Abstract
Background
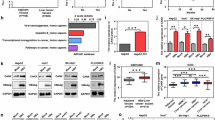
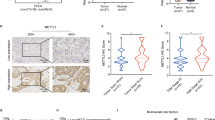
Intrahepatic cholangiocarcinoma (ICC) is the second most prevalent type of primary liver cancer and lacks effective targeted therapy. Previously, we reported that B cell-specific Moloney murine leukaemia virus integration site 1 (Bmi1) drives the formation and development of ICC independent of Ink4A/Arf; however, the underlying mechanism remains unclear. Here, we report that hepatic leukaemia factor (HLF) acts as a tumour-suppressor gene in ICC and Bmi1 represses HLF to drive ICC initiation and progression.
Methods
RNA sequencing was performed to find the downstream target of Bmi1 in ICC. Bioinformatic analysis and molecular biological techniques were used to examine the expression of Bmi1 and HLF in ICC. Effects of HLF silence or overexpression by lentivirus on cell proliferation or development were evaluated in human ICC cells, xenograft and primary ICC mouse models, respectively. The luciferase reporter assay was used to identify that Bmi1 regulates HLF.
Results
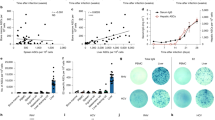
In ICC, HLF expression levels were inversely correlated with Bmi1. Overexpression of HLF inhibited the growth of ICC both in vitro and in vivo, whereas HLF knockout promoted ICC development in ICC mouse models. Importantly, HLF repression reversed the inhibitory effects of Bmi1 knockdown on cell survival, proliferation and colony formation. Luciferase reporter assay results indicated that Bmi1 represses HLF by directly binding to its promoter.
Conclusions
These findings revealed the molecular mechanism through which Bmi1 promotes ICC formation and development and uncovered the role of HLF as a tumour suppressor in ICC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its Supplementary Materials]. The raw data of the RNA sequence were uploaded to the NCBI SRA Submission platform (Accession number: PRJNA1202219), and the data will be released upon receipt of this article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Beal EW, Tumin D, Moris D, Zhang XF, Chakedis J, Dilhoff M, et al. Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2018;7:270–6.
Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198–222.
European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181–208.
Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN Guidelines(R) Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Cancer Netw. 2023;21:694–704.
Alkema MJ, Bronk M, Verhoeven E, Otte A, van ‘t Veer LJ, Berns A, et al. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 1997;11:226–40.
Buechel M, Dey A, Dwivedi SKD, Crim A, Ding K, Zhang R, et al. Inhibition of BMI1, a therapeutic approach in endometrial cancer. Mol Cancer Ther. 2018;17:2136–43.
Dey A, Dhar Dwivedi SK, Wang L, Hossen MN, Neizer-Ashun F, Bieniasz M, et al. Targeting BMI1 mitigates chemoresistance in ovarian cancer. Genes Dis. 2022;9:1415–8.
Zhu M, Fan H, Deng J, Jiang K, Liao J, Zhang X, et al. BMI1 silencing liposomes suppress postradiotherapy cancer stemness against radioresistant hepatocellular carcinoma. ACS Nano. 2023;17:23405–21.
Sardoiwala MN, Kushwaha AC, Dev A, Shrimali N, Guchhait P, Karmakar S, et al. Hypericin-loaded transferrin nanoparticles induce PP2A-regulated BMI1 degradation in colorectal cancer-specific chemo-photodynamic therapy. ACS Biomater Sci Eng. 2020;6:3139–53.
Zhu S, Zhao D, Li C, Li Q, Jiang W, Liu Q, et al. BMI1 is directly regulated by androgen receptor to promote castration-resistance in prostate cancer. Oncogene. 2020;39:17–29.
Wu Z, Ding Z, Cheng B, Cui Z. The inhibitory effect of human DEFA5 in growth of gastric cancer by targeting BMI1. Cancer Sci. 2021;112:1075–83.
Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, et al. The Bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–6.
Verdoodt B, Sommerer F, Palisaar RJ, Noldus J, Vogt M, Nambiar S, et al. Inverse association of p16 INK4a and p14 ARF methylation of the CDKN2a locus in different Gleason scores of prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:295–301.
Bednar F, Schofield HK, Collins MA, Yan W, Zhang Y, Shyam N, et al. Bmi1 is required for the initiation of pancreatic cancer through an Ink4a-independent mechanism. Carcinogenesis. 2015;36:730–8.
Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, et al. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007;12:328–41.
Guo J, Deng N, Xu Y, Li L, Kuang D, Li M, et al. Bmi1 drives the formation and development of intrahepatic cholangiocarcinoma independent of Ink4A/Arf repression. Pharmacol Res. 2021;164:105365.
Hunger SP, Ohyashiki K, Toyama K, Cleary ML. Hlf, a novel hepatic bZIP protein, shows altered DNA-binding properties following fusion to E2A in t(17;19) acute lymphoblastic leukemia. Genes Dev. 1992;6:1608–20.
Inaba T, Roberts WM, Shapiro LH, Jolly KW, Raimondi SC, Smith SD, et al. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science. 1992;257:531–4.
Lehnertz B, Chagraoui J, MacRae T, Tomellini E, Corneau S, Mayotte N, et al. HLF expression defines the human hematopoietic stem cell state. Blood. 2021;138:2642–54.
Yokomizo T, Watanabe N, Umemoto T, Matsuo J, Harai R, Kihara Y, et al. Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J Exp Med. 2019;216:1599–614.
Roychoudhury J, Clark JP, Gracia-Maldonado G, Unnisa Z, Wunderlich M, Link KA, et al. MEIS1 regulates an HLF-oxidative stress axis in MLL-fusion gene leukemia. Blood. 2015;125:2544–52.
Han T, Chen T, Chen L, Li K, Xiang D, Dou L, et al. HLF promotes ovarian cancer progression and chemoresistance via regulating Hippo signaling pathway. Cell Death Dis. 2023;14:606.
Xiang DM, Sun W, Zhou T, Zhang C, Cheng Z, Li SC. Oncofetal HLF transactivates c-Jun to promote hepatocellular carcinoma development and sorafenib resistance. Gut. 2019;68:1858–71.
Chen J, Liu A, Lin Z, Wang B, Chai X, Chen S, et al. Downregulation of the circadian rhythm regulator HLF promotes multiple-organ distant metastases in non-small cell lung cancer through PPAR/NF-kappab signaling. Cancer Lett. 2020;482:56–71.
Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104:14771–6.
Smith KS, Rhee JW, Naumovski L, Cleary ML. Disrupted differentiation and oncogenic transformation of lymphoid progenitors in E2A-HLF transgenic mice. Mol Cell Biol. 1999;19:4443–51.
Bentley ML, Corn JE, Dong KC, Phung Q, Cheung TK, Cochran AG. Recognition of UbcH5c and the nucleosome by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 2011;30:3285–97.
McGinty RK, Henrici RC, Tan S. Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature. 2014;514:591–6.
Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LLP, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–59.
Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–9.
Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99.
Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS, et al. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett. 2004;203:217–24.
Fan C, He L, Kapoor A, Gillis A, Rybak AP, Cutz JC, et al. Bmi1 promotes prostate tumorigenesis via inhibiting p16(INK4A) and p14(ARF) expression. Biochim Biophys Acta. 2008;1782:642–8.
Inaba T, Shapiro LH, Funabiki T, Sinclair AE, Jones BG, Ashmun RA, et al. DNA-binding specificity and trans-activating potential of the leukemia-associated E2A-hepatic leukemia factor fusion protein. Mol Cell Biol. 1994;14:3403–13.
Magnusson M, Brun AC, Miyake N, Larsson J, Ehinger M, Bjornsson JM, et al. HOXA10 is a critical regulator for hematopoietic stem cells and erythroid/megakaryocyte development. Blood. 2007;109:3687–96.
Wahlestedt M, Ladopoulos V, Hidalgo I, Sanchez Castillo M, Hannah R, Sawen P, et al. Critical modulation of hematopoietic lineage fate by hepatic leukemia factor. Cell Rep. 2017;21:2251–63.
Dorn DC, Kou CA, Png KJ, Moore MA. The effect of cantharidins on leukemic stem cells. Int J Cancer. 2009;124:2186–99.
Lai CK, Norddahl GL, Maetzig T, Rosten P, Lohr T, Sanchez Milde L, et al. Meis2 as a critical player in MN1-induced leukemia. Blood Cancer J. 2017;7:e613.
Wei X, He J, Wang J, Yang X, Ma B. Bmi-1 is essential for the oncogenic potential in CD133(+) human laryngeal cancer cells. Tumour Biol. 2015;36:8931–42.
Liu YL, Jiang SX, Yang YM, Xu H, Liu JL, Wang XS. USP22 acts as an oncogene by the activation of BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem Biophys. 2012;62:229–35.
Jiao K, Jiang W, Zhao C, Su D, Zhang H. Bmi-1 in gallbladder carcinoma: clinicopathology and mechanism of regulation of human gallbladder carcinoma proliferation. Oncol Lett. 2019;18:1365–71.
Hu C, Zhang Q, Tang Q, Zhou H, Liu W, Huang J, et al. CBX4 promotes the proliferation and metastasis via regulating BMI-1 in lung cancer. J Cell Mol Med. 2020;24:618–31.
Kim BR, Kwon Y, Rho SB. BMI-1 interacts with sMEK1 and inactivates sMEK1-induced apoptotic cell death. Oncol Rep. 2017;37:579–86.
Wang R, Fan H, Sun M, Lv Z, Yi W. Roles of BMI1 in the initiation, progression, and treatment of hepatocellular carcinoma. Technol Cancer Res Treat. 2022;21:15330338211070689.
Li B, Chen Y, Wang F, Guo J, Fu W, Li M, et al. Bmi1 drives hepatocarcinogenesis by repressing the TGFbeta2/SMAD signalling axis. Oncogene. 2020;39:1063–79.
Gao F, Huang W, Zhang Y, Tang S, Zheng L, Ma F, et al. Hes1 promotes cell proliferation and migration by activating Bmi-1 and PTEN/Akt/GSK3beta pathway in human colon cancer. Oncotarget. 2015;6:38667–80.
Yamashita M, Kuwahara M, Suzuki A, Hirahara K, Shinnaksu R, Hosokawa H, et al. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J Exp Med. 2008;205:1109–20.
Kimura M, Takenobu H, Akita N, Nakazawa A, Ochiai H, Shimozato O, et al. Bmi1 regulates cell fate via tumor suppressor WWOX repression in small-cell lung cancer cells. Cancer Sci. 2011;102:983–90.
Kameyama A, Nishijima R, Yamakoshi K. Bmi-1 regulates mucin levels and mucin O-glycosylation in the submandibular gland of mice. PLoS ONE. 2021;16:e0245607.
Yu F, Zhou C, Zeng H, Liu Y, Li S. BMI1 activates WNT signaling in colon cancer by negatively regulating the WNT antagonist IDAX. Biochem Biophys Res Commun. 2018;496:468–74.
Acknowledgements
The authors thank all the members of the Dr Xu laboratory for their technical assistance and helpful discussions.
Funding
This work was supported by the National Science Foundation of China (82372667, 82273059 and 82073091) and Guizhou Province Science and Technology Project (ZK-2024-Key-097).
Author information
Authors and Affiliations
Contributions
CX contributed to the conception and the design of the study. JG, XS and RL performed the experiments and contributed to the acquisition of data. HW contributed to the analysis and interpretation of data. FY contributed to manuscript drafting or critical revisions on the intellectual content. CX finalised the manuscript. JG and CX confirm the authenticity of all the raw data. YS contributed to the revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Shi, X., Sun, Y. et al. Bmi1 represses HLF to drive the formation and development of intrahepatic cholangiocarcinoma. Br J Cancer 134, 33–44 (2026). https://doi.org/10.1038/s41416-025-03234-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03234-8