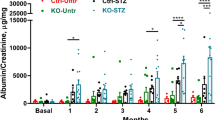
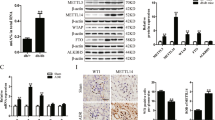
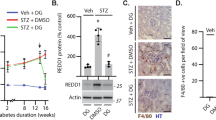
Abstract
Impaired glucose uptake regulated by suppressed insulin receptor signaling is a key driving force of podocytopathies. The identification of potential therapeutic targets that mediate podocyte insulin receptor signaling holds significant clinical importance. Here, we observed a substantial reduction in PR domain-containing 16 (PRDM16) expression within damaged podocytes in both humans and mice. Podocyte-specific Prdm16 deletion aggravated podocyte injury, albuminuria, and glomerulosclerosis in diabetic nephropathy (DN) mice. Conversely, exogenous PRDM16 delivered by lentivirus mitigated these pathological changes in DN mice and adriamycin (ADR) nephropathy mice. Furthermore, we demonstrated that loss of PRDM16 blocked glucose uptake of podocytes by inhibiting insulin receptor signaling. Mechanistically, PRDM16 deficiency downregulated the transcription of NEDD4L, subsequently enhancing the stability of IKKβ protein. The accumulation of IKKβ caused by the loss of PRDM16 led to the phosphorylation of serine residues on insulin receptor substrate-1 (IRS-1), thereby promoting IRS-1 degradation. Exogenous NEDD4L mitigated podocyte injury induced by PRDM16 knockdown in vitro and attenuated ADR nephropathy in vivo. Our study clarified the role and mechanism of PRDM16 in insulin receptor signaling and podocyte injury, providing a potential therapeutic target for podocytopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Materials availability
The materials used in this study should be directed to and will be fulfilled by the Lead Contac, Doctor Chun Zhang: drzhangchun@hust.edu.cn. The accession number for the RNA sequencing data reported in this paper is GEO: GSE252685. The link is as follows: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSE252685, password: mjcdmioohforvof.
References
Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Podocytopathies. Nat Rev Dis Primers. 2020;61:68.
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Investig. 2014;1246:2333–40.
Chen Z, Zhu Z, Liang W, Luo Z, Hu J, Feng J, et al. Reduction of anaerobic glycolysis contributes to angiotensin II-induced podocyte injury with foot process effacement. Kidney Int. 2023;1034:735–48.
Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;236:753–62.
Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;191:31–44.
Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;124:329–40.
Mohandes S, Doke T, Hu H, Mukhi D, Dhillon P, Susztak K. Molecular pathways that drive diabetic kidney disease. J Clin Investig. 2023;1334:e165654.
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;1212:721–37.
Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun. 2019;101:2692.
Ishibashi J, Seale P. Functions of Prdm16 in thermogenic fat cells. Temperature. 2015;21:65–72.
Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, et al. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 2018;271:180–194.e186.
Wang W, Ishibashi J, Trefely S, Shao M, Cowan AJ, Sakers A, et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 2019;301:174–189.e175.
Stine RR, Sakers AP, TeSlaa T, Kissig M, Stine ZE, Kwon CW, et al. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell. 2019;256:830–845.e838.
Wang Q, Li H, Tajima K, Verkerke ARP, Taxin ZH, Hou Z, et al. Post-translational control of beige fat biogenesis by PRDM16 stabilization. Nature. 2022;6097925:151–8.
Rogacka D. Insulin resistance in glomerular podocytes: potential mechanisms of induction. Arch Biochem Biophys. 2021;710:109005.
Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;5411:3095–102.
Sun H, Li H, Yan J, Wang X, Xu M, Wang M, et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat Commun. 2022;131:1600.
Lu J, Chen PP, Zhang JX, Li XQ, Wang GH, Yuan BY, et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics. 2021;1110:4728–42.
Kim SJ, DeStefano MA, Oh WJ, Wu CC, Vega-Cotto NM, Finlan M, et al. mTOR complex 2 regulates proper turnover of insulin receptor substrate-1 via the ubiquitin ligase subunit Fbw8. Mol Cell. 2012;486:875–87.
Denhez B, Rousseau M, Spino C, Dancosst DA, Dumas M, Guay A, et al. Saturated fatty acids induce insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1. Sci Rep. 2020;101:21628.
Schmitz-Peiffer C, Whitehead JP. IRS-1 regulation in health and disease. IUBMB Life. 2003;557:367–74.
Xu F, Jiang H, Li X, Pan J, Li H, Wang L, et al. Discovery of PRDM16-Mediated TRPA1 induction as the mechanism for low tubulo-interstitial fibrosis in diabetic kidney disease. Adv Sci. 2024;11:2306704.
Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;896:1221–30.
Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;1561-2:304–16.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Investig. 2011;1211:96–105.
Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;5047478:163–7.
Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;7312:1385–93.
Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;167:1936–47.
He M, Li Y, Wang L, Guo B, Mei W, Zhu B, et al. MYDGF attenuates podocyte injury and proteinuria by activating Akt/BAD signal pathway in mice with diabetic kidney disease. Diabetologia. 2020;639:1916–31.
Zhao C, Tang J, Li X, Yan Z, Zhao L, Lang W, et al. Beneficial effects of procyanidin B2 on adriamycin-induced nephrotic syndrome mice: the multi-action mechanism for ameliorating glomerular permselectivity injury. Food Funct. 2022;1316:8436–64.
Lay A, Coward RJ. Recent advances in our understanding of insulin signalling to the podocyte. Nephrol Dial Transplant. 2014;296:1127–33.
Yuan Q, Tang B, Zhang C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct Target Ther. 2022;71:182.
Du P, Fan B, Han H, Zhen J, Shang J, Wang X, et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int. 2013;842:265–76.
Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun. 2015;6:6496.
Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Investig. 2001;1072:181–9.
Alharbi I, Alhowail A, Aldubayan M, Mani V, Almogbel Y, Felemban S, et al. DOX and MET treatment induces cognitive impairment through downregulation of IL-1-alpha and IRS-1 in the rat brain. Eur Rev Med Pharmacol Sci. 2021;2511:4106–12.
Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;256:1142–7.
Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–68.
Shirata N, Ihara KI, Yamamoto-Nonaka K, Seki T, Makino SI, Oliva Trejo JA, et al. Glomerulosclerosis induced by deficiency of membrane-associated guanylate kinase inverted 2 in kidney podocytes. J Am Soc Nephrol. 2017;289:2654–69.
Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, et al. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Investig. 2013;1232:657–65.
Henshall TL, Manning JA, Alfassy OS, Goel P, Boase NA, Kawabe H, et al. Deletion of Nedd4-2 results in progressive kidney disease in mice. Cell Death Differ. 2017;2412:2150–60.
Gao S, Alarcón C, Sapkota G, Rahman S, Chen PY, Goerner N, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;363:457–68.
Fukushima T, Yoshihara H, Furuta H, Kamei H, Hakuno F, Luan J, et al. Nedd4-induced monoubiquitination of IRS-2 enhances IGF signalling and mitogenic activity. Nat Commun. 2015;6:6780.
Kim M, Chen SW, Park SW, Kim M, D’Agati VD, Yang J, et al. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;758:809–23.
Qiu Y, Lei C, Zeng J, Xie Y, Cao Y, Yuan Q, et al. Asparagine endopeptidase protects podocytes in adriamycin-induced nephropathy by regulating actin dynamics through cleaving transgelin. Mol Ther. 2023;3111:3337–54.
Liu BC, Song X, Lu XY, Li DT, Eaton DC, Shen BZ, et al. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta. 2013;18336:1434–42.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2024YFC3044900), National Natural Science Foundation of China (82370728, 81974097, 82170773, 82100729, 82100794, 82200808, 82200841, 81800610, 82300843, 82300851, and 82300786), Key Research and Development Program of Hubei Province (2023BCB034), and National Key Research and Development Program of China (2021YFC2500200). We thank the Medical Subcenter of the HUST analytical & testing center in data acquisition. We thank the Cancer Center Laboratory, Wuhan Union Hospital, for sharing the laboratory and equipment.
Author information
Authors and Affiliations
Contributions
QY and CZ designed the study; QY, BT, and HS collected and analyzed the clinical data; QY, BT, YRX, YJX, and YTZ performed animal models; QY, BT, and YRX performed in vitro experiments; QY and BT prepared figures and tables; QY and BT wrote the paper; CZ and HYL revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research investigations involving human tissue samples received approval from the Research Ethics Committee of Huazhong University of Science and Technology. All animal experimental procedures were ethically reviewed and approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Q., Tang, B., Xie, Y. et al. PRDM16 deficiency promotes podocyte injury by impairing insulin receptor signaling. Cell Death Differ 32, 1536–1554 (2025). https://doi.org/10.1038/s41418-025-01477-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01477-9