Abstract
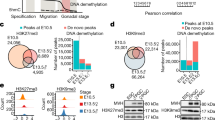
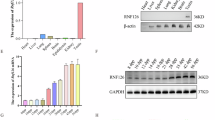
Lethal (3) malignant brain tumor like 2 (L3MBTL2) is a member of the MBT-domain proteins, which are involved in transcriptional repression and implicated in chromatin compaction. Our previous study has shown that L3MBTL2 is highly expressed in the testis, but its role in spermatogenesis remains unclear. In the present study, we found that L3MBTL2 was most highly expressed in pachytene spermatocytes within the testis. Germ cell-specific ablation of L3mbtl2 in the testis led to increased abnormal spermatozoa, progressive decrease of sperm counts and premature testicular failure in mice. RNA-sequencing analysis on L3mbtl2 deficient testes confirmed that L3MBTL2 was a transcriptional repressor but failed to reveal any significant changes in spermatogenesis-associated genes. Interestingly, L3mbtl2 deficiency resulted in increased γH2AX deposition in the leptotene spermatocytes, subsequent inappropriate retention of γH2AX on autosomes, and defective crossing-over and synapsis during the pachytene stage of meiosis I, and more germ cell apoptosis and degeneration in aging mice. L3MBTL2 interacted with the histone ubiquitin ligase RNF8. Inhibition of L3MBTL2 reduced nuclear RNF8 and ubH2A levels in GC2 cells. L3mbtl2 deficiency led to decreases in the levels of the RNF8 and ubH2A pathway and in histone acetylation in elongating spermatids, and in protamine 1 deposition and chromatin condensation in sperm. These results suggest that L3MBTL2 plays important roles in chromatin remodeling during meiosis and spermiogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15.
Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–6.
Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, et al. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–42.
Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet. 2005;37:41–7.
Garcia-Cruz R, Roig I, Caldes MG. Maternal origin of the human aneuploidies. Are homolog synapsis and recombination to blame? Notes (learned) from the underbelly. Genome Dyn. 2009;5:128–36.
Blanco-Rodriguez J. Programmed phosphorylation of histone H2AX precedes a phase of DNA double-strand break-independent synapsis in mouse meiosis. Reproduction. 2012;144:699–712.
Balhorn R. A model for the structure of chromatin in mammalian sperm. J Cell Biol. 1982;93:298–305.
Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, et al. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol. 1999;207:322–33.
Hazzouri M, Pivot-Pajot C, Faure AK, Usson Y, Pelletier R, Sele B, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur J Cell Biol. 2000;79:950–60.
Godmann M, Auger V, Ferraroni-Aguiar V, Di Sauro A, Sette C, Behr R, et al. Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol Reprod. 2007;77:754–64.
Moriniere J, Rousseaux S, Steuerwald U, Soler-Lopez M, Curtet S, Vitte AL, et al. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature. 2009;461:664–8.
Montellier E, Boussouar F, Rousseaux S, Zhang K, Buchou T, Fenaille F, et al. Chromatin-to-nucleoprotamine transition is controlled by the histone H2B variant TH2B. Genes Dev. 2013;27:1680–92.
Lu LY, Wu J, Ye L, Gavrilina GB, Saunders TL, Yu X. RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev Cell. 2010;18:371–84.
Gou LT, Kang JY, Dai P, Wang X, Li F, Zhao S, et al. Ubiquitination-deficient mutations in human piwi cause male infertility by impairing histone-to-protamine exchange during spermiogenesis. Cell. 2017;169:1090–104 e1013.
Bonasio R, Lecona E, Reinberg D. MBT domain proteins in development and disease. Semin Cell Dev Biol. 2010;21:221–30.
Qin J, Whyte WA, Anderssen E, Apostolou E, Chen HH, Akbarian S, et al. The polycomb group protein L3mbtl2 assembles an atypical PRC1-family complex that is essential in pluripotent stem cells and early development. Cell Stem Cell. 2012;11:319–32.
Wismar J, Loffler T, Habtemichael N, Vef O, Geissen M, Zirwes R, et al. The Drosophila melanogaster tumor suppressor gene lethal(3)malignant brain tumor encodes a proline-rich protein with a novel zinc finger. Mech Dev. 1995;53:141–54.
Wismar J. Molecular characterization of h-l(3)mbt-like: a new member of the human mbt family. FEBS Lett. 2001;507:119–21.
Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–6.
Trojer P, Li G, Sims RJ 3rd, Vaquero A, Kalakonda N, Boccuni P, et al. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell. 2007;129:915–28.
Frankenberg S, Smith L, Greenfield A, Zernicka-Goetz M. Novel gene expression patterns along the proximo-distal axis of the mouse embryo before gastrulation. BMC Dev Biol. 2007;7:8.
Trojer P, Cao AR, Gao Z, Li Y, Zhang J, Xu X, et al. L3MBTL2 protein acts in concert with PcG protein-mediated monoubiquitination of H2A to establish a repressive chromatin structure. Mol Cell. 2011;42:438–50.
Bergink S, Salomons FA, Hoogstraten D, Groothuis TA, de Waard H, Wu J, et al. DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A. Genes Dev. 2006;20:1343–52.
Chou DM, Adamson B, Dephoure NE, Tan X, Nottke AC, Hurov KE, et al. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci USA. 2010;107:18475–80.
Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30:10096–111.
Ismail IH, Andrin C, McDonald D, Hendzel MJ. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J Cell Biol. 2010;191:45–60.
Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M, et al. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol Cell Biol. 2011;31:1972–82.
Leung JW, Agarwal P, Canny MD, Gong F, Robison AD, Finkelstein IJ, et al. Nucleosome acidic patch promotes RNF168- and RING1B/BMI1-dependent H2AX and H2A ubiquitination and DNA damage signaling. PLoS Genet. 2014;10:e1004178.
Huang H, Xu C, Wang Y, Meng C, Liu W, Zhao Y, et al. Lethal (3) malignant brain tumor-like 2 (L3MBTL2) protein protects against kidney injury by inhibiting the DNA damage-p53-apoptosis pathway in renal tubular cells. Kidney Int. 2018;93:855–70.
Takada Y, Isono K, Shinga J, Turner JM, Kitamura H, Ohara O, et al. Mammalian Polycomb Scmh1 mediates exclusion of Polycomb complexes from the XY body in the pachytene spermatocytes. Development. 2007;134:579–90.
Hasegawa K, Sin HS, Maezawa S, Broering TJ, Kartashov AV, Alavattam KG, et al. SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev Cell. 2015;32:574–88.
Luo M, Zhou J, Leu NA, Abreu CM, Wang J, Anguera MC, et al. Polycomb protein SCML2 associates with USP7 and counteracts histone H2A ubiquitination in the XY chromatin during male meiosis. PLoS Genet. 2015;11:e1004954.
Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–36.
Lu LY, Xiong Y, Kuang H, Korakavi G, Yu X. Regulation of the DNA damage response on male meiotic sex chromosomes. Nat Commun. 2013;4:2105.
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705.
Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci. 2002;115(Pt 8):1611–22.
Adams SR, Maezawa S, Alavattam KG, Abe H, Sakashita A, Shroder M, et al. RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genet. 2018;14:e1007233.
Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900.
Nowsheen S, Aziz K, Aziz A, Deng M, Qin B, Luo K, et al. L3MBTL2 orchestrates ubiquitin signalling by dictating the sequential recruitment of RNF8 and RNF168 after DNA damage. Nat Cell Biol. 2018;20:455–64.
Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10:207–16.
Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8.
Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46:738–42.
Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–8.
Acknowledgements
We are grateful to Dr. P. Jeremy Wang (University of Pennsylvania) for helpful suggestion and critical reading and editing of the manuscript. We thank Josie Lai and Jean Kung (The Chinese University of Hong Kong) for technical assistance with the electronic microscopy. We also thank Dr. Wenhui Zhao (Peking University) for the CMV-HA-RNF8 plasmid, and Dr. Winnie Shum (ShanghaiTech University) for helpful discussions. Mr. Geng Tian (Shanghai Jiao Tong University) helped with the bioinformatics analysis. This work was supported by Health and Medical Research Fund (HMRF) grants 04153586 and 05161376 (to Y. X.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by V D'Angiolella
Supplementary information
Rights and permissions
About this article
Cite this article
Meng, C., Liao, J., Zhao, D. et al. L3MBTL2 regulates chromatin remodeling during spermatogenesis. Cell Death Differ 26, 2194–2207 (2019). https://doi.org/10.1038/s41418-019-0283-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0283-z
This article is cited by
-
Elucidating the susceptibility genes between insomnia and migraine by integrating genetic data and transcriptomes
The Journal of Headache and Pain (2026)
-
Dynamic architecture of mammalian paternal chromatin: histone-to-protamine exchange and post-fertilization reprogramming
Epigenetics & Chromatin (2025)
-
Evaluation of circANKLE2 & circL3MBTL4 -RNAs Expression in Fertile and Infertile Men
Biochemical Genetics (2025)
-
Male germ cells with Bag5 deficiency show reduced spermiogenesis and exchange of basic nuclear proteins
Cellular and Molecular Life Sciences (2025)