Abstract
Background
The open-label, single-arm, multicentre ORZORA trial (NCT02476968) evaluated maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer (PSR OC) with a germline (g) or somatic (s) BRCA1 and/or BRCA2 mutation (BRCAm) or a non-BRCA homologous recombination repair mutation (non-BRCA HRRm).
Methods
Patients were in response to platinum-based chemotherapy after ≥2 prior lines of treatment and underwent prospective central screening for tumour BRCA status, then central gBRCAm testing to determine sBRCAm or gBRCAm status. An exploratory cohort evaluated non-BRCA HRRm in 13 predefined genes. Patients received olaparib 400 mg (capsules) twice daily until investigator-assessed disease progression. Secondary endpoints included overall survival (OS) and safety.
Results
177 patients received olaparib. At the final data cutoff (25 June 2021), median OS from study enrolment was 46.8 (95% confidence interval [CI] 37.9–54.4), 43.2 (31.7–NC [not calculated]), 47.4 (37.9–NC) and 44.9 (28.9–NC) months in the BRCAm, sBRCAm, gBRCAm and non-BRCA HRRm cohorts, respectively. No new safety signals were identified.
Conclusion
Maintenance olaparib showed consistent clinical activity in the BRCAm and sBRCAm cohorts; exploratory analysis suggested similar activity in the non-BRCA HRRm cohort. These findings highlight that patients with PSR OC, beyond those with gBRCAm, may benefit from maintenance olaparib.
Similar content being viewed by others
Introduction
Most patients with high-grade epithelial ovarian cancer typically respond to debulking surgery followed by platinum-based chemotherapy as a first-line treatment; however, around 70% of patients will eventually experience relapse [1]. Maintenance therapy with the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib is a standard of care in platinum-sensitive relapsed ovarian cancer [2]. BRCA1 and/or BRCA2 mutations (BRCAm) are established mechanisms of homologous recombination deficiencies (HRDs) known to play a role in ovarian cancer and may be germline (g) or somatic (s) in origin. Approximately 20% of ovarian cancers have a BRCAm [3], with 15–17% of ovarian tumours reported to have a gBRCAm and 3–8% reported to have an sBRCAm [3, 4], although recent analysis of a large genomic dataset of ~75,000 tumour samples across six major tumour types suggested a 1:1 ratio of gBRCAm to sBRCAm in ovarian cancer [4].
Olaparib is a globally approved maintenance therapy for patients with platinum-sensitive relapsed ovarian cancer; in the USA olaparib is approved for patients with a BRCAm, whereas in Europe it is approved irrespective of BRCAm or other biomarker status [5,6,7,8,9]. Benefits of maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer have previously been reported. The Phase III SOLO2 trial demonstrated a clinically meaningful improvement in overall survival (OS) in patients with platinum-sensitive relapsed ovarian cancer and a gBRCAm receiving maintenance olaparib compared with placebo (hazard ratio [HR] 0.74; 95% confidence interval [CI] 0.54–1.00; P = 0.054). Although the inclusion criteria of the SOLO2 trial permitted enrolment of patients with an sBRCAm, no patients with an sBRCAm were enroled [10]. Furthermore, in the Phase II Study 19 trial, in patients with platinum-sensitive relapsed serous ovarian cancer, maintenance olaparib provided an apparent OS advantage compared with placebo regardless of BRCAm status (HR 0.73; 95% CI 0.55–0.95; nominal P = 0.021 in the full analysis set), although it did not meet the predefined threshold for statistical significance [11]. In Study 19, the OS Kaplan–Meier curves in the full analysis set, as well as in BRCAm and BRCA wildtype subgroups, separated in favour of olaparib as duration of follow-up increased, albeit without statistical significance being met [11]. This indicated the presence of a subset of patients experiencing long-term benefit from maintenance olaparib within the BRCA wildtype subgroup, suggestive of non-BRCA homologous recombination repair mutation (HRRm) [11].
Data evaluating the efficacy and safety of olaparib in patients with sBRCAm are limited [12, 13]; the ORZORA trial (NCT02476968) was designed to confirm the benefit of and provide rationale for the use of olaparib in this patient cohort and also included an exploratory cohort assessing maintenance olaparib in patients with a non-BRCA HRRm.
In the primary analysis of ORZORA (data cutoff [DCO] 17 April 2020), maintenance olaparib showed activity in patients with platinum-sensitive relapsed ovarian cancer and a BRCAm, including those with an sBRCAm [14]. Additionally, exploratory analyses showed activity in patients with a non-BRCA HRRm. Median progression-free survival (PFS) of 18.0 months (95% CI 14.3–22.1), 16.6 months (95% CI 12.4–22.2), 19.3 months (95% CI 14.3–27.6) and 16.4 months (95% CI 10.9–19.3) was reported in the BRCAm, sBRCAm, gBRCAm and non-BRCA HRRm cohorts, respectively [14].
In this analysis of the ORZORA trial, we report the final OS results alongside other secondary efficacy endpoints and updated safety and tolerability data.
Methods
Study design and patients
Study methods and full patient screening and enrolment criteria have previously been described in detail [14]. In brief, ORZORA was a Phase IV, open-label, single-arm, multicentre study of maintenance olaparib in patients with platinum-sensitive relapsed high-grade epithelial ovarian, primary peritoneal and/or fallopian tube cancer with a deleterious or suspected deleterious gBRCAm or sBRCAm, or a qualifying deleterious or suspected deleterious non-BRCA HRRm. Patients had platinum-sensitive disease, defined as disease progression ≥6 months after completion of the penultimate platinum-based chemotherapy regimen, and had completed ≥2 prior lines of platinum-based chemotherapy. Patients were in response, either complete or partial, following the last line of chemotherapy prior to study enrolment.
Patients were screened to determine BRCAm and HRRm status, undergoing prospective central screening for tumour BRCA status (MyChoice®CDx, Myriad Genetic Laboratories, Inc., Salt Lake City, UT, USA), then central gBRCAm testing (BRACAnalysis CDx®, Myriad Genetic Laboratories, Inc., Salt Lake City, UT, USA) to determine sBRCAm or gBRCAm status. Patients with previously confirmed gBRCAm were excluded to limit their inclusion and ensure ≥50 patients with sBRCAm were recruited. Following a protocol amendment (July 2016), patients without a tumour BRCAm were eligible for entry into the exploratory non-BRCA HRRm cohort. Non-BRCA HRRm status was determined via central tumour testing using the FoundationOne®CDx assay (Foundation Medicine, Inc., Cambridge, MA, USA). Qualifying genetic alterations were predicted deleterious or suspected deleterious loss of function mutations in any of the following 13 predefined genes involved (directly and indirectly) in the HRR pathway: ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D and RAD54L.
Within 8 weeks after their last dose of platinum-based chemotherapy, patients received maintenance olaparib capsules 400 mg twice daily (total daily dose: 800 mg) until investigator-assessed radiological disease progression (modified Response Evaluation Criteria in Solid Tumours [RECIST] version 1.1) or unacceptable toxicity. Some patients were permitted to continue olaparib beyond progression if the investigator deemed that the patient was benefitting from treatment in relation to other clinical assessments and did not meet other discontinuation criteria.
The trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines and the AstraZeneca policy of bioethics [15] and was approved by the Ethics Committee of Istituto Nazionale Tumori ‘Fondazione G Pascale’ (Reference no. 25/15). All patients provided written informed consent.
Endpoints and assessment
The co-primary endpoints of investigator-assessed PFS (modified RECIST version 1.1) in the BRCAm and sBRCAm cohorts have been reported previously [14].
OS and PFS2 were secondary endpoints; OS was defined as the time from date of study enrolment until death due to any cause and PFS2 was defined as the time to second progression or death. OS and PFS2 were reported in the any BRCAm and sBRCAm cohorts. Time to first subsequent therapy or death (TFST), time to second subsequent therapy or death (TSST), and safety and tolerability were also included in the secondary analyses in the any BRCAm and sBRCAm cohorts. OS and safety and tolerability were also assessed in the exploratory non-BRCA HRRm cohort. PFS2, TFST and TSST were not prespecified endpoints in the exploratory non-BRCA HRRm cohort.
The safety follow-up period was during study treatment and for 30 days after the last dose of olaparib. Myelodysplastic syndrome (MDS) events, acute myeloid leukaemia (AML) and new primary malignancy events were required to be reported even if they occurred after the end of the 30-day safety follow-up period.
Statistical analysis
The DCO was 25 June 2021. OS was reported as Kaplan–Meier estimates in the BRCAm, sBRCAm, gBRCAm and non-BRCA HRRm cohorts. PFS2, TFST and TSST were reported as Kaplan–Meier estimates in the BRCAm, sBRCAm and gBRCAm cohorts.
Efficacy results were reported using the full analysis set, which included all patients who were assigned to olaparib treatment. Safety results were reported using the safety analysis set, which included all patients who received at least one dose of olaparib.
Results
Patients
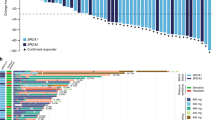
Of the 872 patients screened, 181 were enroled into the ORZORA study and 177 received olaparib and were therefore included in the safety analyses. One hundred and forty-five patients were included in the BRCAm cohort, with 55 and 87 patients included in the sBRCAm and gBRCAm cohorts, respectively; three patients were assigned to the BRCAm cohort with unknown s/gBRCAm status. Three enroled patients had neither a BRCAm nor a non-BRCA HRRm and were unassigned. Thirty-three patients were included in the exploratory non-BRCA HRRm cohort (Supplementary Fig. S1); the breakdown of individual non-BRCA HRRm genes in ORZORA is shown in Fig. 1. Baseline characteristics have been reported previously [14] and were consistent across cohorts (Supplementary Table S1). At the final DCO (25 June 2021), 34 of 177 patients (19.2%) were still receiving maintenance olaparib, including 12 (21.8%) patients with an sBRCAm and 6 (18.8%) with a non-BRCA HRRm. One hundred and forty-three patients had discontinued olaparib (79.0%), for whom disease progression, observed in 102 patients (71.3%), was the most frequently reported reason for olaparib discontinuation (Supplementary Table S2).
Overall survival
The final OS analysis was performed after 68 of 145 patients had died (46.9% maturity) (BRCAm cohort), including 28 (50.9%) deaths in the sBRCAm cohort. In the non-BRCA HRRm cohort, 14 of 33 (42.4%) patients had died. The median duration of follow-up in patients censored for OS was 42.6 (range 0.03–68.3) months in the BRCAm cohort (39.5 [5.8–57.4] months in sBRCAm; 45.3 [1.0–68.3] months in gBRCAm) and 39.3 (0.03–51.7) months in the non-BRCA HRRm cohort. Median OS was consistent across all cohorts at more than 3.5 years. In the BRCAm, sBRCAm, gBRCAm and non-BRCA HRRm cohorts, median OS was 46.8 (95% CI 37.9–54.4), 43.2 (95% CI 31.7–NC [not calculated]), 47.4 (95% CI 37.9–NC) and 44.9 (95% CI 28.9–NC) months, respectively (Fig. 2). At 30 months, Kaplan–Meier estimates for OS rates were 69.8% (95% CI 61.3–76.8) in the BRCAm cohort, 64.6% (95% CI 50.3–75.8) in the sBRCAm cohort, 73.0% (95% CI 61.9–81.3) in the gBRCAm cohort and 64.7% (95% CI 45.4–78.7) in the non-BRCA HRRm cohort (Fig. 2). Kaplan–Meier estimates for 36-month OS rates were 60.4% (95% CI 51.6–68.2), 56.8% (95% CI 42.4–68.8), 62.6% (95% CI 51.0–72.2) and 56.6% (95% CI 36.8–72.4) in the BRCAm, sBRCAm, gBRCAm and exploratory non-BRCA HRRm cohort, respectively (Fig. 2). Patient-level OS varied across individual non-BRCA HRRm gene mutations (Fig. 3).
Other secondary efficacy endpoints
At the time of final DCO, the maturity of PFS2 was 42.8% after 62 events in the BRCAm cohort. PFS2 was consistent across the BRCAm, sBRCAm and gBRCAm cohorts. At 30 months, the PFS2 rate was 58.1% (95% CI 48.1–66.8) in the BRCAm cohort, 48.7% (95% CI 32.9–62.8) in the sBRCAm cohort and 63.8% (95% CI 50.8–74.3) in the gBRCAm cohort (Kaplan–Meier estimates) (Supplementary Fig. S2A, B).
TFST analysis was performed at 50.3% maturity (BRCAm cohort). Consistent TFST results were observed in the BRCAm, sBRCAm and gBRCAm cohorts. At 30 months, the Kaplan–Meier estimate of the rate of freedom from the use of a first subsequent therapy or from death was 52.7% (95% CI 43.3–61.2) in the BRCAm cohort (51.2% [95% CI 36.3–64.3] in sBRCAm and 53.1% [95% CI 40.8–64.0] in gBRCAm) (Supplementary Fig. S3A, B). TSST was analysed at 41.4% maturity (BRCAm cohort); at 30 months, the Kaplan–Meier estimate of the rate of freedom from the use of a second subsequent therapy or from death was 61.1% (95% CI 51.3–69.4) in the BRCAm cohort (57.9% [95% CI 41.9–70.9] in sBRCAm and 62.6% [95% CI 49.9–73.0] in gBRCAm) (Kaplan–Meier estimates) (Supplementary Fig. S4A, B). The prespecified secondary efficacy endpoints of PFS2, TFST and TSST in the BRCAm, sBRCAm and gBRCAm cohorts are summarised in Supplementary Fig. S5. PFS2, TFST and TSST were not prespecified secondary endpoints in the exploratory non-BRCA HRRm cohort (data not shown).
Across all cohorts, patients received subsequent anticancer therapy; platinum-based chemotherapy was the most frequently reported subsequent therapy (Table 1). The proportion of patients receiving subsequent anticancer therapy was similar across the BRCAm cohorts.
Safety
The safety analyses included 177 patients and the median total treatment duration was 17.7 months (range 0.0–68.1 months). The adverse event (AE) profile was consistent with the primary analysis [14]. The majority of patients (93.8%) experienced at least one AE; however, most AEs were low-grade and only 11 patients (6.2%) had an AE leading to olaparib discontinuation at the final DCO. Treatment-emergent AEs leading to dose interruptions and reductions occurred in 91 patients (51.4%) and 51 patients (28.8%), respectively. The most common AEs of any grade included nausea (54.8% of patients), fatigue/asthenia (53.7%), anaemia (44.1%) and vomiting (28.2%). Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3 AEs were reported in 37.9% of patients and the most common grade ≥3 AE was anaemia, occurring in 16.4% of patients across the cohorts (Supplementary Table S3). Serious AEs were reported in 48 patients (27.1%) and AEs with an outcome of death were reported in 4 patients (2.3%). Neutropenia, diarrhoea, AML and MDS each led to discontinuation of olaparib in two patients (1.1%) (Supplementary Table S4).
Since the primary DCO (17 April 2020), MDS was reported in four patients (2.3%; in whom two cases of MDS were reported after the last dose of olaparib) and no new cases of AML were reported (two cases of AML were reported at the primary DCO [14]). Therefore, a total of six patients (3.4%) had experienced MDS/AML events at the final DCO (25 June 2021). One patient (0.6%) had experienced a new primary malignancy since the primary DCO, non-Hodgkin lymphoma. The total incidence of new primary malignancies was low; three patients (1.7%) experienced a new primary malignancy in the ORZORA study at the final DCO (one case of Burkitt’s lymphoma and one case of papillary thyroid cancer were reported at the primary DCO [14]).
Discussion
In the Phase IV ORZORA trial, maintenance olaparib for patients with platinum-sensitive relapsed ovarian cancer, who were in response to their most recent platinum-based chemotherapy after ≥2 prior lines of platinum-based chemotherapy, demonstrated consistent clinical activity in patients with a BRCAm, irrespective of germline or somatic status, or a non-BRCA HRRm. The primary analysis demonstrated the clinical activity of maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer and an sBRCAm and expanded on the limited data available for this patient cohort [14]. The final OS analysis reported here further defined the clinical activity of maintenance olaparib for patients with platinum-sensitive relapsed ovarian cancer, beyond those with a gBRCAm, with consistent OS and supportive clinical efficacy results reported across the different patient cohorts.
In the ORZORA study, median OS data reported in the BRCAm (46.8 months), sBRCAm (43.2 months), gBRCAm (47.4 months) and non-BRCA HRRm (44.9 months) cohorts were consistent with results reported in patients with platinum-sensitive relapsed ovarian cancer and a gBRCAm receiving maintenance olaparib in the SOLO2 trial (median OS 51.7 months vs. 38.8 months with placebo [HR 0.74; 95% CI 0.54–1.00; P = 0.054]) [10]. In the OS analysis of the Study 19 trial, maintenance olaparib provided a trend in OS compared with placebo, with median OS of 29.8 months in the olaparib arm and 27.8 months in the placebo arm (full analysis set; HR 0.73; 95% CI 0.55–0.95; nominal P = 0.021); however, the predefined threshold of statistical significance was not met [11]. Additionally, in the single-arm OPINION trial, the first study to prospectively assess maintenance olaparib monotherapy in patients with platinum-sensitive relapsed ovarian cancer without a gBRCAm, median OS of 32.7 months was reported for patients receiving maintenance olaparib [13, 16]. OPINION included a small group of patients with confirmed sBRCAm status; the 30-month Kaplan–Meier OS rate was 70.4% (95% CI 49.4–83.9) [16]. The OS results reported in the ORZORA trial may provide supportive evidence for the use of maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer and an sBRCAm or non-BRCA HRRm. In the newly diagnosed ovarian cancer setting, olaparib is approved for use as a first-line maintenance treatment. Maintenance olaparib improved OS versus placebo in patients with a BRCAm after 7 years of follow-up in the SOLO1 study (HR 0.55; 95% CI 0.40–0.76; P = 0.0004 [P < 0.0001 required to declare statistical significance]) [17], and maintenance olaparib plus bevacizumab provided a clinically meaningful OS improvement versus placebo plus bevacizumab in patients who tested HRD-positive after 5 years of follow-up in the PAOLA-1 study (HR 0.62; 95% CI 0.45–0.85) [18].
While comparisons between ORZORA and Phase III randomised controlled trials such as NOVA and ARIEL3 should be avoided due to differences in study design and patient populations, it is worth noting that although a PFS benefit was demonstrated in the primary analysis of NOVA, which evaluated maintenance niraparib versus placebo in patients with platinum-sensitive relapsed ovarian cancer with or without a gBRCAm [19], no OS benefit was seen in the non-gBRCAm cohort (including HRD-positive non-gBRCAm) who received niraparib [20, 21]. Similarly, although a PFS benefit was demonstrated in the primary analysis of ARIEL3, which evaluated maintenance rucaparib versus placebo in the relapsed disease setting [22], no OS benefit was observed in the HRD-positive cohort [23, 24]. These studies were not statistically powered for OS and crossover confounded these results; thus, interpretation is limited [23, 24]. However, these OS results from NOVA and ARIEL3 have led to restriction of maintenance niraparib to patients with gBRCAm platinum-sensitive relapsed ovarian cancer [25] and maintenance rucaparib to patients with BRCAm platinum-sensitive relapsed ovarian cancer [26] in the USA. Moreover, based on these data from NOVA and ARIEL3, maintenance olaparib was restricted to patients with BRCAm platinum-sensitive relapsed ovarian cancer in the USA [7].
Interestingly, in ORZORA, the exploratory non-BRCA HRRm analyses suggest a similar level of activity in patients with a non-BRCA HRRm to that seen in patients with BRCAm. Exploratory subgroup analyses from Study 19 of patients with platinum-sensitive relapsed ovarian cancer and a non-BRCA HRRm (n = 21) suggested a PFS benefit for maintenance olaparib versus placebo with an HR of 0.21 (95% CI 0.04–0.86) (HR for OS of 0.77 [95% CI 0.28–2.28]) compared with patients without a BRCAm or HRRm (n = 58) (HR for PFS of 0.71 [95% CI 0.37–1.35]; HR for OS of 1.19 [95% CI 0.66–2.10]) [27]. However, the small number of patients with a non-BRCA HRRm limits the interpretation of these results. Exploratory analyses from NOVA and ARIEL3 also suggested a PFS benefit in the subgroup of patients with non-BRCA HRRm (HR for PFS with maintenance niraparib vs. placebo of 0.31 [95% CI 0.13–0.77], n = 41 [28] and for maintenance rucaparib vs. placebo of 0.21 [95% CI 0.09–0.50], n = 43 [29]), although small patient numbers limit interpretation of these data. By contrast, non-BRCA HRRm gene panels were not predictive of PFS benefit with maintenance olaparib plus bevacizumab in the newly diagnosed advanced ovarian cancer setting in the PAOLA-1 study [30].
In the ORZORA study, 40.7% of patients in the BRCAm cohort received a subsequent anticancer therapy; similar levels of subsequent anticancer therapy were reported in the other patient cohorts. These data are important for the contextualisation of the OS results. Assessing improvements in OS can prove difficult in ovarian cancer trials as patients often receive multiple subsequent anticancer therapies post-progression, including experimental agents [31, 32]. Moreover, mortality unrelated to cancer or the treatment under investigation can confound OS results. Therefore, evaluation of supportive efficacy endpoints is useful to provide a comprehensive evaluation of efficacy and could be considered an appropriate surrogate for OS [32]. The supportive efficacy endpoints (PFS2, TFST and TSST) reported in this analysis are comparable with values published for SOLO2 and Study 19 [10, 11]. PFS2, TFST and TSST also favoured PARP inhibitor maintenance over placebo in NOVA and ARIEL3 [33, 34].
The safety profile of maintenance olaparib was consistent with the primary analysis [14] and with previous reports in this setting [10, 16], and no new safety findings were identified. Consistent with previous PARP inhibitor studies, AEs in ORZORA were usually managed by dose interruption [35]; dose interruptions due to AEs were reported in 51.4% of patients. In line with the primary analysis, the incidence of MDS/AML remained low, with a total incidence of 3.4% at the final DCO. An imbalance in MDS/AML was observed between maintenance olaparib and placebo (8% vs. 4% of patients) after approximately 5 years’ follow-up in the relapsed disease setting in SOLO2 [10]. Factors that may have contributed to this imbalance include potential baseline risk factors, MDS/AML being a long latency event and the longer duration of OS observed in the olaparib arm. The incidence of MDS/AML remained low with longer-term follow-up in the newly diagnosed ovarian cancer setting. In SOLO1, the incidence of MDS/AML after 7 years of follow-up was 1.5% in the maintenance olaparib group and 0.8% in the placebo group [17]. In PAOLA-1, the incidence of MDS/AML/aplastic anaemia after 5 years of follow-up was 1.7% in the olaparib plus bevacizumab group and 2.2% in the placebo plus bevacizumab group [18]. The incidence of new primary malignancies was low (1.7%) at the final DCO in the ORZORA trial.
The limitations of the ORZORA trial have been described fully by Pignata et al. and include the lack of comparator arm [14]; however, given the benefit of maintenance olaparib, a comparator arm was deemed inappropriate. Moreover, the recent succession of olaparib capsules by olaparib tablets resulted in early termination of the study due to discontinuation of olaparib capsules prior to the pre-planned final OS analysis at 60% maturity (OS results reported here at 46.9% maturity). The small size and heterogeneity of the exploratory non-BRCA HRRm cohort limits the ability to assess the predictive benefit of olaparib monotherapy, which may be associated with individual non-BRCA HRR genes. Finally, the supportive efficacy endpoints reported here have methodological limitations, with PFS2 also affected by subsequent therapy and TSFT and TSST influenced by the investigator and patient [32]. However, the inclusion of intermediate clinical endpoints, including PFS2, TFST and TSST, can further define efficacy and provide supportive evidence for the clinical activity of maintenance olaparib demonstrated by PFS and OS [32].
OS results reported from the final analysis continue to demonstrate the clinical activity of maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer with an sBRCAm or BRCAm. Exploratory OS analyses suggest similar olaparib activity can be achieved in patients with platinum-sensitive relapsed ovarian cancer and a non-BRCA HRRm compared with patients with a BRCAm. The safety profile observed was consistent with previous studies of maintenance olaparib in the relapsed disease setting and the primary analysis, with no new safety findings identified. Taken together, these results highlight that patients with platinum-sensitive relapsed ovarian cancer, beyond those with a gBRCAm, may benefit from maintenance olaparib.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
References
Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus. 2016;5:1197.
Tew WP, Lacchetti C, Ellis A, Maxian K, Banerjee S, Bookman M, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468–93.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Lai Z, Brosnan M, Sokol ES, Xie M, Dry JR, Harrington EA, et al. Landscape of homologous recombination deficiencies in solid tumours: analyses of two independent genomic datasets. BMC Cancer. 2022;22:13.
AstraZeneca. Lynparza receives approval in Japan for the treatment of advanced ovarian cancer. 2018. https://www.astrazeneca.com/media-centre/press-releases/2018/lynparza-receives-approval-in-japan-for-the-treatment-of-advanced-ovarian-cancer-19012018.html. Accessed 25 February 2025.
AstraZeneca. Lynparza approved in China as a 1st-line maintenance therapy in BRCA-mutated advanced ovarian cancer. 2019. https://www.astrazeneca.com/media-centre/press-releases/2019/lynparza-approved-in-china-as-a-1st-line-maintenance-therapy-in-brca-mutated-advanced-ovarian-cancer.html. Accessed 25 February 2025.
AstraZeneca. Lynparza prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/208558s029lbl.pdf. Accessed 25 February 2025.
AstraZeneca. Lynparza prescribing information (capsules). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206162s011lbl.pdf. Accessed 25 February 2025.
AstraZeneca. Lynparza summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf. Accessed 25 February 2025.
Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:620–31.
Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018;119:1075–85.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61.
Poveda A, Lheureux S, Colombo N, Cibula D, Lindemann K, Weberpals J, et al. Olaparib maintenance monotherapy in platinum-sensitive relapsed ovarian cancer patients without a germline BRCA1/BRCA2 mutation: OPINION primary analysis. Gynecol Oncol. 2022;164:498–504.
Pignata S, Oza A, Hall G, Pardo B, Madry R, Cibula D, et al. Maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer: outcomes by somatic and germline BRCA and other homologous recombination repair gene mutation status in the ORZORA trial. Gynecol Oncol. 2023;172:121–9.
AstraZeneca. Global policy: bioethics. https://www.astrazeneca.com/content/dam/az/Sustainability/Bioethics_Policy.pdf. Accessed 25 February 2025.
Poveda Velasco AM, Lheureux S, Colombo N, Cibula D, Elstrand M, Weberpals J, et al. 531P Maintenance olaparib monotherapy in patients (pts) with platinum-sensitive relapsed ovarian cancer (PSR OC) without a germline BRCA1/BRCA2 mutation (non-gBRCAm): final overall survival (OS) results from the OPINION trial. Ann Oncol. 2022;33:S790.
DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol. 2023;41:609–17.
Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martín A, Marth C, et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 2023;34:681–92.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64.
GlaxoSmithKline. Dear health care provider letter (niraparib). 2022. https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter.pdf. Accessed 25 February 2025.
GlaxoSmithKline. Zejula (niraparib) Important Prescribing Information for the maintenance treatment of adult patients with non-gBRCAmut recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy in second or later line setting. 2022. https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20November%202022.pdf. Accessed 25 February 2025.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61.
Coleman RL, Oza A, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Overall survival results from ARIEL3: a Phase 3 randomized, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma (abstract O003/#557). Int J Gynecol Cancer. 2022;32:A3–4
Matulonis U, Herrstedt J, Oza A, Mahner S, Redondo A, Berton D, et al. Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecol Oncol. 2021;162:S24–5.
GlaxoSmithKline. ZEJULA® (niraparib) capsules, for oral use: prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214876s000lbl.pdf. Accessed 25 February 2025.
Clovis Oncology. RUBRACA® (rucaparib) tablets, for oral use: prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209115s013lbl.pdf. Accessed 25 February 2025.
Hodgson DR, Dougherty BA, Lai Z, Fielding A, Grinsted L, Spencer S, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119:1401–9.
Mirza MR, Feng B, Shan M, Sun K, Yalcin I, Coleman K, et al. Elucidation of PARP inhibitor activity in BRCAwt recurrent ovarian cancer by HRR mutational gene profile analysis. J Clin Oncol. 2019;37:abstr 5568.
O’Malley DM, Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, et al. Results from the phase 3 study ARIEL3: mutations in non-BRCA homologous recombination repair genes confer sensitivity to maintenance treatment with the PARP inhibitor rucaparib in patients with recurrent platinum-sensitive high-grade ovarian carcinoma. Mol Cancer Ther. 2018;17:abstr LB-A12.
Pujade-Lauraine E, Brown J, Barnicle A, Wessen J, Lao-Sirieix P, Criscione SW, et al. Homologous recombination repair gene mutations to predict olaparib plus bevacizumab efficacy in the first-line ovarian cancer PAOLA-1/ENGOT-ov25 trial. JCO Precis Oncol. 2023;7:e2200258.
Wilson MK, Pujade-Lauraine E, Aoki D, Mirza MR, Lorusso D, Oza AM, et al. Fifth ovarian cancer consensus conference of the gynecologic cancer InterGroup: recurrent disease. Ann Oncol. 2017;28:727–32.
Matulonis UA, Oza AM, Ho TW, Ledermann JA. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer. 2015;121:1737–46.
Ledermann JA, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:710–22.
Matulonis U, Herrstedt J, Oza A, Mahnerd S, Redondo A, Berton D, et al. Final overall survival and long-term safety in the ENGOT-OV16/NOVA phase III trial of niraparib in patients with recurrent ovarian cancer (LBA 6). Gynecol Oncol. 2023;176:S31–S2.
Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;29:728–60.
Acknowledgements
We thank the patients who participated in this trial and their families. Medical writing assistance was provided by Abbie Newman BSc, of AMICULUM, funded by AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Prior presentation: Topline results from the final overall survival analysis of the ORZORA study were presented as a Poster Discussion at the American Society of Clinical Oncology Annual Meeting, 3–7 June 2022 (Pignata S et al. J Clin Oncol. 2022;40(Suppl 16):abstr 5519).
Funding
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Sandro Pignata: Conceptualisation, methodology, investigation, resources, writing – original draft, writing – review & editing. Amit Oza: Investigation, resources, writing – original draft, writing – review & editing. Geoff Hall: Investigation, resources, writing – original draft, writing – review & editing. Beatriz Pardo: Investigation, resources, writing – original draft, writing – review & editing. Radoslaw Madry: Investigation, resources, writing – original draft, writing – review & editing. David Cibula: Investigation, resources, writing – original draft, writing – review & editing. Jaroslav Klat: Investigation, resources, writing – original draft, writing – review & editing. Ana Montes: Investigation, resources, writing – original draft, writing – review & editing. Rosalind Glasspool: Investigation, resources, writing – original draft, writing – review & editing. Nicoletta Colombo: Investigation, resources, writing – original draft, writing – review & editing. Imre Pete: Investigation, resources, writing – original draft, writing – review & editing. Ana Herrero Ibáñez: Investigation, resources, writing – original draft, writing – review & editing. Margarita Romeo: Investigation, resources, writing – original draft, writing – review & editing. Rumyana Ilieva: Investigation, resources, writing – original draft, writing – review & editing. Constanta Timcheva: Investigation, resources, writing – original draft, writing – review & editing. Massimo Di Maio: Investigation, writing – original draft, writing – review & editing. Zahid Bashir: Project administration, writing – original draft, writing – review & editing. Rosie Taylor: Formal analysis, writing – original draft, writing – review & editing. Alan Barnicle: Formal analysis, writing – original draft, writing – review & editing. Andrew Clamp: Investigation, resources, writing – original draft, writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines and the AstraZeneca policy of bioethics (https://www.astrazeneca.com/content/dam/az/PDF/2019/Bioethics%20Policy%20final.pdf) was approved by the Ethics Committee of Istituto Nazionale Tumori ‘Fondazione G Pascale’ (Reference no. 25/15). All patients provided written informed consent.
Competing interests
Sandro Pignata: Honoraria from AstraZeneca, Merck Sharp & Dohme (MSD), Roche, Pfizer, GlaxoSmithKline (GSK) and Clovis Pharma; and research funding from Roche, MSD, AstraZeneca and Pfizer. Amit Oza: Principal investigator of investigator-initiated studies with AstraZeneca; institutional grant funding from AstraZeneca; and steering committee member (non-compensated) for AstraZeneca, Clovis Oncology and GSK. Geoff Hall: Research funding from IQVIA. Beatriz Pardo: Research funding from AstraZeneca, GSK, Clovis and MSD. Radoslaw Madry: Advisory board participation for AstraZeneca, Roche and GSK; and personal fees from AstraZeneca, Roche and GSK. David Cibula: Advisory board participation for AstraZeneca, Roche, Genmab, SOTIO, Merck and GSK. Jaroslav Klat: Reports no disclosures. Ana Montes: Reports no disclosures. Rosalind Glasspool: Principal investigator for trials sponsored by AstraZeneca, Clovis, Tesaro, Immunogen, Pfizer and Lilly; research funding from Clovis, Boehringer Ingelheim and Lilly/Ignyta; institution research funding from Tesaro; institutional fees from Novartis; and personal fees from AstraZeneca, MSD, Clovis, Tesaro, GSK, Immunogen and SOTIO. Nicoletta Colombo: Personal fees from AstraZeneca, MSD, Roche, Tesaro, GSK, Clovis Oncology, PharmaMar, Pfizer, Amgen, Novartis, BIODCAD and Immunogen. Imre Pete: Reports no disclosures. Ana Herrero Ibáñez: Consulting/advisory board participation for GSK; speakers’ bureau participation for Clovis, GSK, MSD, PharmaMar; and research funding from AstraZeneca and Roche. Margarita Romeo: Institution research funding from AstraZeneca, GSK, Clovis and Pfizer; personal fees from MSD and GSK; and AstraZeneca employment (this author became an AstraZeneca employee in July 2024; her contributions to this manuscript were made while working at her previous institution). Rumyana Ilieva: Reports no disclosures. Constanta Timcheva: Principal investigator of clinical trials of AstraZeneca, Roche, Novartis, Pfizer and Merck; and member of advisory boards of Astellas, Servier and Novartis. Massimo Di Maio: Principal investigator of clinical trials of Roche, Merck, BeiGene, Novartis, Pfizer and Exelixis; member of advisory boards of AstraZeneca, Roche, Novartis, Pfizer, Eisai, Astellas, Janssen, Merck and Amgen; and institutional research funding from GSK/Tesaro. Zahid Bashir: AstraZeneca employment (at time of study); and stock ownership of Omniac Pharm Consult Ltd. Rosie Taylor: AstraZeneca employment and stock ownership. Alan Barnicle: AstraZeneca employment and stock ownership. Andrew Clamp: Research funding from AstraZeneca; and honoraria from AstraZeneca, Clovis Oncology, GSK/Tesaro and Eisai.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
At time of study: Margarita Romeo, Massimo Di Maio, Zahid Bashir.
Supplementary information
41416_2025_2966_MOESM1_ESM.pdf
Online Supplementary Appendix to: Overall survival with maintenance olaparib in platinum-sensitive relapsed ovarian cancer by somatic or germline BRCA and homologous recombination repair mutation stat
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pignata, S., Oza, A., Hall, G. et al. Overall survival with maintenance olaparib in platinum-sensitive relapsed ovarian cancer by somatic or germline BRCA and homologous recombination repair mutation status. Br J Cancer 132, 725–732 (2025). https://doi.org/10.1038/s41416-025-02966-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-02966-x
This article is cited by
-
Enhancing PARP inhibitor efficacy in ovarian cancer: targeting the PI3K/AKT/mTOR pathway
Journal of Ovarian Research (2025)