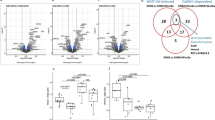
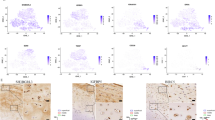
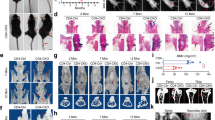
Abstract
Dysregulation of Wnt signaling has been implicated in developmental defects and in the pathogenesis of many diseases such as osteoarthritis; however, the underlying mechanisms are poorly understood. Here, we report that non-canonical Wnt signaling induced loss of chondrocyte phenotype through activation of Fz-6/DVL-2/SYND4/CaMKIIα/B-raf/ERK1/2 cascade. We show that in response to Wnt-3a, Frizzled 6 (Fz-6) triggers the docking of CaMKIIα to syndecan 4 (SYND4) and that of B-raf to DVL-2, leading to the phosphorylation of B-raf by CaMKIIα and activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling, which leads to chondrocyte de-differentiation. We demonstrate that CaMKIIα associates and phosphorylates B-raf in vitro and in vivo. Our study reveals the mechanism by which non-canonical Wnt activates ERK1/2 signaling that induces loss of chondrocyte phenotype, and demonstrates a direct functional relationship between CaMKIIα and B-raf during chondrocyte de-differentiation. The identification of Fz-6, SYND4, and B-raf as novel physiological regulators of chondrocyte phenotype may provide new potential anti-osteoarthritic targets.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
MacDonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131:1204–1204.
Semenov MV, Habas R, MacDonald BT, He X. SnapShot: non-canonical Wnt signaling pathways. Cell. 2007;131:1378.
MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–80.
Gao B. Wnt regulation of planar cell polarity (PCP). Curr Top Dev Biol. 2012;101:263–95.
Kühl M, Sheldahl LC, Malbon CC, Moon RT. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–11.
Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C, et al. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and non-canonical pathways. J Cell Biol. 2011;193:551–64.
Vermeulen L, Felipe De Sousa EM, Van Der Heijden M, Cameron K, De Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76.
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18.
Leonova E, Galzitskaya O. Structure and functions of syndecans in vertebrates. Biochemistry (Mosc). 2013;78:1071–85.
Elfenbein A, Simons M. Syndecan-4 signaling at a glance. J Cell Sci. 2013;126:3799–804.
Barre PE, Redini F, Boumediene K, Vielpeau C, Pujol JP. Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and-4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthr Cartil. 2000;8:34–43.
Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–6.
Nakamura Y, Nawata M, Wakitani S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol. 2005;167:97–105.
Yun MS, Kim S-E, Jeon SH, Lee JS, Choi K-Y. Both ERK and Wnt/β-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118:313–22.
Halleskog C, Schulte G. Pertussis toxin-sensitive heterotrimeric G αi/o proteins mediate Wnt/β-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell Signal. 2013;25:822–8.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38.
Lee YN, Gao Y, Wang HY. Differential mediation of the Wnt canonical pathway by mammalian dishevelleds-1, -2, and -3. Cell Signal. 2008;20:443–52.
Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259
Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18.
Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–36.
Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–94.
Horowitz A, Simons M. Phosphorylation of the cytoplasmic tail of syndecan-4 regulates activation of protein kinase Cα. J Biol Chem. 1998;273:25548–51.
White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem. 1998;273:3166–72.
Haag J, Gebhard PM, Aigner T. SOX gene expression in human osteoarthritic cartilage. Pathobiology. 2008;75:195–9.
Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216.
Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat Rev Rheumatol. 2015;11:606–15.
Ryu JH, Chun JS. Opposing roles of WNT-5A and WNT-11 in interleukin-1β regulation of type II collagen expression in articular chondrocytes. J Biol Chem. 2006;281:22039–47.
Lefebvre V, Li P, De Crombrugghe B. A new long form of Sox5 (L‐Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–33.
Taschner MJ, Rafigh M, Lampert F, Schnaiter S, Hartmann C. Ca2+/Calmodulin-dependent kinase II signaling causes skeletal overgrowth and premature chondrocyte maturation. Dev Biol. 2008;317:132–46.
Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt/β-catenin signaling cascade. J Biol Chem. 2004;279:14879–88.
Möller S, Vilo J, Croning MD. Prediction of the coupling specificity of G protein coupled receptors to their G proteins. Bioinformatics. 2001;17:S174–181.
Koval A, Katanaev VL. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem J. 2011;433:435–40.
Kilander MBC, Petersen J, Andressen KW, Ganji RS, Levy FO, Schuster J, et al. Disheveled regulates precoupling of heterotrimeric G proteins to Frizzled 6. FASEB J. 2014;28:2293–305.
Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94.
Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Jt Surg Am. 1971;53:523–37.
Acknowledgements
This work was supported by Agence Nationale de la Recherche (ANR: Solv-CDG) and by Région Lorraine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xie, Z., Khair, M., Shaukat, I. et al. Non-canonical Wnt induces chondrocyte de-differentiation through Frizzled 6 and DVL-2/B-raf/CaMKIIα/syndecan 4 axis. Cell Death Differ 25, 1442–1456 (2018). https://doi.org/10.1038/s41418-017-0050-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-017-0050-y