Abstract
Background
Colorectal cancer (CRC) incidence is rising among adults under 55 years, but its causes remain unclear. Large-scale prospective studies are needed to identify risk factors for early-onset CRC (EOCRC).
Methods
We pooled three large European prospective cohort studies, examining 14 known or suspected risk factors with EOCRC (diagnosed <55 years, N = 1369) and later-onset CRCs (LOCRC) (diagnosed ≥55 years, N = 13,490). Cox proportional hazards models estimated hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Higher body mass index (BMI, per 5 kg/m2 increase), was strongly associated with EOCRC in men (HR 1.33, 95% CI: 1.18–1.51), particularly for early-onset colon cancer (HR 1.55, 95% CI: 1.32-1.82), compared to later-onset disease (HR 1.25, 95% CI: 1.19–1.31) (Phet = 0.01). Weaker associations with BMI were observed for women and rectal cancers. Similar sex and subsite specific trends were observed for waist circumference and waist-to-hip ratio. Current smoking (HR 1.24, 95% CI: 1.07–1.44) and alcohol use (HR 1.15, 95% CI: 1.06–1.25) increased EOCRC risk, and physical activity (HR 0.71, 95% CI: 0.54–0.95) was protective.
Discussion
Adiposity, physical inactivity, smoking, and alcohol consumption are risk factors for EOCRC. Risk factors were largely similar between EOCRC and LOCRC, except for adiposity, with stronger EOCRC association in men.
Similar content being viewed by others
Introduction
The incidence of colorectal cancer (CRC) in young adults (usually defined as a diagnosis prior to age 50 years) has increased over recent decades in many countries [1,2,3,4]. Data from the United States (U.S) shows that CRC incidence is also rising recently in the 50-54 year age group, mirroring the trends reported in those <50 years, with underlying risk factors and mechanisms speculated to be similar in both age groups [5]. Studying this age group alongside those under 50 is therefore important to capture the full scope of early-onset disease. In the U.S and several other high-income countries, higher incidence rates of early-onset CRC (EOCRC) have been observed across successive birth cohorts since the 1960s [1, 6], which points to secular changes in exposure to suspected risk factors from this period contributing to the increasing disease rates in younger adults [7].
Candidate risk factors that have been proposed to explain the rise in EOCRC incidence rates include obesity, the adoption of unhealthy lifestyle habits– such as high alcohol consumption, and changes in reproductive and menstrual risk factors [8, 9]. However, for most suspected risk factors, prior evidence on how they are associated with EOCRC is derived from case-control studies [10,11,12,13], small-scale cohort analyses (<120 incident EOCRC cases) [14, 15], or clinical databases [16,17,18] that lack high-quality data on risk factors and covariates. In a recent Mendelian randomisation (MR) analysis, we found that adiposity traits and alcohol consumption were positively associated with risk of EOCRC, but for other lifestyle risk factors evidence was less clear, possibly due to insufficient statistical power [19]. Consequently, the role of most lifestyle-related risk factors in EOCRC development remains largely uncertain. High-quality and large-scale prospective studies examining how these risk factors relate to risk of developing EOCRC are lacking.
Here, we evaluated 14 lifestyle-related risk factors that have been associated with CRC [20] in relation to EOCRC risk (herein defined as CRC diagnosed at <55 years) and compared the associations between EOCRC and later-onset CRC (LOCRC, diagnosed ≥55 years). We performed a comprehensive investigation of risk factors by pooling individual-level data from three large-scale prospective European cohorts (the European Prospective Investigation into Cancer and Nutrition [EPIC], the Norwegian Women and Cancer Study [NOWAC], and the UK Biobank [UKB]), across nine countries resulting in more than 14,859 incident CRC cases, 1369 of which were diagnosed <55 years of age. The large-scale pooled dataset afforded sufficient statistical power to compare EOCRC risk factor relationships with LOCRC disease and across anatomical subsites.
Methods
Study participants
EPIC is a cohort comprised of 521,448 participants, mostly aged 35 years and older, recruited between 1992 and 2000 from the general population of 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom) [21]. Written informed consent was provided by all study participants, and ethical approval for EPIC was provided by the International Agency for Research on Cancer and local participating centres.
NOWAC enroled a random sample of 172,000 Norwegian National Population Registry women, mostly aged 35–70 years old, in three waves between 1991 and 2007 [22]. Written informed consent was provided by all study participants, and the NOWAC study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate.
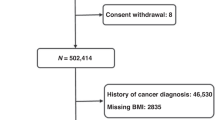
UKB is a prospective cohort of 502,536 adults aged between 40 and 69 years old who were recruited between 2006 and 2010. UKB has approval from the North West Multi-centre Research Ethics Committee, the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. In addition, an independent Ethics and Governance Council was formed in 2004 to oversee UKB’s continuous adherence to the Ethics and Governance Framework, which was developed for the study (http://www.ukbiobank.ac.uk/ethics/). All participants provided written informed consent at recruitment, and this research has been conducted under UKB application number 25897.
During the baseline recruitment visits, participants from all three cohorts were asked to complete a self-administered questionnaire, which included questions on sociodemographic information (including age, sex, education), health/medical history, and lifestyle exposures (including smoking habits, dietary intakes, and alcohol consumption). After removing prevalent cancer cases (participants with cancer diagnoses before recruitment) (n = 6327), participants with missing follow-up evaluation (n = 59,755), participants from Greece (EPIC) due to lack of available data (n = 26,916), overlapping or duplicated participants of NOWAC from EPIC (n = 34,666), and UKB participants who withdrew consent (n = 223), a total of 430,410 from EPIC, 166,134 from NOWAC and 472,159 participants from UKB remained.
Exposures
For all three cohorts, the fourteen risk factors of interest were measured at recruitment. The exposures considered in the current analysis were defined as follows: height (per 10 cm), body mass index (BMI) (per 5 kg/m2), waist circumference (per 10 cm), waist-to-hip ratio (per 0.1), smoking status (never, former, current), alcohol consumption (per 30 g/d), physical activity index (inactive, moderately inactive, moderately active, active); prevalent diabetes (no, yes), prevalent hypertension (no, yes), and in women only, age at menarche (<12, 12–15, >15 years), oral contraceptive (OC) use (never, ever), and parity (nulliparous, ever parity), age at menopause (≤50, 51–55, >55 years), and ever postmenopausal hormone replacement therapy (HRT) use (no, yes). To assess the assumption of linearity, alcohol consumption was evaluated using restricted cubic spline regression and sex-specific quartiles of anthropometric measures in relation to CRC risk. Details of measurements and variable harmonisation are included in the Supplementary material.
Follow-up evaluation for cancer incidence and vital status
In EPIC, cancer incidence was determined through record linkage with regional cancer registries (Denmark, Italy, the Netherlands, Spain, Sweden, and the United Kingdom) or via a combination of methods, including the use of health insurance records, contacts with cancer and pathology registries, and active follow-up evaluation through participants and their next of kin (France and Germany). The end of follow-up for cancer related outcomes and mortality ranged from 2008-2013 for different centers. For NOWAC, we obtained information on cancer incidence, death, and emigration in the cohort through linkage to the Norwegian Cancer Registry, the Cause of Death Registry, and the Norwegian Central Population Register, respectively. Participants were followed-up from the date of return of the baseline questionnaire until date of death, emigration, or the end of follow-up in December 2018, whichever occurred first. In UKB, incident cancer cases and cancer cases recorded first in death certificates were identified through linkage to national cancer and death registries. Participants were followed-up from the date of baseline assessment until either date of death, cancer diagnosis, date of loss to follow-up or end of follow-up for cancer incidence (November 2014). CRC cases were defined using the 10th Revision of the International Classification of Diseases (ICD-10 codes C18–C20). Colon cancer was defined as ICD-10 code C18, and rectal cancer was defined as ICD-10 codes C19 - C20.EOCRC was defined as an incident CRC diagnosis <55 years, consistent with recent evidence showing similar incidence trends in individuals aged 50–54 years as those <50, and to increase statistical power; while later-onset CRC was defined as a diagnosis ≥55 years in our pooled analysis.
Statistical analysis
Using age as the underlying timescale in Cox proportional hazards models, we calculated hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for each of the fourteen risk factors with EOCRC and LOCRC. Age at recruitment was the time of entry, and age at exit was determined by the age at the earliest of the following events: CRC diagnosis, death, or the final date at which follow-up evaluation in each study was deemed complete. All the multivariable models were stratified by cohort for sex-specific analysis, cohort and sex for sex-combined analysis, and adjusted for age and country. The models were further adjusted as follows: models evaluating height and BMI were mutually adjusted, other body size variables were adjusted for height, as well as for physical activity, smoking, alcohol, hypertension, diabetes and HRT. To reduce potential bias from collinearity between body size variables, BMI, waist circumference, and waist-to-hip ratio were modelled separately and were not included simultaneously in the same regression models. Models for physical activity, smoking, alcohol, hypertension, diabetes were mutually adjusted and adjusted for height, BMI and HRT. Reproductive factors were adjusted further for height, BMI, physical activity, smoking, alcohol, hypertension, diabetes and HRT, except the models evaluating HRT. Multivariable joint Cox proportional hazards model was used to estimate HRs and 95% CIs for the analyses by anatomic site, as described previously with similar covariate adjustment as above [23]. Briefly, the model accounts each anatomical subsite as competing risk, allows different baseline hazard functions for each subsite (i.e., colon and rectum) and directly compares the associations with outcome subtypes using Wald’s test. Since the association between body size measurements and CRC was found to vary by sex in earlier studies [24,25,26], as well as in our study (Supplementary Table 1), separate models were used for men and women. Statistical tests used in the analysis were all 2-sided, and a P value less than 0.05 was considered statistically significant. Since the analyses were hypothesis-driven, evaluating previously established or suspected risk factors of CRC, correction for multiple testing was not applied.
Ethics approval and consent to participate
For EPIC, ethical approval was provided by the International Agency for Research on Cancer and local participating centres. For NOWAC, the study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. UK Biobank has approval from the North West Multi-centre Research Ethics Committee, the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. In addition, an independent Ethics and Governance Council was formed in 2004 to oversee UKB’s continuous adherence to the Ethics and Governance Framework, which was developed for the study (http://www.ukbiobank.ac.uk/ethics/). This research has been conducted under UK-Biobank application number 25897. All participants in each of the cohorts provided written informed consent at recruitment.
Results
After a median follow-up time of 11.5 years, 14,859 (9360 women and 5499 men) incident CRC cases occurred; of which 1369 were EOCRC cases (diagnosed <55 years) and included 792 early-onset colon (EO-CC) and 526 early-onset rectal cancer (EO-RC) cases. Table 1 shows the main characteristics of participants at time of recruitment, and Supplementary Tables 2 and 3 show the characteristics by sex and each cohort.
Higher BMI was associated with an increased risk of EOCRC among men (per 5 kg/m², HR 1.33, 95% CI: 1.18–1.51) but not among women (per 5 kg/m², HR 1.03, 95% CI: 0.95–1.13) (Fig. 1). The positive association between BMI and EOCRC was stronger for colon cancer in men (per 5 kg/m² HR 1.55, 95% CI: 1.32–1.82) compared to rectal cancer (per 5 kg/m² HR 1.12, 95% CI: 0.94–1.35) (P-hetCCvsRC = 0.008). For LOCRC, BMI was positively associated with risk in both women (per 5 kg/m², HR 1.08, 95% CI: 1.05–1.11) and men (per 5 kg/m², HR 1.19, 95% CI: 1.15–1.24). However, similar to EOCRC, for LOCRC the association in men was stronger for colon cancer (per 5 kg/m² HR 1.25, 95% CI: 1.19–1.31) than for rectal cancer (per 5 kg/m² HR 1.10, 95% CI: 1.03–1.17) (P-hetCCvsRC = 0.001). Among men, the association between BMI and EO-CC was stronger (per 5 kg/m², HR 1.55, 95% CI: 1.32–1.82) than the association observed for later-onset colon cancer (LO-CC) (per 5 kg/m², HR 1.25, 95% CI: 1.19–1.31) (P-hetEOvsLO = 0.01). For rectal cancer, however, associations with BMI did not differ significantly by sex or between early-onset and later-onset disease, and they were all broadly lower than colon cancer specific associations with BMI. Similar to BMI, a higher risk was observed for larger waist circumference for EOCRC in men only, with a stronger association for colon cancers compared to rectal cancers. The positive association between waist circumference and EO-CC (per 10 cm, HR 1.41, 95% CI: 1.24–1.62) was stronger than for LO-CC (per 10 cm, HR 1.21, 95% CI: 1.17–1.25) (P-het = 0.02). For rectal cancer, weaker positive associations were found with waist circumference. The pattern of results for waist-to-hip ratio (WHR) in EOCRC and LOCRC, as well as by anatomical subsite, was largely consistent with those observed for waist circumference. Height did not exhibit a significant association with EOCRC risk in either men or women, and the lack of association was consistent across both sexes and anatomical subsites. However, a positive association with height was observed for LO-CC, but not later-onset rectal cancer (LO-RC). Sensitivity analyses by sex-specific quartiles of BMI, waist circumference, and WHR showed similar positive associations for both EOCRC and LOCRC with statistical significant test for linear trend (Supplementary Table 4), as well as for each cohort analysed (Supplementary Table 5).
Multivariable Cox regression models for overall colorectal cancer using age as the underlying time variable stratified by cohort, adjusted for age at recruitment, country, physical activity, smoking status, intakes of alcohol, diabetes, hypertension and ever use of postmenopausal hormone replacement therapy. The multivariable model for height was adjusted further for body mass index. Multivariable models for body mass index, waist circumference, and waist-to-hip ratio were adjusted further for height. For multivariable joint Cox models for anatomical subsite, similar variable adjustments were made as above. EOCRC: early-onset colorectal cancer, LOCRC: later-onset colorectal cancer, P-het: heterogeneity p-value between subsites.
Current smoking was similarly associated with risk of both EOCRC (current vs. never smokers, HR 1.24, 95% CI: 1.07–1.44) and LOCRC (current vs. never smokers, HR 1.30, 95% CI: 1.23–1.37), with a similar pattern of associations generally observed for colon and rectal cancer (Fig. 2). Former smoking vs. never smoking was positively associated with LOCRC (HR 1.21, 95% CI: 1.16–1.26) but not EOCRC risk (HR 1.06, 95% CI: 0.92–1.23). A similar pattern of associations was found for colon and rectal cancer, with statistically significant heterogeneity by early-onset and later onset cancers found for former smoking in rectal cancer (P-hetEOvsLO = 0.02). Similar magnitude positive associations were also observed between alcohol consumption and EOCRC (per 30 g/day, HR 1.15, 95% CI: 1.06–1.25) and LOCRC (per 30 g/day, HR 1.16, 95% CI: 1.13–1.20), and for colon and rectal cancer (Fig. 2). Restricted cubic spline analyses indicated a linear association between alcohol consumption and CRC risk (Supplementary Fig. 1a, b).
Multivariable Cox regression models was constructed using age as the underlying time variable stratified by sex and cohort. For smoking, alcohol consumption and physical activity, the above models were mutually adjusted, and adjusted for age at recruitment, country, height, body mass index, diabetes, hypertension and ever use of postmenopausal hormone replacement therapy and physical activity. Multivariable model for diabetes and hypertension was mutually adjusted and adjusted further for age at recruitment, country, height, body mass index, smoking, alcohol and physical activity. For multivariable joint Cox models for anatomical subsite, similar variable adjustments were made as above. EOCRC early-onset colorectal cancer, LOCRC later-onset colorectal cancer, P-het: heterogeneity p value between subsites.
Higher levels of physical activity were associated with a lower risk of LOCRC (active vs. inactive, HR 0.92, 95% CI: 0.86-0.98) and EOCRC (active vs. inactive, HR 0.83, 95% CI: 0.69-1.03), although the latter association did not reach statistical significance despite a lower point estimate. Inverse associations for physical activity were observed for EO-CC (active vs. inactive, HR 0.71, 95% CI: 0.54-0.95) and LO-CC (active vs. inactive, HR 0.84, 95% CI: 0.78-0.91), but not for rectal cancer. Diabetes was positively associated with LOCRC (no vs yes; HR 1.17, 95%CI: 1.07-1.29), and EOCRC albeit the risk estimate did not attain statistical significance (no vs yes; HR 1.44, 95% CI: 0.95-2.16), with a similar pattern observed for EO-CC (Fig. 2). Although not statistically significant, a stronger positive association was observed for diabetes and EO-RC (no vs yes; HR 1.74, 95%CI: 0.97-3.11) compared to LO-RC (no vs yes; HR 1.08, 95% CI: 0.92-1.27) (Phet = 0.03). Similar magnitude positive associations were observed for hypertension with EOCRC and LOCRC, with only the latter endpoint reaching the threshold of statistical significance (yes vs. no), HR 1.08, 95% CI: 1.03-1.13); this positive association was stronger for colon cancer than rectal cancer in both age groups, but the difference did not reach statistical significance (early-onset: Phet = 0.43; later-onset: Phet = 0.17) (Fig. 2).
Reproductive and menstrual factors were not associated with EOCRC risk (Fig. 3). In contrast, for LOCRC, later age at menarche (>15 vs. <12 years, HR 0.87, 95% CI: 0.77–0.98), parity (ever vs. nulliparous, HR 0.89, 95% CI: 0.82–0.97) and ever HRT use (yes vs. no), HR 0.91, 95% CI: 0.86–0.96) were associated with lower risk. No heterogeneity was observed in the pattern of results for reproductive factors for colon and rectal cancer across both age groups.
Multivariable Cox regression models were constructed using age as the underlying time variable stratified by cohort, and adjusted for age at recruitment, country, physical activity, smoking status, intakes of alcohol, diabetes, hypertension and ever use of postmenopausal hormone replacement therapy (HRT). For HRT use, the models were adjusted for all the above except for postmenopausal hormone replacement therapy use. For multivariable joint Cox models for anatomical subsite, similar variable adjustments were made as above. EOCRC early-onset colorectal cancer, LOCRC later-onset colorectal cancer, P-het: heterogeneity p value between subsites.
Discussion
In this multi-country analysis that pooled data from three large-scale prospective European cohorts, we comprehensively assessed associations between various anthropometric, lifestyle and reproductive factors and the risk of EOCRC. BMI and central adiposity measurements (men only), low physical activity levels, current smoking, and alcohol consumption were associated with greater EOCRC risk. For BMI and waist circumference in men, stronger positive associations were observed for EO-CC than for LO-CC. Overall, we generally observed associations of similar direction and magnitude for EOCRC as for LOCRC.
In recent decades, obesity rates have surged among younger adults, mirroring the rising incidence rates of EOCRC [9]. Although BMI is the most commonly reported anthropometric index, waist circumference and WHR may better capture central adiposity and have been suggested to be stronger predictors of colorectal cancer in some studies [27]. BMI has been generally associated with higher EOCRC risk in previous prospective cohort and case-control studies [15, 28,29,30,31], but evidence for central adiposity measures, such as waist circumference and WHR, is less common. In our recent MR study, we observed positive effect estimates for genetically predicted BMI, waist circumference, and WHR with EOCRC risk [19]. However, due to sample size constraints, we were unable to stratify these analyses by sex. In the current pooled analysis that included data from three prospective cohorts, we only observed positive associations for these adiposity measurements with EOCRC risk among men and not women. Notably, for BMI and waist circumference, stronger positive associations for men were observed for EO-CC risk than for later-onset disease, but not in EO-RC. The observed disparity by sex and anatomical subsite is in accordance with a large body of prior epidemiologic evidence showing similar results for CRC in all age groups, which primarily comprises older persons [20, 26, 32, 33]. Reasons for these sex differences for the associations of BMI with CRC risk are unknown but have been postulated to include a risk-reducing effect of adipose-tissue derived oestrogen for women that may not be experienced by men with obesity [34, 35]. Moreover, men are more likely to accumulate visceral fat [36], which is metabolically active and associated with insulin resistance, chronic inflammation, and higher circulating IGF-1 levels, all implicated in CRC development [37]. Women, by contrast, tend to accumulate more subcutaneous fat, which is considered less deleterious metabolically [38]. However, obesity alone is unlikely to explain the increasing EOCRC trend fully, given that the increase in incidence rates has been primarily in rectal and distal colon cancers and the rates are high, if not higher, in women as opposed to men [3].
Higher levels of physical activity have been consistently associated with lower CRC risk [39,40,41]; however, few studies have investigated the association with EOCRC. To our knowledge, the current investigation is the first prospective study to report an inverse association between physical activity and EO-CC risk. A previous prospective investigation in the Nurses’ Health Study II reported that prolonged sedentary activity (i.e., television viewing time) was associated with greater EOCRC risk [14]. A larger pooled study, mostly comprising case-control studies, reported a positive association for sedentary behaviour (defined as absence of physical activity less than 1 h per week) and EOCRC that did not reach the threshold of statistical significance [10].
Collectively, these results are largely supportive of high adiposity and low physical activity levels being implicated in EOCRC development. Both risk factors have been found to influence key processes underlying colorectal tumorigenesis, including inflammation [42,43,44], oxidative stress [45,46,47], insulin resistance [48,49,50], hormonal dysregulation [51] and gut microbiota alterations [52,53,54]. Obesity, particularly visceral adiposity, promotes expression of pro-inflammatory cytokines, such as IL-6 and TNF-α, fostering chronic inflammation and oxidative stress [42, 55]. Dysfunctional adipose tissue disrupts adipokines, impacting insulin sensitivity and cell proliferation [56]. Insulin resistance in obesity leads to hyperinsulinemia and elevated bioavailable IGF-1 levels, fuelling tumour growth [57]. Conversely, physical activity has been shown to improve insulin sensitivity and lower circulating levels of inflammatory markers [58,59,60].
We found that current smoking was associated with greater risk of both EOCRC and LOCRC, while former smoking increased the risk of LOCRC only. Although smoking has long been associated with CRC risk [61, 62], only a limited number of smaller case-control studies have investigated its impact on EOCRC risk, with null or strong positive associations mostly reported [10, 63,64,65]. Heavier alcohol consumption, typically defined as >25–30 g/day, has been associated with EOCRC in two case-control studies, exhibiting comparable or stronger effects than in LOCRC [10, 66]. A recent retrospective cohort analysis from Korea found similar increased EOCRC risk in moderate and heavy drinkers [67]. While the current observational analysis revealed similar association strengths between alcohol and EOCRC and LOCRC risk, our recent MR analysis identified a stronger positive effect for younger-onset disease [19].
The mechanisms by which smoking and alcohol consumption contribute to CRC development are not yet fully understood. Cigarette smoke, rich in carcinogens such as polycyclic aromatic hydrocarbons (PAHs) and nitrosamines, can induce DNA damage, oxidative stress, and inflammation, promoting CRC initiation and progression [68,69,70]. Similarly, alcohol consumption can also cause DNA damage, oxidative stress, and lipid peroxidation, leading to DNA adduct formation and mutations in CRC development [71, 72]. Smoking and alcohol can also promote the growth of pathogenic bacteria and the production of pro-inflammatory metabolites, exacerbating inflammation and immune dysregulation in the colon and rectum and creating a pro-inflammatory environment conducive to CRC development [73,74,75].
For height, we found little evidence of a positive association with EOCRC risk, in accordance with a previous pooled analysis including mostly case-control studies [10]. In contrast, we previously observed a positive effect estimate for the association between genetically predicted height and EOCRC risk [19]. The lack of association for height and EOCRC observed in the current analysis may be a consequence of insufficient statistical power for a more modest observational effect size. Larger prospective studies are likely needed to comprehensively investigate the role of anthropometric traits in EOCRC development. Similarly, we observed no associations of HRT use or parity with EOCRC, although both factors were inversely associated with LOCRC. Previous studies have reported mixed results for these reproductive factors, with some suggesting protective effects [76, 77] and others reporting null associations [78]. Our findings, therefore, suggest that reproductive and menstrual factors may have limited relevance for EOCRC, while their influence may be more pronounced in later-onset disease.
Our study represents one of the first large-scale prospective cohort analyses to comprehensively evaluate the association of both well-established and suspected CRC risk factors with EOCRC development. By leveraging data from three well-characterised cohort studies, we were able to assess sex- and subsite-specific differences and compare them with later-onset disease, while minimising between-study heterogeneity. Our findings also have important public health implications. Several EOCRC risk factors, including adiposity, smoking, alcohol use, and low physical activity, are modifiable. Promoting healthy weight, smoking cessation, reduced alcohol intake, and physical activity may support efforts to reduce EOCRC risk in younger adults. Limitations include only a single assessment of exposures at baseline with no repeat measurements and lack of lifestyle or body size trajectory data from adolescence or early adulthood. Although our analyses were hypothesis-driven and focused on established CRC risk factors, results across subsites may still be influenced by multiple testing and warrant independent validation. A further limitation is that our analyses did not capture differences by smoking intensity or duration, or by type of alcoholic beverage. Selection bias, particularly in UK Biobank, cannot be ruled out entirely, and our findings reflect risks observed in the studied age ranges rather than across the full life course. Lastly, our study only included individuals of European ancestry and residing in Europe, which may not generalise to other populations or geographic regions also experiencing increased rates of EOCRC.
In conclusion, adiposity, physical inactivity, current smoking, and alcohol consumption were associated with elevated EOCRC risk. In general, we found a similar pattern of risk factor relationships for EOCRC as with LOCRC, indicating that the changing prevalence of these risk factors may be among the contributing factors to the rising rates of EOCRC. However, these risk factors likely do not explain all the increases in EOCRC incidence rates fully, and hence, larger studies are warranted to examine risk factors for CRC across younger ages of diagnosis and to identify potential novel causes of EOCRC.
Data availability
The data that support the findings of this study are available from each of the three study cohorts used: The UK Biobank, the EPIC study and the NOWAC study but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available for others upon reasonable request and with permission from The UK Biobank (https://www.ukbiobank.ac.uk/about-us/how-we-work/access-to-uk-biobank-data/), the EPIC study (provided by the International Agency for Research on Cancer) and the NOWAC study (provided by https://uit.no/research/nowac_en#region_783025).
References
Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–85.
Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22.
Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1695–8.
Feletto E, Yu XQ, Lew JB, St John DJB, Jenkins MA, Macrae FA, et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: analysis of data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev. 2019;28:83–90.
Zaki TA, Singal AG, May FP, Murphy CC. Increasing incidence rates of colorectal cancer at ages 50-54 years. Gastroenterology. 2022;162:964–5 e3.
Sinicrope FA. Increasing incidence of early-onset colorectal cancer. N Engl J Med. 2022;386:1547–58.
Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230–43.
Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262–74.
Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656–73.
Archambault AN, Lin Y, Jeon J, Harrison TA, Bishop DT, Brenner H, et al. Nongenetic determinants of risk for early-onset colorectal cancer. JNCI Cancer Spectr. (2021);5:pkab029.
Chang VC, Cotterchio M, De P, Tinmouth J. Risk factors for early-onset colorectal cancer: a population-based case-control study in Ontario, Canada. Cancer Causes Control. 2021;32:1063–83.
Gausman V, Dornblaser D, Anand S, Hayes RB, O’Connell K, Du M, et al. Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol. 2020;18:2752–9 e2.
Low EE, Demb J, Liu L, Earles A, Bustamante R, Williams CD, et al. Risk factors for early-onset colorectal cancer. Gastroenterology. 2020;159:492–501 e7.
Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr. 2018;2:pky073.
Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5:37–44.
Imperiale TF, Myers LJ, Barker BC, Larson J, Stump TE, Daggy JK. Risk factors for early-onset sporadic colorectal cancer in male veterans. Cancer Prev Res. 2023;16:513–22.
Tait C, Patel AH, Chen A, Li Y, Minacapelli CD, Rustgi V. Early-onset colorectal cancer: prevalence, risk factors, and clinical features among commercially insured adults in the United States. Cureus. 2023;15:e49432.
Elangovan A, Skeans J, Landsman M, Ali SMJ, Elangovan AG, Kaelber DC, et al. Colorectal cancer, age, and obesity-related comorbidities: a large database study. Dig Dis Sci. 2021;66:3156–63.
Laskar RS, Qu C, Huyghe JR, Harrison T, Hayes RB, Cao Y, et al. Genome-wide association studies and Mendelian randomization analyses provide insights into the causes of early-onset colorectal cancer. Ann Oncol. 2024;35:523–536.
Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault MC, Carbonnel F, et al. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European countries: a multinational cohort study. Clin Gastroenterol Hepatol. 2019;17:1323–31.e6.
Riboli E, Kaaks R. The EPIC project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S6–14.
Lund E, Dumeaux V, Braaten T, Hjartaker A, Engeset D, Skeie G, et al. Cohort profile: the Norwegian Women and cancer study–NOWAC–Kvinner og kreft. Int J Epidemiol. 2008;37:36–41.
Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, et al. A comparison of the polytomous logistic regression and joint Cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2013;22:275–85.
Schlesinger S, Lieb W, Koch M, Fedirko V, Dahm CC, Pischon T, et al. Body weight gain and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Obes Rev. 2015;16:607–19.
Song M, Hu FB, Spiegelman D, Chan AT, Wu K, Ogino S, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol. 2016;45:871–83.
Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28:1–4.
Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obesity. 2004;28:559–67.
Levi Z, Kark JD, Katz LH, Twig G, Derazne E, Tzur D, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: a population-based study. Cancer. 2017;123:4022–30.
Hua H, Jiang Q, Sun P, Xu X. Risk factors for early-onset colorectal cancer: systematic review and meta-analysis. Front Oncol. 2023;13:1132306.
O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, et al. Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1229–40 e5.
Li H, Boakye D, Chen X, Jansen L, Chang-Claude J, Hoffmeister M, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. 2022;162:1088–97 e3.
Seo JY, Jin EH, Chung GE, Kim YS, Bae JH, Yim JY, et al. The risk of colorectal cancer according to obesity status at four-year intervals: a nationwide population-based cohort study. Sci Rep. 2023;13:8928.
Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65.
Campbell PT, Cotterchio M, Dicks E, Parfrey P, Gallinger S, McLaughlin JR. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1735–44.
Simpson ER, Bulun SE, Nichols JE, Zhao Y. Estrogen biosynthesis in adipose tissue: regulation by paracrine and autocrine mechanisms. J Endocrinol. 1996;150:S51–7.
Nauli AM, Matin S. Why do men accumulate abdominal visceral fat?. Front Physiol. 2019;10:1486.
Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12.
Kim H, Kim SE, Sung MK. Sex and gender differences in obesity: biological, sociocultural, and clinical perspectives. World J Mens Health. 2025;43:758–72.
Morris JS, Bradbury KE, Cross AJ, Gunter MJ, Murphy N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118:920–9.
Zhang X, Theodoratou E, Li X, Farrington SM, Law PJ, Broderick P, et al. Genetically predicted physical activity levels are associated with lower colorectal cancer risk: a Mendelian randomisation study. Br J Cancer. 2021;124:1330–8.
Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. 2020;11:597.
Hildebrandt X, Ibrahim M, Peltzer N. Cell death and inflammation during obesity: “Know my methods, WAT(son). Cell Death Differ. 2023;30:279–92.
Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–67.
Burini RC, Anderson E, Durstine JL, Carson JA. Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med Health Sci. 2020;2:1–6.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9:17181–98.
Bardelcikova A, Soltys J, Mojzis J. Oxidative stress, inflammation and colorectal cancer: an overview. Antioxidants. 2023;12:901.
Poloz Y, Stambolic V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015;6:e2037.
Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol. 2021;15:790–800.
Campbell PT, Newton CC, Jacobs EJ, McCullough ML, Wang Y, Rees-Punia E, et al. Prospective associations of hemoglobin A(1c) and c-peptide with risk of diabetes-related cancers in the Cancer Prevention Study-II Nutrition Cohort. Cancer Res Commun. 2022;2:653–62.
Rubinstein MM, Brown KA, Iyengar NM. Targeting obesity-related dysfunction in hormonally driven cancers. Br J Cancer. 2021;125:495–509.
Kang X, Ng SK, Liu C, Lin Y, Zhou Y, Kwong TNY, et al. Altered gut microbiota of obesity subjects promotes colorectal carcinogenesis in mice. EBioMedicine. 2023;93:104670.
Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671–82.
Himbert C, Stephens WZ, Gigic B, Hardikar S, Holowatyj AN, Lin T, et al. Differences in the gut microbiome by physical activity and BMI among colorectal cancer patients. Am J Cancer Res. 2022;12:4789–801.
Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70:i104–8.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Gallagher EJ, LeRoith D. Hyperinsulinaemia in cancer. Nat Rev Cancer. 2020;20:629–44.
Silva FM, Duarte-Mendes P, Teixeira AM, Soares CM, Ferreira JP. The effects of combined exercise training on glucose metabolism and inflammatory markers in sedentary adults: a systematic review and meta-analysis. Sci Rep. 2024;14:1936.
Drummond AE, Swain CTV, Milne RL, English DR, Brown KA, Skinner TL, et al. Linking physical activity to breast cancer risk via the insulin/insulin-like growth factor signaling system, part 2: the effect of insulin/insulin-like growth factor signaling on breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2022;31:2116–25.
Swain CTV, Drummond AE, Milne RL, English DR, Brown KA, Chong JE, et al. Linking physical activity to breast cancer risk via insulin/insulin-like growth factor signaling system, part 1: the effect of physical activity on the insulin/insulin-like growth factor signaling system. Cancer Epidemiol Biomarkers Prev. 2022;31:2106–15.
Botteri E, Borroni E, Sloan EK, Bagnardi V, Bosetti C, Peveri G, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol. 2020;115:1940–9.
Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009;7:682–8 e1-5.
Li H, Chen X, Hoffmeister M, Brenner H. Associations of smoking with early- and late-onset colorectal cancer. JNCI Cancer Spectr. 2023;7:pkad004.
Lee SE, Jo HB, Kwack WG, Jeong YJ, Yoon YJ, Kang HW. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol. 2016;22:2981–92.
Koo JE, Kim KJ, Park HW, Kim HK, Choe JW, Chang HS, et al. Prevalence and risk factors of advanced colorectal neoplasms in asymptomatic Korean people between 40 and 49 years of age. J Gastroenterol Hepatol. 2017;32:98–105.
Chen X, Li H, Guo F, Hoffmeister M, Brenner H. Alcohol consumption, polygenic risk score, and early- and late-onset colorectal cancer risk. EClinicalMedicine. 2022;49:101460.
Jin EH, Han K, Shin CM, Lee DH, Kang SJ, Lim JH, et al. Sex and tumor-site differences in the association of alcohol intake with the risk of early-onset colorectal cancer. J Clin Oncol. 2023;41:3816–25.
Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44.
Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100:1105–12.
Liu Y, Lu L, Yang H, Wu X, Luo X, Shen J, et al. Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: a review. Environ Pollut. 2023;339:122730.
Johnson CH, Golla JP, Dioletis E, Singh S, Ishii M, Charkoftaki G, et al. Molecular mechanisms of alcohol-induced colorectal carcinogenesis. Cancers. 2021;13:4404.
Rossi M, Jahanzaib Anwar M, Usman A, Keshavarzian A, Bishehsari F. Colorectal cancer and alcohol consumption-populations to molecules. Cancers. 2018;10:38.
Bai X, Wei H, Liu W, Coker OO, Gou H, Liu C, et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71:2439–50.
Leite G, Barlow GM, Hosseini A, Parodi G, Pimentel ML, Wang J, et al. Smoking has disruptive effects on the small bowel luminal microbiome. Sci Rep. 2022;12:6231.
Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38:163–71.
Amitay EL, Niedermaier T, Alwers E, Chang-Claude J, Hoffmeister M, Brenner H. Reproductive factors and colorectal cancer risk: a population-based case-control study. JNCI Cancer Spectr. 2022;6:pkac042.
Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130:419–30.
Guan HB, Wu QJ, Gong TT, Lin B, Wang YL, Liu CX. Parity and risk of colorectal cancer: a dose-response meta-analysis of prospective studies. PLoS ONE. 2013;8:e75279.
Acknowledgements
The authors are grateful to all of the European Prospective Investigation into Cancer and Nutrition [EPIC], the Norwegian Women and Cancer Study [NOWAC] and the UK-Biobank participants and personnel involved in data curation and maintenances of the Consortia. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organisation, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policies, or views of the International Agency for Research on Cancer/World Health Organisation. This article is the result of the scientific work of NM while he was affiliated with IARC.
Funding
This work was supported by the Fonds Mondial de Recherche contre le Cancer the French affiliate of World Cancer Research Fund International [grant number IIG_- FULL_2021_026 to NM], the French National Cancer Institute [grant number INCa SHSESP22-015, No2022-132 to NM].
Author information
Authors and Affiliations
Contributions
Conceptualisation MJG, NM, KBB, and RSL; Data curation: NM, TBB, GS, and THN; Formal analysis: RSL; Funding acquisition: NM and MJG; Investigation: RSL, NM, and KBB; Methodology: RSL, NM, KBB, and PF; Project administration: MJG, KBB, NM; Resources: MJG, PB, AJC, MG, VP, KBB, AT, and RTF; Supervision: MJG, KBB; Visualisation: RSL; Writing—original draft: RSL, KBB, and NM,; Writing— review and editing: MJG, KBB, RSL, AJC, PTC, PB, VP, GS, TB, AT, RTF, and THN.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. NM is currently an employee of Merck & Co., Inc. This article is the result of the scientific work of NM while he was affiliated at the International Agency for Research on Cancer (IARC). This study was not funded by any commercial entity. All authors have reviewed and approved the final version of the manuscript before submission. We also confirm that the paper has not been published previously and is not being given consideration or simultaneously submitted elsewhere.
Consent for publication
This is an observational study involving de-identified secondary data analysis only. There was no direct interaction with human subjects for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication
This is an observational study involving de-identified secondary data analysis only. There was no direct interaction with human subjects for this study.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Laskar, R.S., Murphy, N., Ferrari, P. et al. A prospective investigation of early-onset colorectal cancer risk factors–pooled analysis of three large-scale European cohorts. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03303-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41416-025-03303-y