Abstract
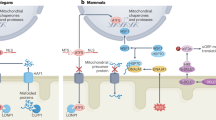
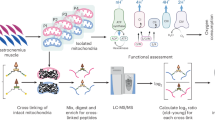
The accumulation of dysfunctional giant mitochondria is a hallmark of aged cardiomyocytes. This study investigated the core mechanism underlying this phenomenon, focusing on the disruption of mitochondrial lipid metabolism and its effects on mitochondrial dynamics and autophagy, using both naturally aging mouse models and etoposide-induced cellular senescence models. In aged cardiomyocytes, a reduction in endoplasmic reticulum-mitochondrial (ER-Mito) contacts impairs lipid transport and leads to insufficient synthesis of mitochondrial phosphatidylethanolamine (PE). A deficiency in phosphatidylserine decarboxylase (PISD) further hinders the conversion of phosphatidylserine to PE within mitochondria, exacerbating the deficit of PE production. This PE shortage disrupts autophagosomal membrane formation, leading to impaired autophagic flux and the accumulation of damaged mitochondria. Modulating LACTB expression to enhance PISD activity and PE production helps maintain mitochondrial homeostasis and the integrity of aging cardiomyocytes. These findings highlight the disruption of mitochondrial lipid metabolism as a central mechanism driving the accumulation of dysfunctional giant mitochondria in aged cardiomyocytes and suggest that inhibiting LACTB expression could serve as a potential therapeutic strategy for mitigating cardiac aging and preserving mitochondrial function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
14 May 2025
The affiliations for Guatam Sethi has been updated to affiliation 10.
References
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–78.
Mutlu AS, Duffy J, Wang MC. Lipid metabolism and lipid signals in aging and longevity. Dev Cell. 2021;56:1394–407.
Papsdorf K, Brunet A. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends Cell Biol. 2019;29:97–116.
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58.
Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15:805–12.
Domingues N, Pires J, Milosevic I, Raimundo N. Role of lipids in interorganelle communication. Trends Cell Biol. 2025;35:46–58.
Csordas G, Weaver D, Hajnoczky G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018;28:523–40.
Barazzuol L, Giamogante F, Cali T. Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role. Cell Calcium. 2021;94:102343.
Cuervo AM, Macian F. Autophagy and the immune function in aging. Curr Opin Immunol. 2014;29:97–104.
Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19:579–93.
Xie Y, Li J, Kang R, Tang D. Interplay between lipid metabolism and autophagy. Front Cell Dev Biol. 2020;8:431.
Duan C, Liu R, Kuang L, Zhang Z, Hou D, Zheng D, et al. Activated Drp1 Initiates the Formation of Endoplasmic Reticulum-Mitochondrial Contacts via Shrm4-Mediated Actin Bundling. Adv Sci (Weinh). 2023;10:e2304885.
Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–7.
Ye Y, Tyndall ER, Bui V, Bewley MC, Wang G, Hong X, et al. Multifaceted membrane interactions of human Atg3 promote LC3-phosphatidylethanolamine conjugation during autophagy. Nat Commun. 2023;14:5503.
Keckesova Z, Donaher JL, De Cock J, Freinkman E, Lingrell S, Bachovchin DA, et al. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature. 2017;543:681–6.
Acharya TK, Kumar A, Kumar S, Goswami C. TRPV4 interacts with MFN2 and facilitates endoplasmic reticulum-mitochondrial contact points for Ca(2+)-buffering. Life Sci. 2022;310:121112.
Song CC, Pantopoulos K, Chen GH, Zhong CC, Zhao T, Zhang DG, et al. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1alpha-PPARgamma pathway. Cell Mol Life Sci. 2022;79:394.
Janikiewicz J, Szymanski J, Malinska D, Patalas-Krawczyk P, Michalska B, Duszynski J, et al. Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis. 2018;9:332.
Lu X, Gong Y, Hu W, Mao Y, Wang T, Sun Z, Su X, Fu G, Wang Y, Lai D. Ultrastructural and proteomic profiling of mitochondria-associated endoplasmic reticulum membranes reveal aging signatures in striated muscle. Cell Death & Disease. 2022;13:296.
Chen YF, Kao CH, Chen YT, Wang CH, Wu CY, Tsai CY, et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23:1183–94.
Martino Adami PV, Nichtova Z, Weaver DB, Bartok A, Wisniewski T, Jones DR, et al. Perturbed mitochondria-ER contacts in live neurons that model the amyloid pathology of Alzheimer’s disease. J Cell Sci. 2019;132:jcs229906.
Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calve B, Augert A, et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun. 2014;5:3792.
Madreiter-Sokolowski CT, Waldeck-Weiermair M, Bourguignon MP, Villeneuve N, Gottschalk B, Klec C, et al. Enhanced inter-compartmental Ca(2+) flux modulates mitochondrial metabolism and apoptotic threshold during aging. Redox Biol. 2019;20:458–66.
Ziegler DV, Vindrieux D, Goehrig D, Jaber S, Collin G, Griveau A, et al. Calcium channel ITPR2 and mitochondria-ER contacts promote cellular senescence and aging. Nat Commun. 2021;12:720.
Dong J, Chen L, Ye F, Tang J, Liu B, Lin J, et al. Mic19 depletion impairs endoplasmic reticulum-mitochondrial contacts and mitochondrial lipid metabolism and triggers liver disease. Nat Commun. 2024;15:168.
Salvador-Gallego R, Hoyer MJ, Voeltz GK. SnapShot: Functions of Endoplasmic Reticulum Membrane Contact Sites. Cell. 2017;171:1224–e1.
Hernandez-Alvarez MI, Sebastian D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell. 2019;177:881–95.e17.
van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:1558–72.
Calzada E, Onguka O, Claypool SM. Phosphatidylethanolamine Metabolism in Health and Disease. Int Rev Cell Mol Biol. 2016;321:29–88.
Qiu B, Zandkarimi F, Bezjian CT, Reznik E, Soni RK, Gu W, et al. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell. 2024;187:1177–90.e18.
Zhao T, Goedhart CM, Sam PN, Sabouny R, Lingrell S, Cornish AJ, et al. PISD is a mitochondrial disease gene causing skeletal dysplasia, cataracts, and white matter changes. Life Sci Alliance. 2019;2.e201900353.
Liu N, Huang L, Xu H, He X, He X, Cao J, et al. Phosphatidylserine decarboxylase downregulation in uric acid‑induced hepatic mitochondrial dysfunction and apoptosis. MedComm (2020). 2023;4:e336.
Xu J, Chen S, Wang W, Man Lam S, Xu Y, Zhang S, et al. Hepatic CDP-diacylglycerol synthase 2 deficiency causes mitochondrial dysfunction and promotes rapid progression of NASH and fibrosis. Sci Bull (Beijing). 2022;67:299–314.
Luo Y, Zhang Y, Pang S, Min J, Wang T, Wu D, et al. PCBP1 protects bladder cancer cells from mitochondria injury and ferroptosis by inducing LACTB mRNA degradation. Mol Carcinog. 2023;62:907–19.
Chen YC, Humphries B, Brien R, Gibbons AE, Chen YT, Qyli T, et al. Functional Isolation of Tumor-Initiating Cells using Microfluidic-Based Migration Identifies Phosphatidylserine Decarboxylase as a Key Regulator. Sci Rep. 2018;8:244.
Zhao H, Wang T. PE homeostasis rebalanced through mitochondria-ER lipid exchange prevents retinal degeneration in Drosophila. PLoS Genet. 2020;16:e1009070.
Rockenfeller P, Koska M, Pietrocola F, Minois N, Knittelfelder O, Sica V, et al. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015;22:499–508.
Nanayakkara R, Gurung R, Rodgers SJ, Eramo MJ, Ramm G, Mitchell CA, et al. Autophagic lysosome reformation in health and disease. Autophagy. 2023;19:1378–95.
Xie S, Xu SC, Deng W, Tang Q. Metabolic landscape in cardiac aging: insights into molecular biology and therapeutic implications. Signal Transduct Target Ther. 2023;8:114.
Miyamoto S. Autophagy and cardiac aging. Cell Death Differ. 2019;26:653–64.
Li X, Ren Z, Huang X, Yu T LACTB, a Metabolic Therapeutic Target in Clinical Cancer Application. Cells. 2022;11:2749.
Hong W, Zeng X, Wang H, Tan X, Tian Y, Hu H, et al. PGC-1alpha loss promotes mitochondrial protein lactylation in acetaminophen-induced liver injury via the LDHB-lactate axis. Pharmacol Res. 2024;205:107228.
Tsugawa H, Ishihara T, Ogasa K, Iwanami S, Hori A, Takahashi M, et al. A lipidome landscape of aging in mice. Nat Aging. 2024;4:709–26.
Victorelli S, Salmonowicz H, Chapman J, Martini H, Vizioli MG, Riley JS, et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature. 2023;622:627–36.
Hong W, Peng X, Zhou X, Li P, Ye Z, Liang W. FXR/ASS1 axis attenuates the TAA-induced liver injury through arginine metabolism. Biochem Biophys Res Commun. 2022;611:31–7.
Acknowledgements
The Multi-SIM work was supported by Nano Insights (https://www.naxi-tech.com). SIM&ODT analyses were supported by CSR-Biotech (https://www.csr-biotech.com). J.C. acknowledges IP national support, through UID/04923 - Comprehensive Health Research Centre.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82272252, 82270378, and 82372192), Chongqing Talents Program under grant number (cstc2022ycjh-bgzxm0007) and Chongqing Natural Science Foundation General Project (No.CSTB2023NSCQ-MSX0192).
Author information
Authors and Affiliations
Contributions
D.C.Y. and H.W.L. wrote the manuscript. H.W.L. was responsible for most of the cellular experiments. Z.X. and T.Y. were responsible for searching, sorting, and summarizing the references. M.H.M. and J.D.P. were responsible for plasmid construction, R.X.P. assisted with the animal experiments. S.R. assisted with the cellular experiments. D.C.Y., M.R.Y. and L.Z.C. were responsible for the funding of this study. H.H., G.S. and M.D.Q. were responsible for the manuscript editing. M.A, S.K.B and J.C wrote the manuscript and provided the English editing. All authors (regardless of their names) have read, edited, collaborated and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All methods were performed in accordance with the relevant guidelines and regulations. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of the Chongqing Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, W., Zeng, X., Ma, R. et al. Age-associated reduction in ER-Mitochondrial contacts impairs mitochondrial lipid metabolism and autophagosome formation in the heart. Cell Death Differ 32, 1900–1914 (2025). https://doi.org/10.1038/s41418-025-01511-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01511-w
This article is cited by
-
Mitochondrial ultrastructural pathology in diabetic cardiomyopathy: integrated analysis via scanning electron microscopy and 3D visualization imaging
Cardiovascular Diabetology (2025)
-
Cellular and molecular mechanisms underlying cardiovascular aging
Cellular & Molecular Biology Letters (2025)
-
Harnessing Adipose Ferroptosis: A Promising Novel Pathway for Obesity Treatment
Current Obesity Reports (2025)