64, 709–716 (2021).
March, L. et al. Burden of disability due to musculoskeletal (MSK) disorders. Best. Pract. Res. Clin. Rheumatol. 28, 353–366 (2014).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Snelling, S. J. B. et al. The chondrocyte-intrinsic circadian clock is disrupted in human osteoarthritis. Chronobiol. Int. 33, 574–579 (2016).
Yang, W. et al. Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology 157, 3096–3107 (2016).
Soul, J. et al. Stratification of knee osteoarthritis: two major patient subgroups identified by genome-wide expression analysis of articular cartilage. Ann. Rheum. Dis. 77, 423 (2018).
Fisch, K. M. et al. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthr. Cartil. 26, 1531–1538 (2018).
Liao, L. et al. Deletion of Bmal1 in aggrecan-expressing cells leads to mouse temporomandibular joint osteoarthritis. J. Bone Min. Miner. 42, 529–537 (2024).
Dudek, M. et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Invest. 126, 365–376 (2016).
Qian, Z. et al. Cartilage-specific deficiency of clock gene Bmal1 accelerated articular cartilage degeneration in osteoarthritis by up-regulation of mTORC1 signaling. Int. Immunopharmacol. 115, 109692 (2023).
Yudoh, K. et al. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 7, R904–R914 (2005).
Pfander, D., Cramer, T., Schipani, E. & Johnson, R. S. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 116, 1819–1826 (2003).
Hu, S. et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 11, 481 (2020).
Qing, L. et al. Expression of hypoxia-inducible factor-1α in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp. Ther. Med. 13, 63–68 (2017).
Bouaziz, W. et al. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. USA 113, 5453–5458 (2016).
Zhang, H. et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 9, eabo7868 (2023).
Leo, C., Giaccia, A. J. & Denko, N. C. The hypoxic tumor microenvironment and gene expression. Semin. Radiat. Oncol. 14, 207–214 (2004).
Wang, C. et al. Stabilization of hypoxia-inducible factor-1α alleviates osteoarthritis via interacting with Per2 and resetting the circadian clock. Tissue Cell 79, 101942 (2022).
Brunette, D. M., Tengvall, P., Textor, M. & Thomsen, P. Properties and Biological Significance of Natural Oxide Films on Titanium and Its Alloys Ch. 7, 171–230 (Springer, Berlin, 2001).
Berglundh, T., Abrahamsson, I., Lang, N. P. & Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral. Implants Res. 14, 251–262 (2003).
Okawa, H., Egusa, H. & Nishimura, I. Implications of the circadian clock in implant dentistry. Dent. Mater. J. 39, 173–180 (2020).
Deng, S., Qi, M., Gong, P. & Tan, Z. The circadian clock component BMAL1 regulates osteogenesis in osseointegration. Front. Pediatr. 10, 1091296 (2022).
Nishimura, I. Genetic networks in osseointegration. J. Dent. Res 92, 109s–18s (2013).
Song, S. et al. HIF-1α increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1α/VEGF/AKT/mTOR signaling pathway. J. Nanobiotechnol. 21, 257 (2023).
Wang, Y. et al. Impact of high-altitude hypoxia on early osseointegration with bioactive titanium. Front. Physiol. 12, 689807 (2021).
Chen, X. et al. Cobalt-doped layered hydroxide coating on titanium implants promotes vascularization and osteogenesis for accelerated fracture healing. Mater. Today Bio 24, 100912 (2024).
Hassan, N. et al. Titanium biomaterials with complex surfaces induced aberrant peripheral circadian rhythms in bone marrow mesenchymal stromal cells. PLoS One 12, e0183359 (2017).
Ye, L. et al. Circadian rhythm disruption aggravates alveolar bone loss in rat apical periodontitis. Int. Endod. J. 58, 744–756 (2025).
Lin, P. et al. A single-cell RNA sequencing guided multienzymatic hydrogel design for self-regenerative repair in diabetic mandibular defects. Adv. Mater. 36, e2410962 (2024).
Cao, Z. et al. Yes-associated protein promotes bone healing after tooth extraction in mice. Biochem. Biophys. Res. Commun. 609, 39–47 (2022).
Dimova, E. Y. et al. The circadian clock protein CRY1 is a negative regulator of HIF-1α. iScience 13, 284–304 (2019).
Vaughan, M. E. et al. Cryptochromes suppress HIF1α in muscles. iScience 23, 101338 (2020).
Gao, L., Mejías, R., Echevarría, M. & López-Barneo, J. Induction of the glucose-6-phosphate dehydrogenase gene expression by chronic hypoxia in PC12 cells. FEBS Lett. 569, 256–260 (2004).
Peng, L. et al. The hepatic clock synergizes with HIF-1α to regulate nucleotide availability during liver damage repair. Nat. Metab. 7, 148–165 (2025).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Beker, M. C. et al. Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci. Rep. 9, 19082 (2019).
Dyar, K. A. et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 3, 29–41 (2014).
Bernhardt, S. et al. Functional proteomics of breast cancer metabolism identifies GLUL as responder during hypoxic adaptation. J. Proteome Res. 18, 1352–1362 (2019).
Lee, S. H., Golinska, M. & Griffiths, J. R. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells 10, 2371 (2021).
Huang, A. et al. Circadian clock gene expression regulates cancer cell growth through glutaminase. Acta Biochim. Biophys. Sin. 46, 409–414 (2014).
Liu, X. et al. Glucose- and glutamine-dependent bioenergetics sensitize bone mechanoresponse after unloading by modulating osteocyte calcium dynamics. J. Clin. Invest 133, e164508 (2023).
Guo, Y. et al. Systematic review with meta-analysis: HIF-1α attenuates liver ischemia-reperfusion injury. Transpl. Rev. 29, 127–134 (2015).
Feng, J. et al. Downregulation of Bmal1 expression in celiac ganglia protects against hepatic ischemia-reperfusion injury. Biomolecules 13, 713 (2023).
Hwang, S. et al. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J. Biol. Chem. 292, 9382–9393 (2017).
Erzurumlu, Y., Catakli, D. & Dogan, H. K. Circadian oscillation pattern of Endoplasmic Reticulum Quality Control (ERQC) components in human embryonic kidney HEK293 cells. J. Circadian Rhythms 21, 1 (2023).
Ezzeddini, R. et al. Clinical importance of FASN in relation to HIF-1α and SREBP-1c in gastric adenocarcinoma. Life Sci. 224, 169–176 (2019).
Liu, M. et al. Circadian clock and lipid metabolism disorders: a potential therapeutic strategy for cancer. Front. Endocrinol. 14, 1292011 (2023).
Hepler, C. et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 378, 276–284 (2022).
Jinteng, L. et al. BMAL1-TTK-H2Bub1 loop deficiency contributes to impaired BM-MSC-mediated bone formation in senile osteoporosis. Mol. Ther. Nucleic Acids 31, 568–585 (2023).
Min, H.-Y. et al. Bmal1 induces osteoblast differentiation via regulation of BMP2 expression in MC3T3-E1 cells. Life Sci. 162, 41–46 (2016).
Fujihara, Y., Kondo, H., Noguchi, T. & Togari, A. Glucocorticoids mediate circadian timing in peripheral osteoclasts resulting in the circadian expression rhythm of osteoclast-related genes. Bone 61, 1–9 (2014).
Harfmann, B. D. et al. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 6, 12 (2016).
Chen, M. et al. Reprogramming of rhythmic liver metabolism by intestinal clock. J. Hepatol. 79, 741–757 (2023).
Gu, W. et al. Metabolic Profile and Lipid Metabolism Phenotype in Mice with Conditional Deletion of Hepatic BMAL1. Int. J. Mol. Sci. 25, 6070 (2024).
Bignon, Y. et al. Multiomics reveals multilevel control of renal and systemic metabolism by the renal tubular. J. Clin. Invest. 133, e167133 (2023).
Acknowledgements
This study was supported by the National Natural Science Foundation of China, No. 82171003 & No. 82171002.
Author information
Authors and Affiliations
Contributions
Yi-Hang Weng was the major contributor in writing the manuscript and making the figures with support from Zhen Tan & Qing Zhao. Jiong Xiong contributed to part of the writing and provided suggestions for the manuscript. Zhen Tan & Qing Zhao reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weng, Y., Xiong, J., Zhao, Q. et al. HIF-1α and BMAL1 in bone regeneration: crosstalk between hypoxia response and circadian rhythm. Bone Res 14, 25 (2026). https://doi.org/10.1038/s41413-026-00506-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41413-026-00506-8