Abstract
Background
Oxaliplatin resistance continues to undermine therapeutic outcomes in colon cancer (CC). Recent investigations point to unc-51 like kinase 1 (ULK1)-mediated disruption of apoptotic pathways as a potential driver of chemoresistance, though the exact mechanisms remain incompletely characterized.
Methods
Using established CC cell lines, we developed oxaliplatin-resistant models through stepwise dose escalation. ULK1 expression was modulated through targeted siRNA knockdown and stable overexpression. Protein interactions were examined via co-immunoprecipitation coupled with mass spectrometry, supplemented by kinase activity assays. Functional impact was assessed through proliferation kinetics, apoptosis profiling, and tumor xenograft studies. Clinical correlations were derived from TCGA analysis and immunohistochemical evaluation of patient tumor specimens.
Results
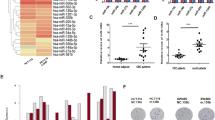
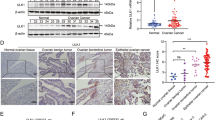
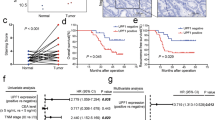
ULK1 overexpression consistently correlated with oxaliplatin resistance and adverse clinical parameters. At the molecular level, ULK1 catalyzed Bcl-2 associated X protein (Bax) phosphorylation at Ser184, creating a recognition motif for Parkin-mediated ubiquitination and proteasomal targeting. Disruption of this axis through ULK1 inhibition restored Bax protein levels, enhanced apoptotic response, and reversed oxaliplatin resistance in both cellular and animal models.
Conclusion
These findings identify ULK1-dependent phosphorylation as a novel regulatory mechanism governing Bax stability in CC. The study provides preclinical rationale for targeting the ULK1-Bax interface to overcome oxaliplatin resistance, while highlighting the need for further investigation into optimal therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data analysed during this study are included in this published article.
References
Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA: a cancer J Clinicians. 2025;75:10–45.
Martinez-Balibrea E, Martínez-Cardús A, Ginés A, Ruiz de Porras V, Moutinho C, Layos L, et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol cancer Therapeutics. 2015;14:1767–76.
Luo ZD, Wang YF, Zhao YX, Yu LC, Li T, Fan YJ, et al. Emerging roles of non-coding RNAs in colorectal cancer oxaliplatin resistance and liquid biopsy potential. World J Gastroenterol. 2023;29:1–18.
Ni J, Hou X, Wang X, Shi Y, Xu L, Zheng X, et al. 3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression. Cell Death Dis. 2019;10:355.
Saul VV, Seibert M, Krüger M, Jeratsch S, Kracht M, Schmitz ML. ULK1/2 Restricts the Formation of Inducible SINT-Speckles, Membraneless Organelles Controlling the Threshold of TBK1 Activation. iScience. 2019;19:527–44.
Xue ST, Li K, Gao Y, Zhao LY, Gao Y, Yi H, et al. The role of the key autophagy kinase ULK1 in hepatocellular carcinoma and its validation as a treatment target. Autophagy. 2020;16:1823–37.
Duan T, Yang X, Kuang J, Sun W, Li J, Ge J, et al. ULK1 Depletion Protects Mice from Diethylnitrosamine-Induced Hepatocarcinogenesis by Promoting Apoptosis and Inhibiting Autophagy. J Hepatocell carcinoma. 2023;10:315–25.
Torii S, Yoshida T, Arakawa S, Honda S, Nakanishi A, Shimizu S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016;17:1552–64.
Hwang DY, Eom JI, Jang JE, Jeung HK, Chung H, Kim JS, et al. ULK1 inhibition as a targeted therapeutic strategy for FLT3-ITD-mutated acute myeloid leukemia. J Exp Clin cancer Res: Cr. 2020;39:85.
Bhattacharya S, Piya S, Ma H, Sharma P, Zhang Q, Baran N, et al. Targeting Unc51-like Autophagy Activating Kinase 1 (ULK1) Overcomes Adaptive Drug Resistance in Acute Myelogenous Leukemia. Mol cancer Res: Mcr. 2023;21:548–63.
Yu L, Shi Q, Jin Y, Liu Z, Li J, Sun W. Blockage of AMPK-ULK1 pathway mediated autophagy promotes cell apoptosis to increase doxorubicin sensitivity in breast cancer (BC) cells: an in vitro study. BMC cancer. 2021;21:195.
Ji X, Zhang X, Li Z. ULK1 inhibitor induces spindle microtubule disorganization and inhibits phosphorylation of Ser10 of histone H3. FEBS open bio. 2020;10:2452–63.
Feng Y, Yang J, Wang Y, Wang X, Ma Q, Li Y, et al. Cafestol inhibits colon cancer cell proliferation and tumor growth in xenograft mice by activating LKB1/AMPK/ULK1-dependent autophagy. J nutritional Biochem. 2024;129:109623.
Zhang Y, Tian L, Huang G, Ge X, Kong F, Wang P, et al. Structural basis of BAX pore formation. Sci (N Y, NY). 2025;388:eadv4314.
Dadsena S, Cuevas Arenas R, Vieira G, Brodesser S, Melo MN, García-Sáez AJ. Lipid unsaturation promotes BAX and BAK pore activity during apoptosis. Nat Commun. 2024;15:4700.
Zhang M, Zheng J, Nussinov R, Ma B. Release of Cytochrome C from Bax Pores at the Mitochondrial Membrane. Sci Rep. 2017;7:2635.
Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–95.
Liu G, Yin T, Kim H, Ding C, Yu Z, Wang H, et al. Structure-activity relationship studies on Bax activator SMBA1 for the treatment of ER-positive and triple-negative breast cancer. Eur J Med Chem. 2019;178:589–605.
Rong Z, Zheng K, Chen J, Jin X. Function and regulation of ULK1: From physiology to pathology. Gene. 2022;840:146772.
Quarato G, Mari L, Barrows NJ, Yang M, Ruehl S, Chen MJ, et al. Mitophagy restricts BAX/BAK-independent, Parkin-mediated apoptosis. Sci Adv. 2023;9:eadg8156.
Mao L, Zhan YY, Wu B, Yu Q, Xu L, Hong X, et al. ULK1 phosphorylates Exo70 to suppress breast cancer metastasis. Nat Commun. 2020;11:117.
Kale J, Kutuk O, Brito GC, Andrews TS, Leber B, Letai A, et al. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO reports. 2018;19:e45235.
Wang H, Wang X, Zhang H, Deng T, Liu R, Liu Y, et al. The HSF1/miR-135b-5p axis induces protective autophagy to promote oxaliplatin resistance through the MUL1/ULK1 pathway in colorectal cancer. Oncogene. 2021;40:4695–708.
Feng Z, Ou Y, Deng X, Deng M, Yan X, Chen L, et al. Deubiquitinase USP10 promotes osteosarcoma autophagy and progression through regulating GSK3β-ULK1 axis. Cell Biosci. 2024;14:111.
Cherradi S, Garambois V, Marines J, Andrade AF, Fauvre A, Morand O, et al. Improving the response to oxaliplatin by targeting chemotherapy-induced CLDN1 in resistant metastatic colorectal cancer cells. Cell Biosci. 2023;13:72.
Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–9.
Xiong Y, Cui MY, Li ZL, Fu YQ, Zheng Y, Yu Y, et al. ULK1 confers neuroprotection by regulating microglial/macrophages activation after ischemic stroke. Int Immunopharmacol. 2024;25:127:111379.
Xiong Y, Cui MY, Li ZL, Fu YQ, Zheng Y, Yu Y, et al. ULK1 confers neuroprotection by regulating microglial/macrophages activation after ischemic stroke. Int Immunopharmacol. 2024:127:111379.
Acknowledgements
Thanks for the technical support of confocal laser scanning microscope (LEICA TCS SP8) and flow cytometer (BECKMAN CytoFlex S) by the Core Facilities (Disen Mei, meidisen@nbu.edu.cn; Danhong Wan, wandanhong@nbu.edu.cn), Health Science Center, Ningbo University. We also thank Chao Dong, Mengjie Yang and Xiqin Guo from the Laboratory Animal Center of Ningbo University for their technical support.
Funding
This study is partly supported by Zhejiang Provincial Natural Science Foundation of China under Grant no.MS26H160044(JC) and LY24C050001(XJ).The National Natural Science Foundation of China grant no. 32270821 (XJ) and 32570835(XJ). The Natural Science Foundation of Ningbo grant no. 2024J037 (XJ) and 2024J413(CJ). The KC Wong Magna Fund in Ningbo University (XJ). The Zhejiang Provincial Medical and Health Science and Technology Plan grant no.2025KY1472 (JC). The Wu Jieping Medical Foundation grant no.320.6750.2022-22-33.
Author information
Authors and Affiliations
Contributions
Conceptualization, ZR and JX; Investigation, ZR, JX, and KFZ; Writing - Original Draft, LP and JX; Writing - Review & Editing, LRP, JC, and XFJ; Supervision, XFJ and JC; Visualization, JX and ZR. The version of the manuscript for publication has been read and is in agreement with all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
CC specimens and paired adjacent normal tissues of human patients were collected from the Yinzhou People’s Hospital after obtaining written consent from the patients. All experimental procedures involving patients were conducted in accordance with the ethical guidelines of the Declaration of Helsinki and were approved by the Ethics Committee of Yinzhou People’s Hospital (No. NBU-2023-213). All animal experimental procedures were approved by the Animal Care and Use Committee of the Ningbo University (No. 12530).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rong, Z., Xing, J., Wu, L. et al. ULK1 promotes oxaliplatin resistance of colon cancer via phosphorylation of Bax S184. Br J Cancer (2026). https://doi.org/10.1038/s41416-025-03223-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41416-025-03223-x