Abstract
Background
We assessed the associations of visceral adiposity indexes such as Chinese Visceral Adiposity Index (CVAI), Visceral Adiposity Index (VAI), Lipid Accumulation Product (LAP), waist circumference (WC), and waist-hip ratio (WHR) with overall and specific cancer incidence in a Chinese population.
Methods
332,297 individuals from the Taiwan MJ cohort (1996–2007) were included. We utilized multivariable Cox proportional hazards models to examine associations of baseline visceral adiposity indexes and cancer incidences. Sex-specific CVAI, VAI, and LAP were calculated, incorporating WC and triglycerides levels. CVAI and VAI also included body mass index and high-density lipoprotein, with CVAI further incorporating age.
Results
Higher CVAI was consistently associated with higher overall cancer incidence, with HRs of 1.45 (95% CI: 1.2–1.76) and 2.03 (95% CI: 1.52–2.72) for males and females, respectively, comparing the fifth quintile to the first. The HRs for WC were 1.27 (95% CI: 1.08–1.49) and 1.19 (95% CI: 1.01–1.40) for males and females, WHR was significantly associated with cancer risk in males (HR:1.28; 95% CI: 1.13–1.45), and LAP was significantly associated with cancer risk in females (HR: 1.25; 95% CI: 1.04–1.5). VAI was not associated with overall cancer incidence.
Discussion
CVAI is a superior clinical biomarker for predicting cancer incidence in the Chinese population compared to traditional visceral obesity indices.

Similar content being viewed by others
Introduction
Obesity increases the risk of chronic diseases, including cancer [1]. According to the available data from the International Agency for Research on Cancer, obesity was a causal factor for 13 types of cancers, which include cancers affecting the colon, liver, kidney, and others [2]. In 2023, among 15.8 million Chinese adults, 34.8% were overweight and 14.1% were obese according to the Chinese BMI classification [3]. Obesity, especially abnormal visceral adipose tissue (VAT), could lead to a hormonal, metabolic, and inflammatory environment that promotes tumor development [4,5,6]. An expanding body of research indicates that VAT, as opposed to the overall body size, was a stronger predictor of cancer incidence [7] and all-cancer risks [8].
There are several ways that VAT is measured. Clinically, VAT is often assessed by MRI or CT. The ability to access medical imaging facilities limits its application to the general population [9]. While waist circumference (WC) and waist-hip ratio (WHR) are easily obtainable, they could not distinguish between VAT and trunk subcutaneous adipose tissue [10]. Therefore, integrated indices like visceral adiposity index (VAI) [11] and lipid accumulation product (LAP) [12] have been established to measure VAT. Both VAI and LAP have been applied in the context of diseases such as metabolic syndrome [13, 14] and type 2 diabetes mellitus (T2DM) [15,16,17]. However, these indices were developed based on specific ethnic populations, i.e., VAI from Italian [11] and LAP from Brazilian [12]. Limited information is available in terms of the ability of these indices to translate into the Asian population.
Chinese visceral adiposity index (CVAI) is a newly developed biomarker that considers the characteristics of body fat in Asians [18]. CVAI incorporated age, WC, body mass index (BMI), and blood lipid markers [18], and has been confirmed by CT for its relevance to VAT [18]. Studies have demonstrated associations between CVAI and the incidence of T2DM [19, 20], hypertension [20, 21], nonalcoholic fatty liver disease [22], and other chronic diseases[23, 24]. In some cases, CVAI exhibited superior predictive performance in disease prognosis compared to BMI, WC, and WHR [19, 25, 26]. Considering CVAI as a more suitable index of VAT in the Asian population, we wondered whether the index would be a better VAT biomarker for predicting cancer risk in Chinese populations. Therefore, we aimed to assess the associations of VAT biomarkers with cancer incidence in the MJ cohort, a large prospective cohort in Taiwan. We further compared CVAI with other VAT measurements, such as VAI, LAP, WC, and WHR.
Methods
Study population
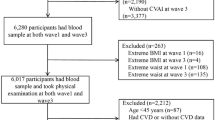
Our study cohort was derived from the ongoing Taiwan MJ cohort study. This longitudinal study has enrolled participants who have undergone extensive medical screenings throughout Taiwan since 1996. Each individual within the MJ cohort completed questionnaires and underwent physical examinations. Additionally, they provided blood and urine samples for laboratory testing. The exclusion criteria for this study comprised individuals under the age of 18 (n = 13,866), those who had previously been diagnosed with cancer when enrolled (n = 4999), and those with a follow-up period of less than one year. (n = 1563). The study included 408,708 participants, with an average follow-up period of 6.7 years, varying from 1 to 11 years. Each adult was required to possess at least one of the five specific targeted visceral obesity indices (VAI, CVAI, LAP, WC, WHR). The ultimate study cohort comprised 332,297 participants, details regarding the selection procedure were illustrated in Fig. 1.
Data collection
Trained staff collected data on sociodemographic characteristics (including age, sex, educational attainment, occupation, and marital status), lifestyles (such as smoking, drinking, and physical activity), as well as personal and family cancer history through questionnaires. Measurements for height, weight, WC, and hip circumference (HC) were documented. Moreover, laboratory tests were conducted on a fasting blood sample obtained during enrollment, encompassing the analysis of TG and HDL.
Definition of visceral obesity indices
Three novel indices (i.e., VAI, CVAI, and LAP) have been developed by previous studies [11, 12, 18] to estimate VAT. These indices provided a more nuanced understanding of visceral obesity by incorporating various factors such as WC, BMI, TG, and HDL. The formulas for males and females are as follows:
For males:
For females:
WHR is computed by dividing WC by hip circumference (HC). In this study, participants were initially categorized into two groups based on the WHO-recommended cut-off values for Asian populations for WC and WHR. Then, they were further categorized into four groups as categorical variables, determined by the median value of each subgroup [26,27,28]. In Asian populations, the WC cutoff is 90 cm for males and 80 cm for females. Similarly, the WHR cutoff is 0.9 for males and 0.85 for females. Individuals whose WC and WHR exceeded the defined cutoff points were identified as having central obesity. WC and WHR were further classified into four ascending groups according to the mentioned cutoff points, with Group 1 representing the lowest measurement.
Study covariables
Occupation (blue collar, white collar, self-employed, and others), marital status (single, married, separated or divorced or widowed), educational attainment (middle school or below, high school, junior school, college or higher), drinking (never, former, or current), smoking (never, former, or current), and family cancer history (yes, no) were modeled as categorical variables. BMI was divided into four categories following a Chinese standard [29]: <18.49, 18.5–23.9, 24–27.9, and ≥28 kg/m2. Leisure-time physical activity was determined by multiplying intensity (metabolic equivalent, MET) and exercise duration (hours), and was grouped into four categories [30]: inactive (<3.75 MET hrs /week), low (3.75–7.49 MET hrs/week), medium (7.5–16.49 MET hrs/week), and high (≥16.5 MET hrs/week).
Ascertainment of all-cancer incidence and cancer-specific incidence
The primary outcome evaluated was the overall cancer incidence. The follow-up period was determined from the date of recruitment to December 31, 2007, date of death, date of cancer incidence, or loss to follow-up, whichever came first. Information regarding follow-up procedures has been previously documented [30, 31].
We gathered data on cancer incidence by connecting participants in our study to both the Taiwan Cancer Registry and the Taiwan Death File from 1996 to 2007. This linkage was established through the unique IDs. Comprehensive details regarding the study design of the MJ cohort are available in other publications [31,32,33]. Cancer cases were based on the International Classification of Diseases, Ninth Revision (ICD-9) codes (140-239), and the International Classification of Diseases, Tenth Revision (ICD-10) codes (C00 to C97).
Statistical analysis
Continuous variables were described as medians (interquartile ranges), and categorical variables as counts (percentages). Baseline characteristics were summarized by sex and compared between male and female participants using the χ2 test or Mann–Whitney U test. To address missing data, which accounts for 3% of the dataset, we generated 5 imputed data sets with 10 iterations and pooled the results using the R package “mice.”
VAI, CVAI, and LAP were used to divide participants into five groups based on ascending quintiles, labeled as Quintiles 1 through 5. We calculated the incidence rates per 100,000 person-years.
We compared the overall cancer incidence in participants with different visceral obesity indices using log-rank tests. Cox proportional hazard models were employed to compute hazard ratios (HRs) and their 95% CIs. The model was applied in males and females separately and was adjusted for age, educational attainment, marital status, occupation, drinking, smoking, physical activity, BMI, and family cancer history. We tested for trend by treating the categorical visceral obesity indices as continuous variables in the model (P for trend). The P values for interaction were computed through the comparison of the likelihood of fitted models, considering both with and without interaction terms.
Stratified analyses were applied to investigate the associations of smoking (never vs former or current) and drinking (never vs former or current) with potential associations between sex-specific visceral obesity indices and overall cancer incidence. All covariates mentioned above were adjusted except the study characteristic of the subgroup. Additionally, a cancer-specific analysis was performed for both males and females to explore the correlation between VAT indices and the incidence of specific cancers.
A two-sided P < 0.05 was considered statistically significant. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R software (version 4.0.4).
Results
Baseline characteristics of the population
The study encompassed 332,297 Chinese participants without a history of cancer, the average age was 40.3 years, and comprised 160,275 (48.2%) males and 172,022 (51.8%) females. During the follow-up period, 6879 cases of cancer were reported. Additional information on population characteristics is available in Table 1.
Associations between VAT indices and overall cancer incidence in males and females
Based on the log-rank analysis, all five indices exhibited a positive correlation with the overall cancer incidence (all P < 0.001). Figure 2 shows the HRs for overall cancer incidence based on VAT indices after adjusting for confounders. The multivariable Cox regression models indicated that CVAI levels exhibited a dose-response relationship with overall cancer incidence in both men and women (Both P for trend <0.005). Compared to the Quintile 1 (lowest), the HRs for males were 1.3 (95% CI: 1.09–1.55), 1.23 (95% CI: 1.03–1.47), 1.36 (95% CI: 1.14–1.63), and 1.45 (95% CI: 1.2–1.76) for Quintiles 2, 3, 4, and 5, respectively. For females, the HRs were 1.47 (95% CI: 1.17–1.84), 1.86 (95% CI: 1.48–2.34), 2 (95% CI: 1.56–2.57), and 2.03 (95% CI: 1.52–2.72) for Quintiles 2–5 when compared to Quintile 1 (lowest).
The incidence rate was defined as 100,000 person-years. HR hazard ratio, CI confidence interval; HRs and CIs were from Cox regression models. Marital status, education, occupation, smoking status, drinking status, physical activity (MET-hours/week), BMI (kg/m2), and family history of cancer were adjusted in both the main analysis and trend analysis.
In Fig. 2, no statistically significant associations were evident in either males or females when VAI was used as visceral obesity indices (all P > 0.05). For LAP, significant findings emerged among females when contrasted with Quintile 1 (lowest). Specifically, for Quintiles 2–5, HRs were 1.2 (95% CI: 1.01–1.42), 1.24 (95% CI: 1.04–1.47), 1.3 (95% CI: 1.09–1.55), and 1.25 (95% CI: 1.04–1.5), respectively, and did not reveal a substantial linear trend effect (P for trend = 0.068). Conversely, no significant outcomes were identified in males for Quintile 5 within the LAP category in comparison to Quintile 1 (lowest). Higher WC or WHR were associated with higher risks of cancer among males (both P for trend <0.005). The HRs for Group 4 in terms of WC and WHR were 1.27 (95% CI: 1.08–1.49) and 1.28 (95% CI: 1.13–1.45), respectively, when compared to Group 1. Among females, a significant correlation was observed with WC (HR:1.19, 95% CI: 1.01–1.4, P for trend = 0.031), but not with WHR (HR:0.96, 95% CI: 0.83–1.11, P for trend = 0.567) (Fig. 2).
Interaction analysis of VAT indices with overall cancer incidence
We assessed the interaction between VAT indices and smoking status as well as drinking status in the adjusted model for the risks of overall cancer incidence in males and females, respectively (Figs. 3 and 4). We found interactions among CVAI, WC, WHR, and smoking status in males (all P < 0.05), indicating the associations in ever and current-smoking males were more evident. And no interactions were found in females.
a Associations in males. b Associations in females. HR hazard ratio, CI confidence interval. Stratified analyses were conducted to examine whether the potential association between sex-specific visceral obesity indices and overall cancer incidence differed by drinking status (never vs former or current). All covariates including marital status, education, occupation, smoking status, physical activity (MET-hours/week), BMI (kg/m2), and family history of cancer were adjusted.
a Associations in males. b Associations in females. HR hazard ratio, CI confidence interval. Stratified analyses were conducted to examine whether the potential association between sex-specific visceral obesity indices and overall cancer incidence differed by drinking status (never vs former or current). All covariates including marital status, education, occupation, drinking status, physical activity (MET-hours/week), BMI (kg/m2), and family history of cancer were adjusted.
Analysis of VAT indices and cancer-specific incidence
Regarding cancer-specific incidences, our analysis revealed a noteworthy link between CVAI and heightened risks of colorectal and oral cancers in males. Specifically, when comparing Quintile 5 to Quintile 1 (lowest) of CVAI, the HR for colorectal cancer was 2.81 (95% CI: 1.5–5.82), and for oral cancer, the HR was 3.44 (95% CI: 1.42–8.33). Similarly, among females, CVAI demonstrated a connection with higher risks of breast and uterine cancers. Specifically, when comparing Quintile 5 to Quintile 1 (lowest) of CVAI, the HR for breast cancer was 2.85 (95% CI: 1.64–4.94), and for uterine cancer, the HR was 34.25 (95% CI: 7.04–166.74) (Fig. 5).
The incidence rate was defined as 100,000 person-years. HR hazard ratio, CI confidence interval, HRs and CIs were from Cox regression models. Marital status, education, occupation, smoking status, drinking status, physical activity (MET-hours/week), BMI (kg/m2), and family history of cancer were adjusted in both the main analysis and trend analysis.
Supplementary Figs. 1 and 2 showed our analysis of cancer-specific incidences concerning WC and WHR. Like CVAI, we found notable associations primarily in colorectal, oral, lung, breast, and uterine cancer incidences. However, the results varied slightly between the different indices. WC was positively associated with lung cancer in males (Group 4 vs Group1, HR: 1.62, 95% CI: 1.17–3.03) and uterine cancer incidence in females (Group 4 vs Group1, HR: 2.63, 95% CI: 1.08–6.43), but was negatively associated with breast cancer in females (Group 4 vs Group1, HR: 0.66, 95% CI: 0.47–0.93; Supplementary Fig. 1). WHR was positively associated with colorectal cancer in both males (Group 4 vs Group1, HR: 1.65, 95% CI: 1.17–2.32) and females (Group 4 vs Group1, HR: 1.76, 95% CI: 1.15–2.7). WHR was also associated with lung (Group 4 vs Group1, HR: 1.55, 95% CI: 1.11–2.16) and oral cancer (Group 4 vs Group1, HR: 2.39, 95% CI: 1.39–4.09) in males. Different from CVAI but coherent with WC, a negative association was also observed between breast cancer and WHR (Group 4 vs Group1, HR: 0.57, 95% CI: 0.41–0.78) (Supplementary Fig. 2).
Discussion
Our results validated the positive correlation between VAT and the overall cancer incidence within the large prospective MJ cohort. The findings further underscored the significance of CVAI’s association with overall cancer incidence in both sexes. In addition, CVAI was identified as an indicator for colorectal and oral cancer in males, breast and uterine cancer in females. CVAI demonstrates strong clinical utility as a predictor of cancer incidence in Chinese populations, outperforming both traditional anthropometrics (WC, WHR) and modern composite indices (VAI, LAP). CVAI could serve as a practical tool for cancer risk assessment in health check-up settings. Further validation in diverse populations would help confirm its generalizability.
Similar to our results, previous research has also indicated a link between elevated VAT levels and cancer incidence [7, 34,35,36]. A cohort comprising 68,253 Chinese women reported that they did not find any significant associations between WHR and overall cancer incidence after an adjustment of baseline BMI [37]. A cohort included 22.9 million Korean adults with 769,871 cancer cases found that WC quintiles were associated with cancer incidents in both males and females as well [38].
Despite WC and WHR, comprehensive indices like VAI and LAP have been used in cancer studies. However, many studied mortalities instead of cancer incidence. Within the UK Biobank, a prospective cohort including 357,457 individuals aged 38–73 years, the association between VAI and higher risks of all-cause and cause-specific mortalities was observed [39]. In 82,091 participants aged over 65 from the National Health and Nutrition Examination Survey, VAI levels were found to have a J-shaped relationship with all-cause mortality [40]. In the Tehran Lipid and Glucose Study, encompassing 6751 participants aged 30 years or older, mortality rates did not exhibit variance across LAP quantiles [41]. Although lacking evidence on cancer incidence, we suppose that VAI and LAP might not be accurate VAT predictors in the Chinese population, since they were formulated based on Western populations.
CVAI has been introduced as a proper VAT measurement in Chinese. In our study, CVAI showed better predictive value than VAI, likely due to the differing body fat characteristics among ethnicities. Previous studies have linked CVAI with various diseases, including coronary heart disease [23], cerebrovascular disease, diabetic kidney disease [42], and nonalcoholic fatty liver disease [43]. The link between CVAI and cancer incidence had been significantly under-researched, and our study addressed this gap.
Cigarette smoking has shown a positive association with central adiposity [44, 45]. In our study, the interactive analysis revealed stronger associations between overall cancer incidence and CVAI, WC, and WHR in males who were either current or former smokers, while no significant interaction was observed in females. Endogenous estrogen may attenuate the impact of visceral adiposity by modulating adipokine profiles and reducing inflammation. Visceral fat promotes carcinogenesis by secreting pro-inflammatory cytokines, which might interact with smoking-induced DNA damage, amplifying cancer risk [46, 47]. Interestingly, estrogen has been shown to mitigate adipose-related inflammation and metabolic dysfunction, potentially explaining the weaker VAT-cancer link in females [48]. Given our female cohort’s median age of 40.3 years, many likely retained estrogen’s protective effects. Despite sex differences, varying risk effects of VAT and smoking on cancer incidence have been reported. In a 7-year study of 22.9 million Korean adults, WC showed continued significance in cancers related to smoking, like oral and laryngeal cancers in males. However, its significance diminished in other cancers, such as brain cancer [49]. Furthermore, a study involving 162,679 males and females reported that WC was not linked to lung cancer within any smoking group after adjusting for BMI [50]. The diversity in cancer types across studies might contribute to the differing effects observed between smokers and non-smokers.
In line with our cancer-specific findings, an analysis based on 245,009 females, among whom there were 5402 cases of breast cancer in the UK Biobank also revealed a protective trend related to WC concerning breast cancer incidence among premenopausal women [51]. Differently, a study involving 116 breast cancer cases and 226 controls found VAI positively associated with breast cancer [42]. Significantly, our study primarily involved premenopausal participants, whose fat distribution were different from postmenopausal women [52]. Hence, CVAI, rather than anthropometric measurements such as WC and WHR, is potentially a more proper VAT metric in premenopausal women.
This study possesses a few strengths. Primarily, we are the first study to identify the associations of VAT indices, especially CVAI with cancer incidence in the Chinese population. Second, the prospective study enrolled a substantial sample size, potentially rendering the results more representative of the general population. Third, our prospective study design, with baseline measurements of adiposity traits, minimizes the impact of reverse causation. Fourth, this study collected sufficient lifestyle co-variates to largely attenuate its confounding effect. Moreover, mortality data were sourced from death registry records, ensuring minimal unavailability of individuals for follow-up.
However, there are also some limitations. First, we did not include a comparison with imaging data, which is the gold standard for measuring VAT. Secondly, our findings, based on Chinese individuals with higher socioeconomic status undergoing routine physical exams, may not apply to other ethnic or socioeconomic groups.
Conclusion
In summary, increased VAT levels were related to elevated overall cancer incidence in the MJ cohort. CVAI was a superior clinical marker for predicting cancer incidence in the Chinese population, surpassing other VAT indices like WC, WHR, VAI, and LAP.
Data availability
The Taiwan MJ Cohort is available to the worldwide research community and offers collaboration. Applicants for data access should contact the MJ Health Research Foundation at [http://www.mjhrf.org/]. Further inquiries can be directed to the corresponding author.
Change history
05 June 2025
In this article the institution listed in the first affiliation was incorrectly given as ‘Center of Clinical Big Data and Analytics of School of Public Health and the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China’; the correct affiliation listing is ‘Center of Clinical Big Data and Analytics of the Second Affiliated Hospital and School of Public Health, Zhejiang University School of Medicine, Hangzhou 310058 Zhejiang, China’.
References
De Pergola G, Silvestris F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013;2013:291546.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer-viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8.
Chen K, Shen Z, Gu W, Lyu Z, Qi X, Mu Y, et al. Prevalence of obesity and associated complications in China: a cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab. 2023;25:3390–9.
Crudele L, Piccinin E, Moschetta A. Visceral adiposity and cancer: role in pathogenesis and prognosis. Nutrients. 2021;13:2101.
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48.
Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–9.
Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: an integrative review of the epidemiological evidence. Obes Rev. 2021;22:e13088.
Barberio AM, Alareeki A, Viner B, Pader J, Vena JE, Arora P, et al. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat Commun. 2019;10:383.
Park HJ, Shin Y, Park J, Kim H, Lee IS, Seo DW, et al. Development and validation of a deep learning system for segmentation of abdominal muscle and fat on computed tomography. Korean J Radio. 2020;21:88–100.
Brown JC, Harhay MO, Harhay MN. The value of anthropometric measures in nutrition and metabolism: comment on anthropometrically predicted visceral adipose tissue and blood-based biomarkers: a cross-sectional analysis. Nutr Metab Insights. 2019;12:1178638819831712.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–2.
Li H, Zhang Y, Luo H, Lin R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front Endocrinol. 2022;13:977625.
Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, et al. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23:13–21.
Zhang L, Zhang Z, Wang B, Yuan Y, Sun L, Gao H, et al. Relative children’s lipid accumulation product is a novel indicator for metabolic syndrome. Front Endocrinol. 2021;12:645825.
Liu PJ, Ma F, Lou HP, Chen Y. Visceral adiposity index is associated with pre-diabetes and type 2 diabetes mellitus in Chinese adults aged 20-50. Ann Nutr Metab. 2016;68:235–43.
Bozorgmanesh M, Hadaegh F, Azizi F. Predictive performance of the visceral adiposity index for a visceral adiposity-related risk: type 2 diabetes. Lipids Health Dis. 2011;10:88.
Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9:9693.
Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214.
Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, et al. Chinese visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev. 2021;37:e3370.
Ren Y, Cheng L, Qie R, Han M, Kong L, Yan W, et al. Dose–response association of Chinese visceral adiposity index with comorbidity of hypertension and diabetes mellitus among elderly people. Front Endocrinol. 2023;14:1187381.
Han M, Qie R, Li Q, Liu L, Huang S, Wu X, et al. Chinese visceral adiposity index, a novel indicator of visceral obesity for assessing the risk of incident hypertension in a prospective cohort study. Br J Nutr. 2021;126:612–20.
Shen S, Huang H, Wang J, Tang Z, Shen C, Xu C. Positive association between the Chinese visceral adiposity index and nonalcoholic fatty liver disease in lean adults. Digest Dis Sci. 2023;68:656–64.
Xie Y, Zhang Y, Qin P, Ping Z, Wang C, Peng X, et al. The association between Chinese Visceral Adipose Index and coronary heart disease: a cohort study in China. Nutr, Metab Cardiovasc Dis. 2022;32:550–9.
Jin J, Woo H, Jang Y, Lee W-K, Kim J-G, Lee I-K, et al. Novel Asian-specific visceral adiposity indices are associated with chronic kidney disease in Korean adults. Diabetes Metab J. 2023;47:426–36.
Liu Z, Huang Q, Deng B, Wei M, Feng X, Yu F, et al. Elevated Chinese visceral adiposity index increases the risk of stroke in Chinese patients with metabolic syndrome. Front Endocrinol. 2023;14:1218905.
Li B, Wang J, Zhou X, Liu Y, Wang W, Gao Z, et al. Chinese visceral adiposity index is more closely associated with hypertension and prehypertension than traditional adiposity indices in Chinese population: results from the REACTION study. Front Endocrinol. 2022;13:921997.
Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. 2012;13:287–96.
Chen Y, Peng Q, Yang Y, Zheng S, Wang Y, Lu W. The prevalence and increasing trends of overweight, general obesity, and abdominal obesity among Chinese adults: a repeated cross-sectional study. BMC Public Health. 2019;19:1293.
Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults-study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53.
Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82.
Wu X, Tsai SP, Tsao CK, Chiu ML, Tsai MK, Lu PJ, et al. Cohort Profile: The Taiwan MJ Cohort: half a million Chinese with repeated health surveillance data. Int J Epidemiol. 2017;46:1744–g.
Aparecida Silveira E, Vaseghi G, de Carvalho Santos AS, Kliemann N, Masoudkabir F, Noll M, et al. Visceral obesity and its shared role in cancer and cardiovascular disease: a scoping review of the pathophysiology and pharmacological treatments. Int J Mol Sci. 2020;21:9042.
Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–47.
Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–5.
Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270.
Liu Y, Warren Andersen S, Wen W, Gao YT, Lan Q, Rothman N, et al. Prospective cohort study of general and central obesity, weight change trajectory and risk of major cancers among Chinese women. Int J Cancer. 2016;139:1461–70.
Lee KR, Seo MH, Do Han K, Jung J, Hwang IC. Waist circumference and risk of 23 site-specific cancers: a population-based cohort study of Korean adults. Br J Cancer. 2018;119:1018–27.
He Q, Liu S, Feng Z, Li T, Chu J, Hu W, et al. Association between the visceral adiposity index and risks of all-cause and cause-specific mortalities in a large cohort: Findings from the UK biobank. Nutr Metab Cardiovasc Dis. 2022;32:2204–15.
Wang L, Yi Z. Obesity paradox and aging: Visceral Adiposity Index and all-cause mortality in older individuals: a prospective cohort study. Front Endocrinol. 2022;13:975209.
Bozorgmanesh M, Hadaegh F, Azizi F. Predictive performances of lipid accumulation product vs. adiposity measures for cardiovascular diseases and all-cause mortality, 8.6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010;9:100.
Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19:118.
Chen X, Shi F, Xiao J, Huang F, Cheng F, Wang L, et al. Associations between abdominal obesity indices and nonalcoholic fatty liver disease: Chinese visceral adiposity index. Front Endocrinol. 2022;13:831960.
Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat: III. Effects of cigarette smoking. JAMA. 1989;261:1169–73.
Jee SH, Lee SY, Nam CM, Kim SY, Kim MT. Effect of smoking on the paradox of high waist‐to‐hip ratio and low body mass index. Obes Res. 2002;10:891–5.
Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–9.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Miller CN, Brown LM, Rayalam S, Della-Fera MA, Baile CA. Estrogens, inflammation and obesity: an overview. Front Biol. 2012;7:40–7.
Lee KR, Seo MH, Do Han K, Jung J, Hwang IC, on behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Waist circumference and risk of 23 site-specific cancers: a population-based cohort study of Korean adults. Br J Cancer. 2018;119:1018–27.
Patel AV, Carter BD, Stevens VL, Gaudet MM, Campbell PT, Gapstur SM. The relationship between physical activity, obesity, and lung cancer risk by smoking status in a large prospective cohort of US adults. Cancer Causes Control. 2017;28:1357–68.
Cao Y, Xia B, Zhang Z, Hu D, Huang X, Yuan J, et al. Association of body fat distribution and risk of breast cancer in pre-and postmenopausal women. Obes Facts. 2023;16:356–63.
Douchi T, Yonehara Y, Kawamura Y, Kuwahata A, Kuwahata T, Iwamoto I. Difference in segmental lean and fat mass components between pre-and postmenopausal women. Menopause. 2007;14:875–8.
Acknowledgements
We thank everyone who contributed to this research for their help.
Funding
This work was supported by Healthy Zhejiang One Million People Cohort (K-20230085), Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01007), Zhejiang Key Laboratory of Intelligent Preventive Medicine (2020E10004), and Key Research and Development Program of Zhejiang Province(2020C03002).
Author information
Authors and Affiliations
Contributions
XW designed the study, acquired funding, and supervised the project. CPW and DTC conducted data collection. MW, JP, and GS performed data analysis. MW, HT, WL and XW drafted the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all cohort participants, and the study received approval from the Institutional Review Boards at the China Medical University (CRREC107-092). And the study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, M., Wen, C.P., Pan, J. et al. Chinese visceral adiposity index outperforms other obesity indexes in association with increased overall cancer incidence: findings from prospective MJ cohort study. Br J Cancer 133, 227–238 (2025). https://doi.org/10.1038/s41416-025-03041-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03041-1