Abstract
Background
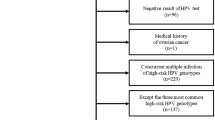
Persistent HPV infection causes cervical cancer, but most infections are transient. Triage methods identify high-risk women needing further evaluation or treatment. We assessed short-term repeat HPV testing as an alternative triage option.
Methods
In ESTAMPA, women aged 30–64 years were screened with HPV testing (HC2 or Cobas) and cytology. Screen positives were referred to colposcopy approximately two months after screening, where cervical samples were collected again for repeat HPV testing. We evaluated the performance of repeat HPV for CIN3+ among HPV-positive women and explored its combination with limited HPV genotyping (HPV16/18).
Results
Among 5390 HPV-positive women (including 629 CIN3 cases and 53 cancers), 61% retested positive at ~2 months (median: 1.8, interquartile range: 1.2–2.8). Repeat HPV sensitivity for CIN3+ was 81.5% (95% CI 77.2–85.2) for HC2 and 87.7% (83.7–90.8) for Cobas. Specificity was <50% with referral rates of 57.4% (55.7–59.0) and 68.2% (66.1–70.2) for HC2 and Cobas. HPV16/18 genotyping followed by repeat HPV among non-HPV16/18-positive women did not greatly improve performance. However, HPV16/18 positivity doubled the risk of CIN3+, supporting its combination with repeat HPV when available.
Conclusions
Short-term repeat HPV testing could be a practical option for triaging HPV-positive women, either alone or in combination with limited HPV genotyping.
Trial registration
ClinicalTrials.gov, NCT01881659
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others

Data availability
Anonymised individual participant data or aggregate data would be available from the corresponding author upon reasonable request, requiring signing a specific contract with the approval of the ESTAMPA principal investigators and local investigators of study centres involved.
References
Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomark Prev. 2013;22:553–60.
Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30:F88–99.
WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention [Internet]. 2nd ed. Geneva: World Health Organization; 2021.
WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention: Use of dual-stain cytology to triage women after a positive test for human papillomavirus (HPV) [Internet]. 2nd ed. Geneva: World Health Organization; 2024.
Arbyn M. Systematic review annex - WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition [Internet]. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Supplement to: WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd ed. Geneva: World Health Organization; 2021.
Baena A, Mesher D, Salgado Y, Martinez S, Villalba GR, Amarilla ML, et al. Performance of visual inspection of the cervix with acetic acid (VIA) for triage of HPV screen-positive women: results from the ESTAMPA study. Int J Cancer. 2023;152:1581–92.
Valls J, Baena A, Venegas G, Celis M, Gonzalez M, Sosa C, et al. Performance of standardised colposcopy to detect cervical precancer and cancer for triage of women testing positive for human papillomavirus: results from the ESTAMPA multicentric screening study. Lancet Glob Health. 2023;11:e350–60.
Ramirez AT, Mesher D, Baena A, Salgado Y, Kasamatsu E, Cristaldo C, et al. Impact of knowledge of HPV positivity on cervical cytology performance in Latin America. J Natl Cancer Inst. 2025;17:644–52.
Taghavi K, Zhao F, Downham L, Baena A, Basu P. Molecular triaging options for women testing HPV positive with self-collected samples. Front Oncol. 2023;13:1243888.
Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–7.
Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101:88–99.
Egemen D, Perkins RB, Clarke MA, Guido R, Huh W, Saraiya M, et al. Risk-based cervical consensus guidelines: methods to determine management if less than 5 Years of data are available. J Low Genit Trac Dis. 2022;26:195–201.
Almonte M, Murillo R, Sanchez GI, Gonzalez P, Ferrera A, Picconi MA, et al. Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol. BMJ Open. 2020;10:e035796.
Arbyn M, Simon M, Peeters E, Meijer C, Berkhof J, Cuschieri K, et al. 2020 List of human papillomavirus assays Suitable for primary cervical cancer screening. Clin Microbiol Infect. 2021. https://doi.org/10.1016/j.cmi.2021.04.031.
Rol ML, Picconi MA, Ferrera A, Sánchez GI, Hernández ML, Lineros J, et al. Implementing HPV testing in 9 Latin American countries: the laboratory perspective as observed in the ESTAMPA study. Front Med. 2022;9:1006038.
Heideman DA, Hesselink AT, Berkhof J, van Kemenade F, Melchers WJ, Daalmeijer NF, et al. Clinical validation of the cobas 4800 HPV test for cervical screening purposes. J Clin Microbiol. 2011;49:3983–5.
Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–63.
Liu SH, Cummings DA, Zenilman JM, Gravitt PE, Brotman RM. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short-interval sampling. Cancer Epidemiol Biomark Prev. 2014;23:200–8.
Demarco M, Hyun N, Carter-Pokras O, Raine-Bennett TR, Cheung L, Chen X, et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine. 2020;22:100293.
Gyllensten U, Sanner K, Gustavsson I, Lindell M, Wikstrom I, Wilander E. Short-time repeat high-risk HPV testing by self-sampling for screening of cervical cancer. Br J Cancer. 2011;105:694–7.
Gustavsson I, Aarnio R, Berggrund M, Hedlund-Lindberg J, Strand AS, Sanner K, et al. Randomised study shows that repeated self-sampling and HPV test has more than two-fold higher detection rate of women with CIN2+ histology than Pap smear cytology. Br J Cancer. 2018;118:896–904.
Arbyn M. Systematic review annex - WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention: Web annex B: Evidence summaries [Internet]. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO. Supplement to: World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention: Use of dual-stain cytology to triage women after a positive test for human papillomavirus (HPV). 2nd ed. Geneva: World Health Organization; 2024.
Wang J, Elfstrom KM, Dillner J. Human papillomavirus-based cervical screening and long-term cervical cancer risk: a randomised health-care policy trial in Sweden. Lancet Public Health. 2024;9:e886–95.
Malagon T, Trottier H, El-Zein M, Villa LL, Franco EL, Ludwig-McGill Cohort S. Human papillomavirus intermittence and risk factors associated with first detections and redetections in the Ludwig-McGill cohort study of adult women. J Infect Dis. 2023;228:402–11.
Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–115.
Baena A, Rol ML, Barbier S, Darragh T, Gonzalez E, Pilleron S, et al. Adjudication of final histological diagnosis of cervical biopsies using a three-step standardised protocol: an inter-observer reproducibility analysis within the ESTAMPA study (NCT01881659). In: International Papillomavirus Conference; 2020 Jul 20-24; Barcelona, Spain. Geneva: International Papillomavirus Society / Kenes Group; 2020. Available from: https://2020.ipvconference.org/2020s-abstracts/.
Acknowledgements
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer / World Health Organization. Some of the authors are present or former staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the views, decisions, or policies of the institutions. AB was supported in part by an appointment to the US National Cancer Institute (NCI) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the National Institutes of Health (NIH). ORISE is managed by ORAU under DOE contract number DESC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of NIH, NCI, DOE, or ORAU/ORISE. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH). The contributions of the NIH author(s) are considered Works of the United States Government. The findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Funding
This work was supported by IARC/WHO; the UNDP / UNFPA / UNICEF / WHO / World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP/WHO), a cosponsored programme executed by the World Health Organization (WHO); the Pan American Health Organization (PAHO); the National Cancer Institute (NCI) (Grant UH2/3 CA202730); the NCI Center for Global Health; Instituto Nacional del Cáncer from Argentina (Grant 2016-2018); Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación from Argentina (Grant PICT 0364-2016); the National Council for Science and Technology (CONACYT) from Paraguay (Grants 14-INV-036 & PINV18-256); Caja Costarricense del Seguro Social; and the National Cancer Institute of Colombia. The funders had no role in the design of the study, data collection, analysis, interpretation, manuscript preparation, or in the decision to publish the results.
Author information
Authors and Affiliations
Consortia
Contributions
MA and RH conceived the ESTAMPA study and are the principal investigators responsible for its overall conduction and funding acquisition. AB conceptualised the manuscript, formulation of the core idea and research objective, and was responsible for data curation, statistical analysis, and writing the original draft. MAP, LM, AF, GV, VV, ACr, GR, CT, ACa, and CW are the local principal investigators responsible for recruitment, clinical management, and data collection. JL, MB, PM, YC, MDF, OZ, LG, PH, MLB, and MR performed HPV testing locally. MA, RH, MAP, LM, AF, LG, and DM contributed to the finalisation of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The ESTAMPA protocol was approved by the Ethics Committee of the International Agency for Research on Cancer of the World Health Organization (IARC/WHO) (IEC Project 12–27-A7), the Pan American Health Organization (PAHO) Ethical Committee, and Ethical Committees at study centres. The study is considered of minimal risk as procedures are standard clinical practice. All women were informed by trained providers of the procedures and signed informed consent. This study is registered with ClinicalTrials.gov (NCT01881659).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baena, A., Picconi, M.A., Mendoza, L. et al. Short-term repeat HPV testing for triaging HPV-positive women in cervical cancer screening. Br J Cancer 133, 1844–1853 (2025). https://doi.org/10.1038/s41416-025-03193-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03193-0