Abstract
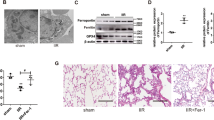
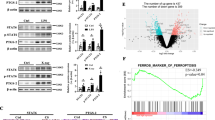
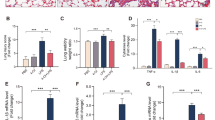
Acute lung injury (ALI) is a life-threatening disorder with high rates of morbidity and mortality. Reactive oxygen species and epithelial apoptosis are involved in the pathogenesis of acute lung injury. Ferroptosis, an iron-dependent non-apoptotic form of cell death, mediates its effects in part by promoting the accumulation of reactive oxygen species. The inhibition of ferroptosis decreases clinical symptoms in experimental models of ischemia/reperfusion-induced renal failure and heart injury. This study investigated the roles of inhibitor of apoptosis-stimulating protein of p53 (iASPP) and Nrf2 in ferroptosis and their potential therapeutic effects in intestinal ischemia/reperfusion-induced acute lung injury. Intestinal ischemia/reperfusion-induced ALI was induced in wild-type and Nrf2−/− mice. The mice were treated with erastin followed by liproxstatin-1. Ferroptosis-related factors in mice with ischemia/reperfusion-induced acute lung injury or in mouse lung epithelial-2 cells with hypoxia/regeneration (HR)-induced ALI were measured by western blotting, real-time PCR, and immunofluorescence. Ferroptosis contributed to intestinal ischemia/reperfusion-induced ALI in vivo. iASPP inhibited ferroptosis and alleviated intestinal ischemia/reperfusion-induced acute lung injury, and iASPP-mediated protection against ischemia/reperfusion-induced ALI was dependent on Nrf2 signaling. HR-induced acute lung injury enhanced ferroptosis in vitro in mouse lung epithelial-2 cells, and ferroptosis was modulated after the enhancement of intestinal ischemia/reperfusion in Nrf2−/− mice. iASPP mediated its protective effects against acute lung injury through the Nrf2/HIF-1/TF signaling pathway. Ferroptosis contributes to intestinal ischemia/reperfusion-induced ALI, and iASPP treatment inhibits ferroptosis in part via Nrf2. These findings indicate the therapeutic potential of iASPP for treating ischemia/reperfusion-induced ALI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ng CSH, Wan S, Arifi AA, Yim APC. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36:205–14.
De-Perrot M, Liu M, Waddell T,S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511.
Axelle C, Kai K, Gilliss BM, Nguyen JX, Marques MB, Marc M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Investig. 2012;122:2661.
Ortiz MG, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124:2625–34.
Rob Mac S, Mark G, Danny MA. Treatment of acute lung injury: current and emerging pharmacological therapies. Semin Respiratory Crit Care Med. 2013;34:487–98.
Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–52.
Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Investig. 2012;122:2731–40.
Marco M, Andrade CF, Bing H, Rashmi S, Yu Z, Xiao HB, et al. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28:227–38.
Feng D, Yao J, Wang G, Li Z, Zu G, Li Y, et al. Inhibition of p66Shc-mediated mitochondrial apoptosis via targeting prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion injury in rats. Clin Sci. 2017;131:759–73.
Lagan AL, Melley DD, Evans TW, Quinlan GJ. Pathogenesis of the systemic inflammatory syndrome and acute lung injury: role of iron mobilization and decompartmentalization. Am J Physiol Lung Cell Mol Physiol. 2008;294:L161.
Dixon S, Lemberg K, Lamprecht M, Skouta R, Zaitsev E, Gleason C, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Andreas L, Rachid S, Nina H, Mulay SR, Christin D, Federica DZ, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111:16836–41.
Jose Pedro FA, Manuela S, Bettina P, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Free Radic Biol Med. 2014;76:1180–91.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol cell. 2015;59:298–308.
Le J, Ning K, Tongyuan L, Shang-Jui W, Tao S, Hanina H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62.
Bruni A, Pepper AR, Pawlick RL, Galalopez B, Gamble AF, Kin T, et al. Ferroptosis-inducing agents compromise in vitro human islet viability and function. Cell Death Dis. 2018;9:595.
Sun X, Ou Z, Chen R, Niu X, Chen, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells.Hepatology. 2016;63:173–84.
MRDL Vega, Dodson M, Gross C, Mansour HM, Lantz RC, Chapman E, et al. Role of Nrf2 and autophagy in acute lung injury. Curr Pharmacol Rep. 2016;2:91–101.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523.
Nicholas Y, Moritz VR, Elma Z, Bauer AJ, Seok YW, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat cell Biol. 2014;16:1180–91.
Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3:232–43.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Rachid S, Dixon SJ, Jianlin W, Dunn DE, Marina O, Kenichi S, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–6.
Mai M, Stefan F, Christoph S, Marcus C, Bornkamm GW, Manfred K. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–68.
Hou B, Zhao Y, Qiang G, Yang X, Xu C, Chen X, et al. Puerarin mitigates diabetic hepatic steatosis and fibrosis by inhibiting TGF-β signaling pathway activation in type 2 diabetic rats. Oxid Med Cell Longev. 2018;2018:1–13.
Yi H, Huang Y, Yang F, Liu W, He S, Hu X. MicroRNA-182 aggravates cerebral ischemia injury by targeting inhibitory member of the ASPP family (iASPP). Arch Biochem Biophysics. 2016;620:S0003986116301412.
Ge W, Zhao K, Wang X, Li H, Yu M, He M, et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 Binding. Cancer Cell. 2017;32:561–73.e566.
Silva-Gomes S, Santos AG, Caldas C, Silva CM, Neves JV, Lopes J, et al. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J Hepatol. 2014;60:354–61.
Bibo K, Xiu-Da S, Yu Z, Haofeng J, Feng G, Shi Y, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59:1200–7.
Zhang LM, Zhang J, Zhang Y, Wang L, Dong L. Interleukin-18 binding protein attenuates lipopolysaccharide-induced acute lung injury in mice via suppression NF-κB and activation Nrf2 pathway. Biochem Biophys Res Commun. 2018;505:837–42.
Liu S, Chen Q, Liu J, Yang X, Zhang Y, Huang F. Sinomenine protects against E.coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed Pharmacother. 2018;107:696–702.
Yang WS, Sriramaratnam R, Welsch M, Shimada K, Skouta R, Viswanathan V, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.
Boshuizen M, Van dPK, Von BL, Biemond BJ, Zeerleder SS, Van BR, et al. Therapeutic use of transferrin to modulate anemia and conditions of iron toxicity. Blood Rev. 2017;31:S0268960X17300413.
Shi W, Feng B, Xu S, Shen X, Zhang T. Inhibitory effect of compound Chuanxiong Kangxian granules on bleomycin-induced pulmonary fibrosis in rats. Biomed Pharmacother. 2017;96:1179.
Kang R, Tang D. Autophagy and ferroptosis—what’s the connection? Curr Pathobiol Rep. 2017;5:153.
Acknowledgements
We thank J. Ludovic Croxford, PhD, of Edanz Medical Writing for providing editorial support.
Funding
This study was supported by the Natural Science Foundation of Shanghai 18ZR1428800 and 81572626.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M. Piacentini
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Cao, Y., Xiao, J. et al. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ 27, 2635–2650 (2020). https://doi.org/10.1038/s41418-020-0528-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-020-0528-x
This article is cited by
-
SLC38A1 Inhibits Ferroptosis of Alveolar Type II Epithelial Cells in Acute Lung Injury by Promoting Autophagic Degradation of Divalent Metal Transporter 1 (DMT1): an In Vivo and In Vitro Study
Inflammation (2026)
-
Nrf2/UBE3B protects against acute lung injury by inhibiting ferritinophagy through the ubiquitination of NCOA4
Biology Direct (2025)
-
Emodin protects against severe acute pancreatitis-associated acute lung injury by activating Nrf2/HO-1/GPX4 signal and inhibiting ferroptosis in vivo and in vitro
BMC Gastroenterology (2025)
-
O-GlcNAcylation attenuates ischemia-reperfusion-induced pulmonary epithelial cell ferroptosis via the Nrf2/G6PDH pathway
BMC Biology (2025)
-
Bridging iron and lung injury: the emerging role of ferroptosis in ARDS
European Journal of Medical Research (2025)