Abstract
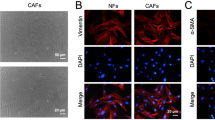
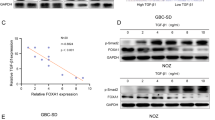
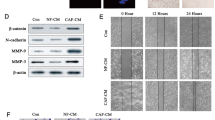
The mesenchymal transcription factor forkhead box F2 (FOXF2) is a critical regulator of embryogenesis and tissue homeostasis. Our previous studies demonstrated that FOXF2 is ectopically expressed in basal-like breast cancer (BLBC) cells and that FOXF2 deficiency promotes the epithelial–mesenchymal transition and aggressiveness of BLBC cells. In this study, we found that FOXF2 controls transforming growth factor-beta (TGF-β)/SMAD signaling pathway activation through transrepression of TGF-β-coding genes in BLBC cells. FOXF2-deficient BLBC cells adopt a myofibroblast-/cancer-associated fibroblast (CAF)-like phenotype, and tend to metastasize to visceral organs by increasing autocrine TGF-β signaling and conferring aggressiveness to neighboring cells by increasing paracrine TGF-β signaling. In turn, TGF-β silences FOXF2 expression through upregulating miR-182-5p, a posttranscriptional regulator of FOXF2 and inducer of metastasis. In addition to mediating a reciprocal repression loop between FOXF2 and TGF-β through direct transrepression by SMAD3, miR-182-5p forms a reciprocal repression loop with FOXF2 that directly transrepresses MIR182 expression. Therefore, FOXF2 deficiency accelerates the visceral metastasis of BLBC through unrestricted increases in autocrine and paracrine TGF-β signaling, and miR-182-5p expression. Our findings provide novel mechanisms underlying the roles of TGF-β, miR-182-5p, and FOXF2 in accelerating BLBC dissemination and metastasis, and may facilitate the development of therapeutic strategies for aggressive BLBC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65.
Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–81.
Bartmann C, Wischnewsky M, Stuber T, Stein R, Krockenberger M, Hausler S, et al. Pattern of metastatic spread and subcategories of breast cancer. Arch Gynecol Obstet. 2017;295:211–23.
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54.
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S. et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468.
Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–75.
Ding Q, Subramanian I, Luckhardt TR, Che P, Waghray M, Zhao XK, et al. Focal adhesion kinase signaling determines the fate of lung epithelial cells in response to TGF-beta. Am J Physiol Lung Cell Mol Physiol. 2017;312:L926–L935.
O’Connor JW, Riley PN, Nalluri SM, Ashar PK, Gomez EW. Matrix rigidity mediates TGFbeta1-induced epithelial-myofibroblast transition by controlling cytoskeletal organization and MRTF-A localization. J Cell Physiol. 2015;230:1829–39.
O’Connor JW, Mistry K, Detweiler D, Wang C, Gomez EW. Cell-cell contact and matrix adhesion promote alphaSMA expression during TGFbeta1-induced epithelial-myofibroblast transition via Notch and MRTF-A. Sci Rep. 2016;6:26226.
Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I. et al.TGF-beta regulates isoform switching of FGF receptors and epithelial-mesenchymal transition.EMBO J. 2011;30:783–95.
Aitola M, Carlsson P, Mahlapuu M, Enerback S, Pelto-Huikko M. Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal interactions. Dev Dynam. 2000;218:136–49.
Tian HP, Lun SM, Huang HJ, He R, Kong PZ, Wang QS, et al. DNA methylation affects the SP1-regulated transcription of FOXF2 in breast cancer cells. J Biol Chem. 2015;290:19173–83.
Wang QS, Kong PZ, Li XQ, Yang F, Feng YM. FOXF2 deficiency promotes epithelial-mesenchymal transition and metastasis of basal-like breast cancer. Breast Cancer Res. 2015;17:30.
Cai J, Tian AX, Wang QS, Kong PZ, Du X, Li XQ, et al. FOXF2 suppresses the FOXC2-mediated epithelial-mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett. 2015;367:129–37.
Kang LJ, Yu ZH, Cai J, He R, Lu JT, Hou C, et al. Reciprocal transrepression between FOXF2 and FOXQ1 controls basal-like breast cancer aggressiveness. FASEB J. 2019;33:6564–73.
Kong PZ, Yang F, Li L, Li XQ, Feng YM. Decreased FOXF2 mRNA expression indicates early-onset metastasis and poor prognosis for breast cancer patients with histological grade II tumor. Plos ONE. 2013;8:e61591.
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110:724–32.
Joseph JV, Conroy S, Tomar T, Eggens-Meijer E, Bhat K, Copray S, et al. TGF-beta is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 2014;5:e1443.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707.
Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–9.
Sachdeva M, Mito JK, Lee CL, Zhang M, Li Z, Dodd RD, et al. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J Clin Investig. 2014;124:4305–19.
Lei R, Tang J, Zhuang X, Deng R, Li G, Yu J, et al. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene. 2014;33:1287–96.
Yu J, Lei R, Zhuang X, Li X, Li G, Lev S, et al. MicroRNA-182 targets SMAD7 to potentiate TGFbeta-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat Commun. 2016;7:13884.
Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin cell Biol. 2016;43:7–13.
Bolte C, Ren XM, Tomley T, Ustiyan V, Pradhan A, Hoggatt A, et al. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290:7563–75.
Seoane J, Gomis RR. TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9:a022277.
Yu ZH, Lun SM, He R, Tian HP, Huang HJ, Wang QS, et al. Dual function of MAZ mediated by FOXF2 in basal-like breast cancer: Promotion of proliferation and suppression of progression. Cancer Lett. 2017;402:142–52.
Meyer-Schaller N, Heck C, Tiede S, Yilmaz M, Christofori G. Foxf2 plays a dual role during transforming growth factor beta-induced epithelial to mesenchymal transition by promoting apoptosis yet enabling cell junction dissolution and migration. Breast Cancer Res. 2018;20:118.
Evans R, Flores-Borja F, Nassiri S, Miranda E, Lawler K, Grigoriadis A, et al. Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Rep. 2019;27:1967–78 e1964.
Li L, Yang L, Wang L, Wang F, Zhang Z, Li J, et al. Impaired T cell function in malignant pleural effusion is caused by TGF-beta derived predominantly from macrophages. Int J Cancer. 2016;139:2261–9.
Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–807.
Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158:564–78.
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, et al. TGF-beta induces miR-182 to sustain NF-kappaB activation in glioma subsets. J Clin Investig. 2012;122:3563–78.
Qiu Y, Luo X, Kan T, Zhang Y, Yu W, Wei Y, et al. TGF-beta upregulates miR-182 expression to promote gallbladder cancer metastasis by targeting CADM1. Mol Biosyst. 2014;10:679–85.
Zhang X, Ma G, Liu J, Zhang Y. MicroRNA-182 promotes proliferation and metastasis by targeting FOXF2 in triple-negative breast cancer. Oncol Lett. 2017;14:4805–11.
Yu J, Shen W, Gao B, Zhao H, Xu J, Gong B. MicroRNA-182 targets FOXF2 to promote the development of triple-negative breast cancer. Neoplasma. 2017;64:209–15.
Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, et al. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. Plos ONE. 2013;8:e55502.
Kundu ST, Byers LA, Peng DH, Roybal JD, Diao L, Wang J, et al. The miR-200 family and the miR-183~96~182 cluster target Foxf2 to inhibit invasion and metastasis in lung cancers. Oncogene. 2016;35:173–86.
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17:1101–U1108.
Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PloS ONE. 2009;4:e7181.
Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Investig. 2014;124:5109–28.
Wang S, Li GX, Tan CC, He R, Kang LJ, Lu JT, et al. FOXF2 reprograms breast cancer cells into bone metastasis seeds. Nat Commun. 2019;10:2707.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 81272357, 81472680, 81672894, and 81872403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by J. P. Medema
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, JT., Tan, CC., Wu, XR. et al. FOXF2 deficiency accelerates the visceral metastasis of basal-like breast cancer by unrestrictedly increasing TGF-β and miR-182-5p. Cell Death Differ 27, 2973–2987 (2020). https://doi.org/10.1038/s41418-020-0555-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-020-0555-7
This article is cited by
-
TGF-β in tumor development and progression: mechanisms and therapeutics
Molecular Biomedicine (2026)
-
Emerging biologic and clinical implications of miR-182-5p in gynecologic cancers
Clinical and Translational Oncology (2024)
-
Role of the Forkhead box family protein FOXF2 in the progression of solid tumor: systematic review
Journal of Cancer Research and Clinical Oncology (2024)
-
Reciprocal regulation of LINC00941 and SOX2 promotes progression of esophageal squamous cell carcinoma
Cell Death & Disease (2023)
-
Identifying miRNA biomarkers for breast cancer and ovarian cancer: a text mining perspective
Breast Cancer Research and Treatment (2023)