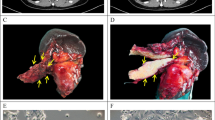
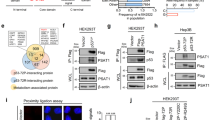
Abstract
Background
The objective of this study was to develop an innovative treatment strategy utilizing antisense oligonucleotides (ASOs) that target the gene encoding protocadherin alpha 11 (PCDHA11) and to elucidate the role of PCDHA11 in gastric cancer cells.
Methods
We designed and screened 54 amido-bridged nucleic acid (AmNA)-modified ASOs, selecting them based on PCDHA11-knockdown efficacy, in vitro and in vivo activity, and off-target effects. We assessed the impact of AmNA-modified anti-PCDHA11 ASOs on cellular functions and signaling pathways, and investigated the effects of Pcdha11 deficiency in mice.
Results
AmNA-modified anti-PCDHA11 ASOs significantly reduced the proliferation of gastric cancer cells and other solid tumors, whereas overexpression of PCDHA11 enhanced cell proliferation. The selected ASOs inhibited cellular functions related to the metastatic potential of gastric cancer cells, including migration, invasiveness, spheroid formation, and cancer stemness. Our findings revealed that AmNA-modified anti-PCDHA11 ASOs disrupted the AKT/mTOR, Wnt/β-catenin, and JAK/STAT signaling pathways. In mouse models of peritoneal metastasis (gastric and pancreatic cancer), systemic metastasis, and established subcutaneous tumors, administration of AmNA-modified anti-PCDHA11 ASOs inhibited tumor growth. ASO treatment induced reversible, dose- and sequence-dependent liver damage. Pcdha11-deficient mice demonstrated normal reproductive, organ, and motor functions.
Conclusions
AmNA-modified anti-PCDHA11 ASOs offer a promising therapeutic strategy for the treatment of gastric cancer and other solid malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data sources and handling of the publicly available datasets used in this study are described in the Materials and Methods. The other data generated in this study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F, Giommoni E, et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38:537–48.
Kanda M, Terashima M, Kinoshita T, Yabusaki H, Tokunaga M, Kodera Y. A multi-institutional study to evaluate the feasibility of next-generation sequencing and genomic analysis using formalin-fixed, paraffin-embedded biopsies of gastric cancer. Gastric Cancer. 2023;26:108–15.
Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–32.
Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–214.
van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–34.
Yu W, Yang L, Li T, Zhang Y. Cadherin signaling in cancer: its functions and role as a therapeutic target. Front Oncol. 2019;9:989.
Sisto M, Ribatti D, Lisi S. Cadherin signaling in cancer and autoimmune diseases. Int J Mol Sci. 2021;22:13358.
Hayashi S, Takeichi M. Emerging roles of protocadherins: from self-avoidance to enhancement of motility. J Cell Sci. 2015;128:1455–64.
Pancho A, Aerts T, Mitsogiannis MD, Seuntjens E. Protocadherins at the crossroad of signaling pathways. Front Mol Neurosci. 2020;13:117.
Yahara A, Shrestha AR, Yamamoto T, Hari Y, Osawa T, Yamaguchi M, et al. Amido-bridged nucleic acids (AmNAs): synthesis, duplex stability, nuclease resistance, and in vitro antisense potency. Chembiochem. 2012;13:2513–6.
Cui L, Nakano K, Obchoei S, Setoguchi K, Matsumoto M, Yamamoto T, et al. Small nucleolar noncoding RNA SNORA23, up-regulated in human pancreatic ductal adenocarcinoma, regulates expression of spectrin repeat-containing nuclear envelope 2 to promote growth and metastasis of xenograft tumors in mice. Gastroenterology. 2017;153:292–306.e2.
Kanda M, Tanaka H, Shimizu D, Miwa T, Umeda S, Tanaka C, et al. SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37:5355-66.
Kanda M, Shimizu D, Sawaki K, Nakamura S, Umeda S, Miwa T, et al. Therapeutic monoclonal antibody targeting of neuronal pentraxin receptor to control metastasis in gastric cancer. Mol Cancer. 2020;19:131.
Szasz AM, Lanczky A, Nagy A, Forster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–33.
Kanda M, Kasahara Y, Shimizu D, Miwa T, Umeda S, Sawaki K, et al. Amido-bridged nucleic acid-modified antisense oligonucleotides targeting SYT13 to treat peritoneal metastasis of gastric cancer. Mol Ther Nucleic Acids. 2020;22:791–802.
Hori S, Yamamoto T, Waki R, Wada S, Wada F, Noda M, et al. Ca2+ enrichment in culture medium potentiates effect of oligonucleotides. Nucleic Acids Res. 2015;43:e128.
Kanda M, Shimizu D, Nakamura S, Sawaki K, Umeda S, Miwa T, et al. Blockade of CHRNB2 signaling with a therapeutic monoclonal antibody attenuates the aggressiveness of gastric cancer cells. Oncogene. 2021;40:5495–504.
Naito Y, Bono H. GGRNA: an ultrafast, transcript-oriented search engine for genes and transcripts. Nucleic Acids Res. 2012;40:W592–6.
Yoshida T, Naito Y, Sasaki K, Uchida E, Sato Y, Naito M, et al. Estimated number of off-target candidate sites for antisense oligonucleotides in human mRNA sequences. Genes Cells. 2018;23:448–55.
Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–54.
Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase IIi trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36:1922–9.
Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, et al. Significance of SYT8 for the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg. 2018;267:495–503.
Ishigami H, Kitayama J, Otani K, Kamei T, Soma D, Miyato H, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311–4.
Sato A, Rahman NIA, Shimizu A, Ogita H. Cell-to-cell contact-mediated regulation of tumor behavior in the tumor microenvironment. Cancer Sci. 2021;112:4005–12.
Wan Z, Rasheed M, Li Y, Li Q, Wang P, Li J, et al. miR-218-5p and miR-320a-5p as biomarkers for brain disorders: focus on the major depressive disorder and Parkinson’s disease. Mol Neurobiol. 2023;60:5642–54.
Gray ME, Johnson ZR, Modak D, Tamilselvan E, Tyska MJ, Sotomayor M. Heterophilic and homophilic cadherin interactions in intestinal intermicrovillar links are species dependent. PLoS Biol. 2021;19:e3001463.
Ning Y, Deng C, Li C, Peng W, Yan C, Ran J, et al. PCDH20 inhibits esophageal squamous cell carcinoma proliferation and migration by suppression of the mitogen-activated protein kinase 9/AKT/β-catenin pathway. Front Oncol. 2022;12:937716.
Ye Z, Yang Y, Wei Y, Li L, Wang X, Zhang J. PCDH1 promotes progression of pancreatic ductal adenocarcinoma via activation of NF-κB signalling by interacting with KPNB1. Cell Death Dis. 2022;13:633.
Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P. Role of cadherins in cancer-a review. Int J Mol Sci. 2020;21:7624.
Santarosa M, Maestro R. The autophagic route of e-cadherin and cell adhesion molecules in cancer progression. Cancers (Basel). 2021;13:6328.
Carrier A, Desjobert C, Lobjois V, Rigal L, Busato F, Tost J, et al. Epigenetically regulated PCDHB15 impairs aggressiveness of metastatic melanoma cells. Clin Epigenetics. 2022;14:156.
Lee JH, Park SA, Park IG, Yoon BK, Lee JS, Lee JM. Stem cell properties of gastric cancer stem-like cells under stress conditions are regulated via the c-Fos/UCH-L3/β-Catenin Axis. Mol Cells. 2023;46:476–85.
Castanotto D, Stein CA. Antisense oligonucleotides in cancer. Curr Opin Oncol. 2014;26:584–9.
Hagedorn PH, Pontoppidan M, Bisgaard TS, Berrera M, Dieckmann A, Ebeling M, et al. Identifying and avoiding off-target effects of RNase H-dependent antisense oligonucleotides in mice. Nucleic Acids Res. 2018;46:5366–80.
Dieckmann A, Hagedorn PH, Burki Y, Brügmann C, Berrera M, Ebeling M, et al. A sensitive in vitro approach to assess the hybridization-dependent toxic potential of high affinity gapmer oligonucleotides. Mol Ther Nucleic Acids. 2018;10:45–54.
Dang Z, Shangguan J, Zhang C, Hu P, Ren Y, Lv Z, et al. Loss of protocadherin-17 (PCDH-17) promotes metastasis and invasion through hyperactivation of EGFR/MEK/ERK signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016;37:2527–35.
De Velasco MA, Kura Y, Sakai K, Hatanaka Y, Davies BR, Campbell H, et al. Targeting castration-resistant prostate cancer with androgen receptor antisense oligonucleotide therapy. JCI Insight. 2019;4:e122688.
Matsumoto S, Yamamichi T, Shinzawa K, Kasahara Y, Nojima S, Kodama T, et al. GREB1 induced by Wnt signaling promotes development of hepatoblastoma by suppressing TGFβ signaling. Nat Commun. 2019;10:3882.
Eckburg A, Dein J, Berei J, Schrank Z, Puri N. Oligonucleotides and microRNAs targeting telomerase subunits in cancer therapy. Cancers (Basel). 2020;12:2337.
Barresi V, Musmeci C, Rinaldi A, Condorelli DF. Transcript-targeted therapy based on RNA interference and antisense oligonucleotides: current applications and novel molecular targets. Int J Mol Sci. 2022;23:8875.
Uehara T, Choong CJ, Nakamori M, Hayakawa H, Nishiyama K, Kasahara Y, et al. Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease. Sci Rep. 2019;9:7567.
Yoshida M, Oda C, Mishima K, Tsuji I, Obika S, Shimojo M. An antisense amido-bridged nucleic acid gapmer oligonucleotide targeting SRRM4 alters REST splicing and exhibits anti-tumor effects in small cell lung cancer and prostate cancer cells. Cancer Cell Int. 2023;23:8.
Acknowledgements
We thank Edanz Group (www.edanzediting.com/ac) and Springer Nature Author Services for editing a draft of this paper.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED; JP20lm02030005, JP21ak0101154, JP22ak0101154 and JP23ak0101154) and a Grant-in-Aid for Scientific Research (20K21629).
Author information
Authors and Affiliations
Contributions
Study concept and design: M.K. (Mitsuro Kanda), Y.K. (Yuuya Kasahara) and S.O. Acquisition of the data: Y.K. (Yuuya Kasahara), S.N., T.S., M.S., Y.I. and M.K. (Masahisa Katsuno). Statistical analysis: D.S. Management of data acquisition: M.K. (Mitsuro Kanda) and Y.K. (Yuuya Kasahara). Analysis of the present data: S.N., T.S., M.S. D.S. and Y.I. Critical interpretation of the present data: M.K. (Mitsuro Kanda) and Y.K. (Yuuya Kasahara). Drafting of the paper: M.K. (Mitsuro Kanda). Critical revision of the paper for important intellectual content: Y.K. (Yuuya Kasahara), S.N., T.S., M.S., D.S., Y.I. and M.K. (Masahisa Katsuno), S.O. and Y.K. (Yasuhiro Kodera). Obtained funding: M.K. (Mitsuro Kanda), Y.K. (Yuuya Kasahara) and S.O. Technical or material support: Y.K. (Yuuya Kasahara) and S.O. Study supervision: Y.K. (Yasuhiro Kodera).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Institutional Review Board of Nagoya University, Japan, approved this study, and written informed consent was obtained from all patients (approval number 2014-0043). The Animal Research Committee of Nagoya University approved experiments using animals, performed according to ARRIVE guidelines (approval number M230102-002).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanda, M., Kasahara, Y., Shimizu, D. et al. Dual-modified antisense oligonucleotides targeting oncogenic protocadherin to treat gastric cancer. Br J Cancer 131, 1555–1566 (2024). https://doi.org/10.1038/s41416-024-02859-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-024-02859-5
This article is cited by
-
Advances in RNA-based cancer therapeutics: pre-clinical and clinical implications
Molecular Cancer (2025)