Abstract
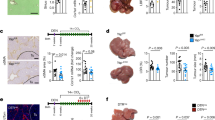
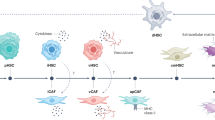
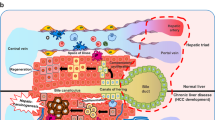
Hepatocellular carcinoma (HCC) generally occurs in the presence of chronic liver injury, often as a sequela of liver fibrosis. Hepatic progenitor cells (HPCs) are known to be capable of forming both hepatocytes and cholangiocytes in chronic liver injury, which are also considered a source of myofibroblasts and tumor-initiating cells, under carcinogenic circumstances. However, the underlying mechanisms that activate HPCs to give rise to HCC are still unclear. In current study, the correlation between HPCs activation and liver fibrosis and carcinogenesis was investigated in rats and human specimens. We analyzed the role of HPCs in tumorigenesis, by transplanting exogenous HPCs in a diethylnitrosamine-induced rat HCC model. Our data indicated that HPC activation correlated with hepatic fibrosis and hepatocarcinogenesis. We further found that exogenous HPC infusion promoted liver fibrosis and hepatocarcinogenesis, while lipopolysaccharides (LPS) played an important role in this process. However, results of our study indicated that LPS did not induce HPCs to form tumor in nude mice directly. Rather, LPS induced myofibroblast-like morphology in HPCs, which enhanced the tumorigenic potential of HPCs. Further experiments showed that LPS/Toll-like receptor 4 (TLR4) signaling mediated the differentiation of HPCs into myofibroblasts and enhanced the production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which led to the aberrant expression of Ras and p53 signaling pathways in HPCs, and finally, promoted the proliferation and malignant transformation of HPCs, by long non-coding RNA regulation. Besides, examination of HCC clinical samples demonstrated that IL-6 and TNF-α production correlated with HPC activation, hepatic fibrosis, and HCC recurrence. Our study indicates that both myofibroblasts and tumor cells are derived from HPCs. HPC-derived myofibroblasts create tumor microenvironment and contribute to the proliferation and malignant transformation of HPCs. Furthermore, LPS present in the chronic liver inflammation microenvironment might play an important role in hepatocarcinogenesis, by regulating the plastic potential of HPCs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127.
Weber A, Boege Y, Reisinger F, Heikenwalder M. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly. 2011;141:w13197.
Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–75.
Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–79.
Turner R, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–45.
Weng HL, et al. IFN-gamma inhibits liver progenitor cell proliferation in HBV-infected patients and in 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-fed mice. J Hepatol. 2013;59:738–45.
Strain AJ, Crosby HA. Hepatic stem cells. Gut. 2000;46:743–45.
Clouston AD, et al. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–18.
Lukacs-Kornek V, Lammert F. The progenitor cell dilemma: cellular and functional heterogeneity in assistance or escalation of liver injury. J Hepatol. 2017;66:619–30.
Chobert MN, et al. Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats. Lab Invest. 2012;92:135–50.
Sekiya S, Miura S, Matsuda-Ito K, Suzuki A. Myofibroblasts derived from hepatic progenitor cells create the tumor microenvironment. Stem Cell Rep. 2016;7:1130–39.
Pan XR, et al. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts via activation of the Hedgehog signaling pathway. Cell Cycle. 2017;16:1357–65.
Arimilli S, Johnson JB, Alexander-Miller MA, Parks GD. TLR-4 and -6 agonists reverse apoptosis and promote maturation of simian virus 5-infected human dendritic cells through NFkB-dependent pathways. Virology. 2007;365:144–56.
Ha T, et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–53.
Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol. 2013;58:385–7.
Dapito DH, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16.
Cirera I, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–7.
Li XY, et al. Lipopolysaccharide promotes tumorigenicity of hepatic progenitor cells by promoting proliferation and blocking normal differentiation. Cancer Lett. 2017;386:35–46.
Libbrecht L, et al. Hepatic progenitor cells in hepatocellular adenomas. Am J Surg Pathol. 2001;25:1388–96.
Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin N Am. 2007;36:831–49.
Li W, et al. STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma. J Clin Invest. 2015;125:4239–54.
Libbrecht L, Desmet V, Van Damme B, Roskams T. Deep intralobular extension of human hepatic ‘progenitor cells’ correlates with parenchymal inflammation in chronic viral hepatitis: can ‘progenitor cells’ migrate? J Pathol. 2000;192:373–8.
Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–41.
Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26:1225–33.
Falkowski O, et al. Regeneration of hepatocyte ‘buds’ in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–64.
Harmey JH, et al. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int J cancer. 2002;101:415–22.
Hamesch K, Borkham-Kamphorst E, Strnad P, Weiskirchen R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 2015;49(1 Suppl.):37–46.
Jing YY, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98.
Lai FB, et al. Lipopolysaccharide supports maintaining the stemness of CD133(+) hepatoma cells through activation of the NF-kappaB/HIF-1alpha pathway. Cancer Lett. 2016;378:131–41.
He G, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–96.
Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208.
Yang L, et al. Transforming growth factor-beta signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology. 2013;144:1042–1054 e1044.
Ji J, et al. MicroRNA expression, survival, and response to interferon in liver cancer. New Engl J Med. 2009;361:1437–47.
Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4–12.
Zhen L, et al. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int J Clin Exp Pathol. 2014;7:6438–46.
Vinciguerra M, et al. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50:1132–41.
Wang Y, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–25.
Yuan JH, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81.
Uthaya Kumar DB, et al. TLR4 signaling via NANOG cooperates with STAT3 to activate Twist1 and promote formation of tumor-initiating stem-like cells in livers of mice. Gastroenterology. 2016;150:707–19.
Zhu XD, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–28.
Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7:986–95.
Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
Acknowledgements
This project was supported by the National Key R&D Program of China (Grant No. 2018YFA0107502); National Natural Science Foundation of China (Grant Nos. 81772940, 81702320, 81630070, 81673641, 81572444, 31700788, 81872243, 81802737); Special Funds for National Key Sci-Tech Special Project of China (Grant No. 2018ZX10723204-005-004); and Shanghai Science and Technology Committee (Grant Nos. 16ZR1400200, 16JC1405200, 16YF1415000); Science Fund for Creative Research Groups, NSFC, China (Grant No. 81521091).
Author contributions
L-xW and Z-pH were responsible for the overall concept, design, and supervision of the study. W-tL, Y-yJ, and LG performed the experiments and data analysis, as well as manuscript writing. RL, XY, Q-dZ, and YY performed the experiments. X-rP, X-jH, and YM analyzed the data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M Piacentini
Supplementary information
41418_2019_340_MOESM5_ESM.tif
LPS/TLR4 signaling pathway contributed to IL-6 and TNF-α production in hepatic progenitor cell-derived myofibroblasts, and malignant transformation of hepatic progenitor cells
Rights and permissions
About this article
Cite this article
Liu, Wt., Jing, Yy., Gao, L. et al. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts constitutes the hepatocarcinogenesis-associated microenvironment. Cell Death Differ 27, 85–101 (2020). https://doi.org/10.1038/s41418-019-0340-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0340-7
This article is cited by
-
Mechanisms, efficacy, and future perspectives of cellular-based therapies for liver fibrosis/cirrhosis: focusing on mesenchymal stromal cells
Cell & Bioscience (2025)
-
Gut microbial metabolites in cancer immunomodulation
Molecular Cancer (2025)
-
Gut microbiome in metabolic dysfunction-associated steatotic liver disease and associated hepatocellular carcinoma
Nature Reviews Gastroenterology & Hepatology (2025)
-
Roles of the gut microbiota in hepatocellular carcinoma: from the gut dysbiosis to the intratumoral microbiota
Cell Death Discovery (2025)
-
ERG mediates the differentiation of hepatic progenitor cells towards immunosuppressive PDGFRα+ cancer-associated fibroblasts during hepatocarcinogenesis
Cell Death & Disease (2025)